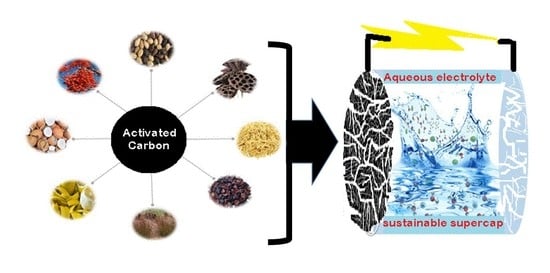
Building towards Supercapacitors with Safer Electrolytes and Carbon Electrodes from Natural Resources
Abstract
:1. Introduction
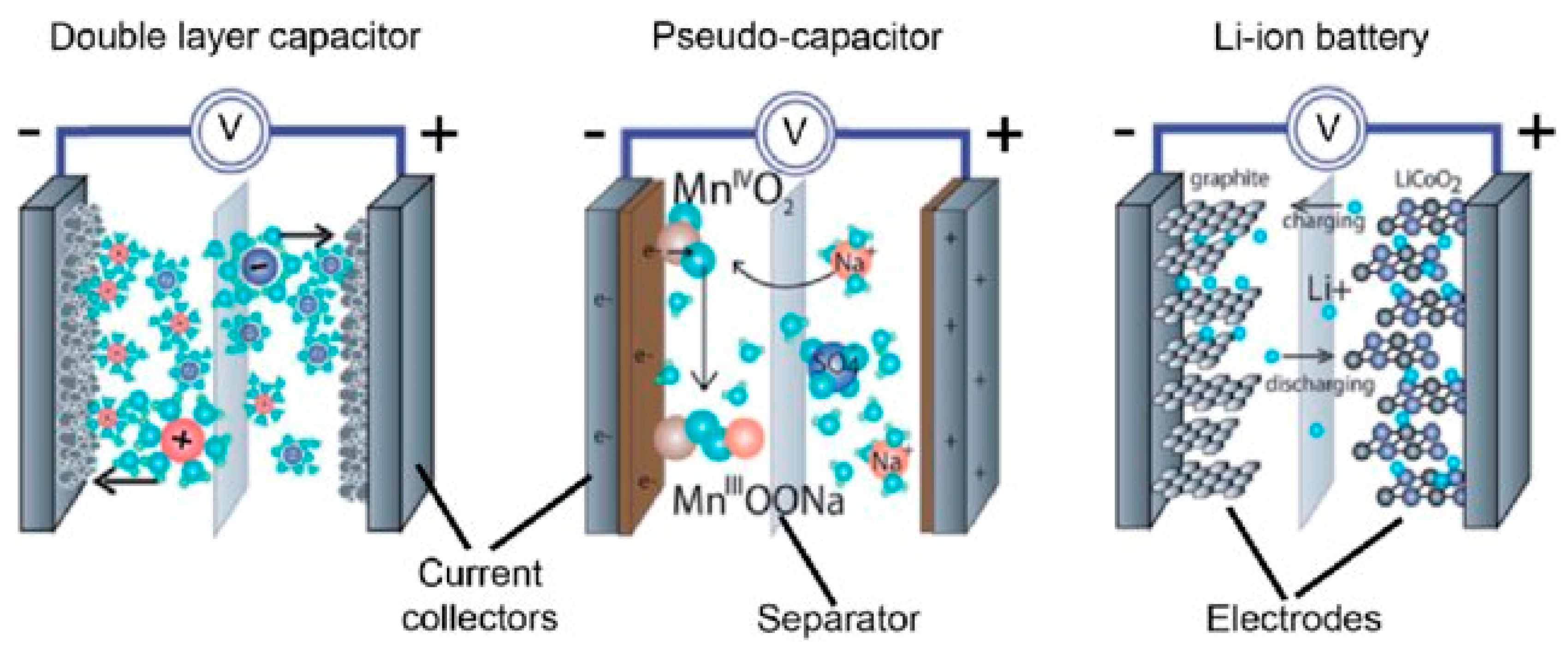
2. Supercapacitors (SCs)
2.1. Electrostatic Double-Layer Capacitor (EDLC)
2.2. Pseudocapacitors (PCs)
2.3. Hybrid Supercapacitors (HSCs)
3. Carbon Materials for Supercapacitors
- -
- Macropores with a diameter greater than 50 nm;
- -
- Mesopores with a diameter between 2 and 50 nm;
- -
- Micropores with a diameter of less than 2 nm.
3.1. Activated Carbon (AC)
3.2. Carbon Nanotubes (CNTs)
3.3. Graphene
3.4. Activated Biochar-Based SCs
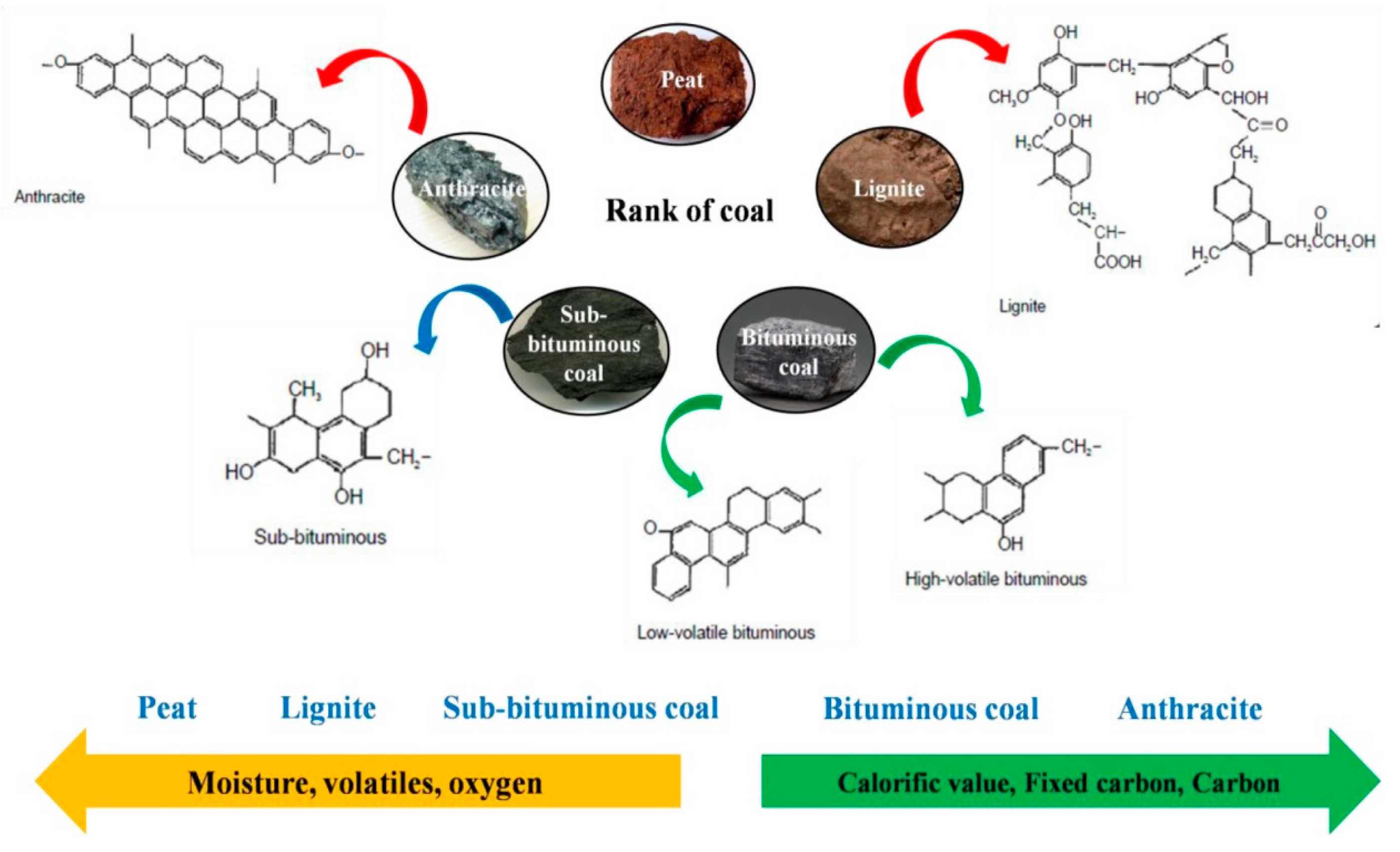
3.5. Coal-Derived AC-Based SCs
4. Electrolytes
- -
- k is ionic conductivity (S cm−1);
- -
- F is the Faraday constant (C mol−1);
- -
- zi is the charge of the ion;
- -
- Ci is the concentration of the ion i (mol cm−3);
- -
- μi is the mobility of the ion i (cm2·V−1·s−1).
4.1. Aqueous Electrolytes
4.2. Organic Electrolytes
4.3. Ionic Liquids
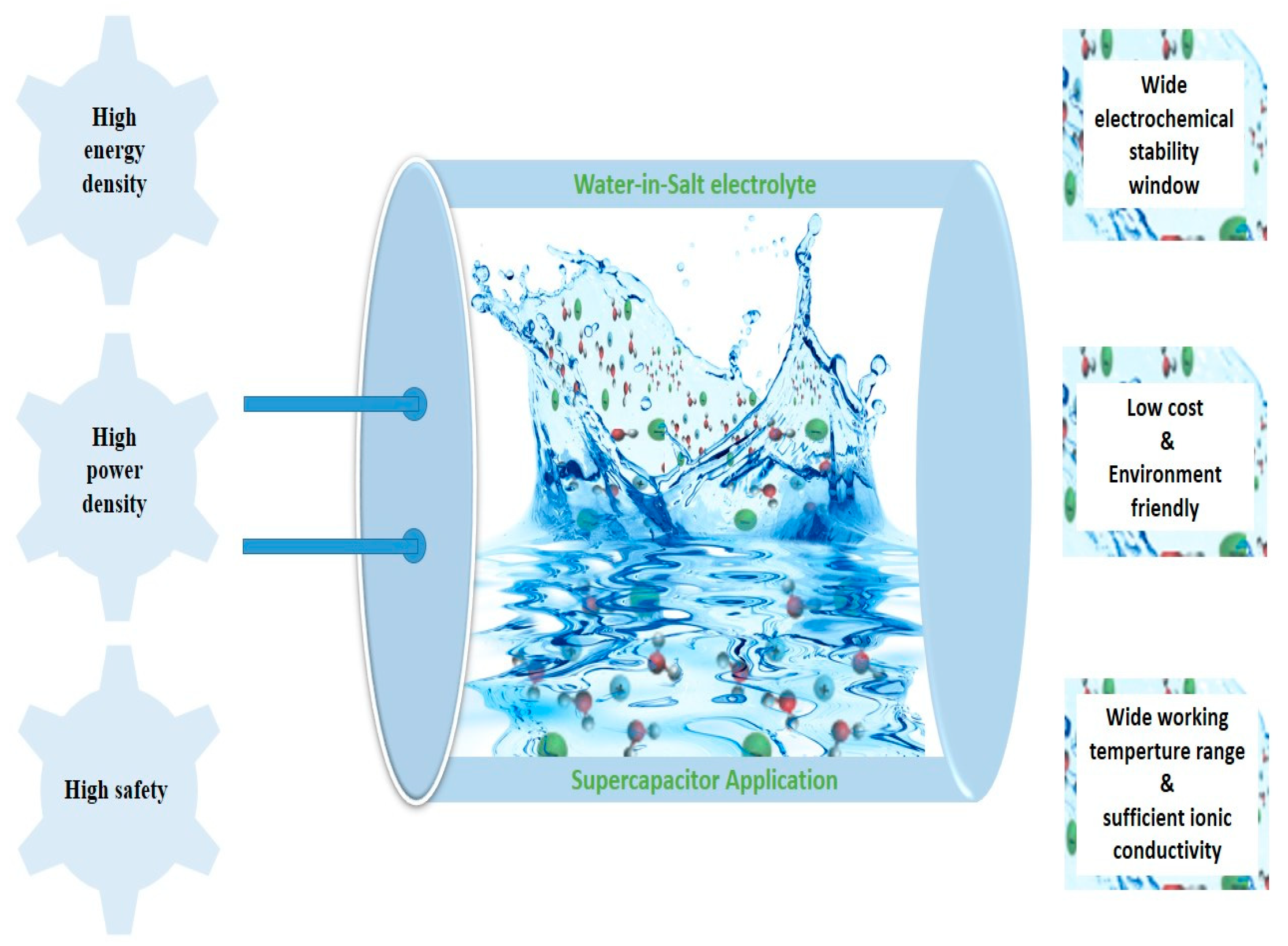
4.4. Water-in-Salt Electrolytes (WiSE)
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dias, R.A.; Mattos, C.R.; Balestieri, J.A. The limits of human development and the use of energy and natural resources. Energy Policy 2006, 34, 1026–1031. [Google Scholar] [CrossRef]
- Sovacool, B.K. The intermittency of wind, solar, and renewable electricity generators: Technical barrier or rhetorical excuse? Util. Policy 2009, 17, 288–296. [Google Scholar] [CrossRef]
- El Shahat, A.; Keyhani, A. Sizing high speed micro generators for smart grid systems. In Smart Power Grids 2011; Springer: Berlin/Heidelberg, Germany, 2012; pp. 177–234. [Google Scholar]
- Ponds, K.T.; Arefi, A.; Sayigh, A.; Ledwich, G. Aggregator of demand response for renewable integration and customer engagement: Strengths, weaknesses, opportunities, and threats. Energies 2018, 11, 2391. [Google Scholar] [CrossRef] [Green Version]
- Cao, W. Biomass-Derived Activated Carbons for Electrical Double Layer Supercapacitors: Performance and Stress Effect. Ph.D. Dissertation, University of Kentucky, Lexington, KY, USA, 2019. [Google Scholar]
- Afif, A.; Rahman, S.M.; Azad, A.T.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced materials and technologies for hybrid supercapacitors for energy storage—A review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Luo, B.; Ye, D.; Wang, L. Recent progress on integrated energy conversion and storage systems. Adv. Sci. 2017, 4, 1700104. [Google Scholar] [CrossRef] [Green Version]
- Gallo, A.; Simões-Moreira, J.; Costa, H.; Santos, M.; Dos Santos, E.M. Energy storage in the energy transition context: A technology review. Renew. Sustain. Energy Rev. 2016, 65, 800–822. [Google Scholar] [CrossRef]
- Dhimish, M.; Schofield, N. Single-switch boost-buck DC-DC converter for industrial fuel cell and photovoltaics applications. Int. J. Hydrogen Energy 2021, 47, 1241–1255. [Google Scholar] [CrossRef]
- Kamel, A.A.; Rezk, H.; Abdelkareem, M.A. Enhancing the operation of fuel cell-photovoltaic-battery-supercapacitor renewable system through a hybrid energy management strategy. Int. J. Hydrogen Energy 2021, 46, 6061–6075. [Google Scholar] [CrossRef]
- Thounthong, P.; Chunkag, V.; Sethakul, P.; Sikkabut, S.; Pierfederici, S.; Davat, B. Energy management of fuel cell/solar cell/supercapacitor hybrid power source. J. Power Source 2011, 196, 313–324. [Google Scholar] [CrossRef]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Chen, T.; Jin, Y.; Lv, H.; Yang, A.; Liu, M.; Chen, B.; Xie, Y.; Chen, Q. Applications of lithium-ion batteries in grid-scale energy storage systems. Trans. Tianjin Univ. 2020, 26, 208–217. [Google Scholar] [CrossRef] [Green Version]
- Nishi, Y. Lithium ion secondary batteries; past 10 years and the future. J. Power Source 2001, 100, 101–106. [Google Scholar] [CrossRef]
- Vandeginste, V. A review of fabrication technologies for carbon electrode-based micro-supercapacitors. Appl. Sci. 2022, 12, 862. [Google Scholar] [CrossRef]
- Smith, B.; Wills, R.; Cruden, A. Aqueous Al-ion cells and supercapacitors—A comparison. Energy Rep. 2020, 6, 166–173. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. Electrochemical capacitors for energy management. Sci. Mag. 2008, 321, 651–652. [Google Scholar] [CrossRef] [Green Version]
- Kötz, R.; Carlen, M. Principles and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Bora, M.; Bhattacharjya, D.; Saikia, B.K. Coal-Derived Activated Carbon for Electrochemical Energy Storage: Status on Supercapacitor, Li-Ion Battery, and Li–S Battery Applications. Energy Fuels 2021, 35, 18285–18307. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. In Nanoscience and Technology: A Collection of Reviews from Nature Journals; World Scientific: Singapore, 2010; pp. 320–329. [Google Scholar]
- Salanne, M.; Rotenberg, B.; Naoi, K.; Kaneko, K.; Taberna, P.-L.; Grey, C.P.; Dunn, B.; Simon, P. Efficient storage mechanisms for building better supercapacitors. Nat. Energy 2016, 1, 16070. [Google Scholar] [CrossRef] [Green Version]
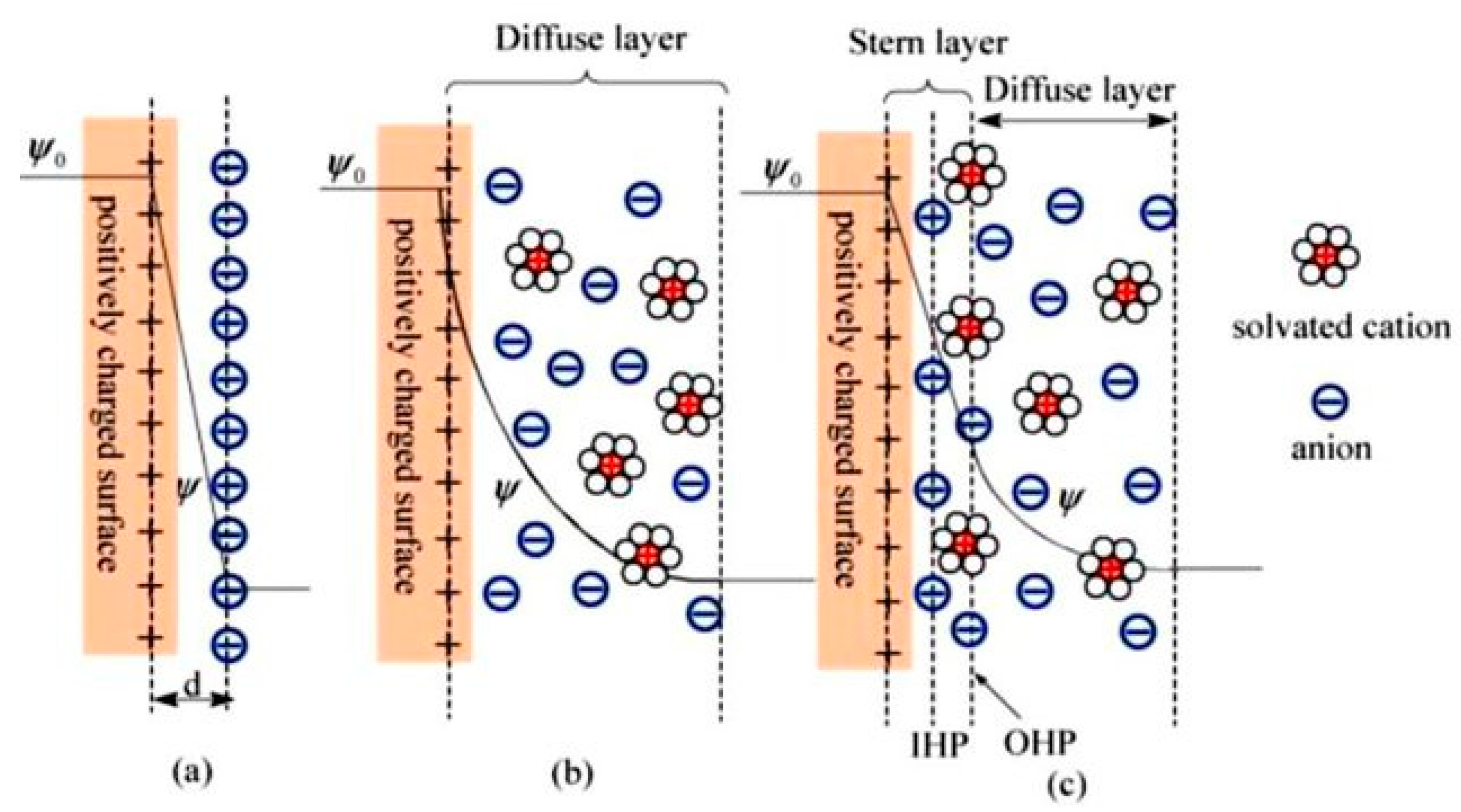
- Helmholtz, H.V. Ueber einige Gesetze der Vertheilung elektrischer Ströme in körperlichen Leitern, mit Anwendung auf die thierisch-elektrischen Versuche (Schluss.). Ann. Phys. 1853, 165, 353–377. [Google Scholar] [CrossRef] [Green Version]
- Bockris, J.; Reddy, A.M. Gamboa-Aldeco in: Modern Electrochemistry Vol. 2A; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2000. [Google Scholar]
- Guoy, G. Constitution of the electric charge at the surface of an electrolyte. J. Phys. 1910, 9, 457–467. [Google Scholar]
- Grahame, D.C. The electrical double layer and the theory of electrocapillarity. Chem. Rev. 1947, 41, 441–501. [Google Scholar] [CrossRef] [PubMed]
- Stern, O. The theory of the electrolytic double-layer. Z. Elektrochem. 1924, 30, 1014–1020. [Google Scholar]
- Luo, X.-Y.; Chen, Y.; Mo, Y. A review of charge storage in porous carbon-based supercapacitors. New Carbon Mater. 2021, 36, 49–68. [Google Scholar] [CrossRef]
- Goel, P.; Sundriyal, S.; Shrivastav, V.; Mishra, S.; Dubal, D.P.; Kim, K.-H.; Deep, A. Perovskite materials as superior and powerful platforms for energy conversion and storage applications. Nano Energy 2021, 80, 105552. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Wang, T.; Shao, J.; Wang, D.; Yang, Y.-W. Mesoporous transition metal oxides for supercapacitors. Nanomaterials 2015, 5, 1667–1689. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gómez-Romero, P.; Korotcenkov, G. Metal Oxides in Supercapacitors; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Karbak, M.; Boujibar, O.; Lahmar, S.; Ah-Lung, G.; Autret-Lambert, C.; Chafik, T.; Ghamouss, F. Nanometric MnO2 and MnO2-Graphene Oxide Materials Enabled by a Solvent-Assisted Synthesis and Their Application in Asymmetric Supercapacitors. Energy Technol. 2023, 11, 2201243. [Google Scholar] [CrossRef]
- Prasad, K.R.; Munichandraiah, N. Fabrication and evaluation of 450 F electrochemical redox supercapacitors using inexpensive and high-performance, polyaniline coated, stainless-steel electrodes. J. Power Source 2002, 112, 443–451. [Google Scholar] [CrossRef]
- Clemente, A.; Panero, S.; Spila, E.; Scrosati, B. Solid-state, polymer-based, redox capacitors. Solid State Ion. 1996, 85, 273–277. [Google Scholar] [CrossRef]
- Mastragostino, M.; Paraventi, R.; Zanelli, A. Supercapacitors based on composite polymer electrodes. J. Electrochem. Soc. 2000, 147, 3167. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.Y.; Liang, M.M.; Ma, H.; Zhang, H.M.; Guo, Z.; Zhao, Y.; Zhao, Y.Z.; RehmanLashari, N.U.; Miao, Z.C. Enhanced cycling performance of surface-amorphized Co3S4 as robust cathode for supercapacitors. J. Energy Storage 2023, 58, 106322. [Google Scholar] [CrossRef]
- Majumdar, D.; Maiyalagan, T.; Jiang, Z. Recent progress in ruthenium oxide-based composites for supercapacitor applications. ChemElectroChem 2019, 6, 4343–4372. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Long, J.W. To be or not to be pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185. [Google Scholar] [CrossRef] [Green Version]
- Jost, K.; Dion, G.; Gogotsi, Y. Textile energy storage in perspective. J. Mater. Chem. A 2014, 2, 10776–10787. [Google Scholar] [CrossRef]
- Conway, B.E.; Birss, V.; Wojtowicz, J. The role and utilization of pseudocapacitance for energy storage by supercapacitors. J. Power Source 1997, 66, 1–14. [Google Scholar] [CrossRef]
- Samantara, A.K.; Ratha, S. Materials Development for Active/Passive Components of a Supercapacitor: Background, Present Status and Future Perspective; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Sundriyal, S.; Kaur, H.; Bhardwaj, S.K.; Mishra, S.; Kim, K.-H.; Deep, A. Metal-organic frameworks and their composites as efficient electrodes for supercapacitor applications. Coord. Chem. Rev. 2018, 369, 15–38. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef]
- Vangari, M.; Pryor, T.; Jiang, L. Supercapacitors: Review of materials and fabrication methods. J. Energy Eng. 2013, 139, 72–79. [Google Scholar] [CrossRef]
- Wu, N.; Bai, X.; Pan, D.; Dong, B.; Wei, R.; Naik, N.; Patil, R.R.; Guo, Z. Recent advances of asymmetric supercapacitors. Adv. Mater. Interfaces 2021, 8, 2001710. [Google Scholar] [CrossRef]
- Peng, H.; Zheng, Y.; Antheaume, C.; Samorì, P.; Ciesielski, A. Novel thiophene-based donor–acceptor scaffolds as cathodes for rechargeable aqueous zinc-ion hybrid supercapacitors. Chem. Commun. 2022, 58, 6689–6692. [Google Scholar] [CrossRef] [PubMed]
- Swain, N.; Balasubramaniam, S.; Ramadoss, A. High energy density supercapattery empowered by efficient binder-free three-dimensional carbon coated NiCo2O4/Ni battery and Fe3S4@NiCo pseudocapacitive electrodes. J. Energy Storage 2023, 58, 106220. [Google Scholar] [CrossRef]
- Wang, H.; Liang, M.; Ma, H.; Zhang, H.; Ma, C.; Duan, W.; Zhao, Y.; Miao, Z. Defect-rich Ni3S4−x as a robust electrode material for supercapacitor and aqueous Ni-Zn battery applications. J. Alloys Compd. 2023, 933, 167733. [Google Scholar] [CrossRef]
- Liang, M.; Li, X.; Kang, Y.; ur RehmanLashari, N.; Zhang, X.; Zhao, Y.; Wang, H.; Miao, Z.; Fu, C. Ni-doped tin disulfide@ Nickel hydroxide as robust cathode toward durable supercapacitor and aqueous Ni-Zn battery. J. Power Source 2022, 535, 231486. [Google Scholar] [CrossRef]
- Kang, J.H. Fabrication and Characterization of Nano Carbon-Based Electrochemical Double-Layer Capacitors. Ph.D. Thesis, University of Waterloo, Waterloo, ON, Canada, 2015. [Google Scholar]
- Cheng, Q.; Tang, J.; Ma, J.; Zhang, H.; Shinya, N.; Qin, L.-C. Graphene and carbon nanotube composite electrodes for supercapacitors with ultra-high energy density. Phys. Chem. Chem. Phys. 2011, 13, 17615–17624. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Zhang, L.; Han, G.; Liu, Z.; Huang, J.; Zhang, L.; Luo, J.; Zhu, Z.; Qiao, Z.A. Emulsion-assisted interfacial polymerization strategy: Controllable architectural engineering of anisotropic and isotropic nanoparticles for high-performance supercapacitors. Battery Energy 2023, 2, 20220058. [Google Scholar] [CrossRef]
- Bizuneh, G.G.; Adam, A.M.; Ma, J. Progress on carbon for electrochemical capacitors. Battery Energy 2023, 2, 20220021. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.; Everett, D.; Haynes, J.; Pernicone, N.; Ramsay, J.; Sing, K.; Unger, K. Recommendations for the characterization of porous solids (Technical Report). Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Wei, L.; Yushin, G. Nanostructured activated carbons from natural precursors for electrical double layer capacitors. Nano Energy 2012, 1, 552–565. [Google Scholar] [CrossRef]
- Boujibar, O.; Ghamouss, F.; Ghosh, A.; Achak, O.; Chafik, T. Activated carbon with exceptionally high surface area and tailored nanoporosity obtained from natural anthracite and its use in supercapacitors. J. Power Source 2019, 436, 226882. [Google Scholar] [CrossRef]
- Boujibar, O.; Ghosh, A.; Achak, O.; Chafik, T.; Ghamouss, F. A high energy storage supercapacitor based on nanoporous activated carbon electrode made from Argan shells with excellent ion transport in aqueous and non-aqueous electrolytes. J. Energy Storage 2019, 26, 100958. [Google Scholar] [CrossRef]
- Chafik, T. Nanoporous Carbonated Materials Prepared from the Shell of the Argan Fruit 1–14. WO2012050411A1, 19 April 2012. [Google Scholar]
- Molina-Sabio, M.; Rodrıguez-Reinoso, F. Role of chemical activation in the development of carbon porosity. Colloids Surf. A Physicochem. Eng. Asp. 2004, 241, 15–25. [Google Scholar] [CrossRef]
- Strand, G. Activated Carbon for Purification of Alcohol; Gert Strand AB: Malmoe, Sweden, 2001. [Google Scholar]
- Markets and Markets. Available online: https://www.researchandmarkets.com/reports/4040498/activated-carbon-market-by-type-powdered (accessed on 20 May 2023).
- Moreira, J.V.S.; Corat, E.J.; May, P.W.; Cardoso, L.D.R.; Lelis, P.A.; Zanin, H. Freestanding aligned multi-walled carbon nanotubes for supercapacitor devices. J. Electron. Mater. 2016, 45, 5781–5788. [Google Scholar] [CrossRef]
- Karbak, M.; Boujibar, O.; Lahmar, S.; Autret-Lambert, C.; Chafik, T.; Ghamouss, F. Chemical Production of graphene oxide with high surface energy for supercapacitor applications. C 2022, 8, 27. [Google Scholar] [CrossRef]
- Xia, Z.Y.; Pezzini, S.; Treossi, E.; Giambastiani, G.; Corticelli, F.; Morandi, V.; Zanelli, A.; Bellani, V.; Palermo, V. The Exfoliation of Graphene in Liquids by Electrochemical, Chemical, and Sonication-Assisted Techniques: A Nanoscale Study. Adv. Funct. Mater. 2013, 23, 4684–4693. [Google Scholar] [CrossRef]
- Xia, Z.Y.; Giambastiani, G.; Christodoulou, C.; Nardi, M.V.; Koch, N.; Treossi, E.; Bellani, V.; Pezzini, S.; Corticelli, F.; Morandi, V.; et al. Synergic Exfoliation of Graphene with Organic Molecules and Inorganic Ions for the Electrochemical Production of Flexible Electrodes. Chempluschem 2014, 79, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Cheaptubes. Available online: https://www.cheaptubes.com/graphene-synthesis-properties-and-applications/ (accessed on 20 May 2023).
- Wang, D.; Sheng, L.; Jiang, M.; Jin, X.; Lin, X.; Lee, S.Y.; Shi, J.; Chen, W. Density and porosity optimization of graphene monoliths with high mass-loading for high-volumetric-capacitance electrodes. Battery Energy 2022, 1, 20220017. [Google Scholar] [CrossRef]
- Azam, M.A.; Ramli, N.S.N.; Nor, N.A.N.M.; Nawi, T.I.T. Recent advances in biomass-derived carbon, mesoporous materials, and transition metal nitrides as new electrode materials for supercapacitor: A short review. Int. J. Energy Res. 2021, 45, 8335–8346. [Google Scholar] [CrossRef]
- Mestre, A.S.; Carvalho, A.P. Nanoporous carbon synthesis: An old story with exciting new chapters. In Porosity; Ghrib, T., Ed.; IntechOpen: London, UK, 2018; pp. 37–68. [Google Scholar]
- Idris-Hermann, K.T.; Raoul, T.T.D.; Giscard, D.; Gabche, A.S. Preparation and characterization of activated carbons from bitter kola (Garcinia kola) nut shells by chemical activation method using H3PO4; KOH and ZnCl2. Chem. Sci. Int. J. 2018, 23, 1–15. [Google Scholar] [CrossRef]
- Jiang, L.; Sheng, L.; Fan, Z. Biomass-derived carbon materials with structural diversities and their applications in energy storage. Sci. China Mater. 2018, 61, 133–158. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.T.; Reed, A.R. Development of activated carbon pore structure via physical and chemical activation of biomass fibre waste. Biomass Bioenergy 2006, 30, 144–152. [Google Scholar] [CrossRef]
- Şentorun-Shalaby, Ç.D.; Uçak-Astarlıoğlu, M.G.; Artok, L.; Sarıcı, Ç. Preparation and characterization of activated carbons by one-step steam pyrolysis/activation from apricot stones. Microporous Mesoporous Mater. 2006, 88, 126–134. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Liu, Z.; Cui, G.; Chen, L. Biomass-derived materials for electrochemical energy storages. Prog. Polym. Sci. 2015, 43, 136–164. [Google Scholar] [CrossRef]
- Ayinla, R.T.; Dennis, J.; Zaid, H.; Sanusi, Y.; Usman, F.; Adebayo, L. A review of technical advances of recent palm bio-waste conversion to activated carbon for energy storage. J. Clean. Prod. 2019, 229, 1427–1442. [Google Scholar] [CrossRef]
- Sundriyal, S.; Shrivastav, V.; Pham, H.D.; Mishra, S.; Deep, A.; Dubal, D.P. Advances in bio-waste derived activated carbon for supercapacitors: Trends, challenges and prospective. Resour. Conserv. Recycl. 2021, 169, 105548. [Google Scholar] [CrossRef]
- Guo, Z.; Yan, N.; Lapkin, A.A. Towards circular economy: Integration of bio-waste into chemical supply chain. Curr. Opin. Chem. Eng. 2019, 26, 148–156. [Google Scholar] [CrossRef]
- Dugmore, T.I.; Clark, J.H.; Bustamante, J.; Houghton, J.A.; Matharu, A.S. Valorisation of biowastes for the production of green materials using chemical methods. In Chemistry and Chemical Technologies in Waste Valorization; Springer: Berlin/Heidelberg, Germany, 2017; pp. 73–121. [Google Scholar]
- Yahya, M.A.; Al-Qodah, Z.; Ngah, C.Z. Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: A review. Renew. Sustain. Energy Rev. 2015, 46, 218–235. [Google Scholar] [CrossRef]
- Liu, S.; Ge, L.; Gao, S.; Zhuang, L.; Zhu, Z.; Wang, H. Activated carbon derived from bio-waste hemp hurd and retted hemp hurd for CO2 adsorption. Compos. Commun. 2017, 5, 27–30. [Google Scholar] [CrossRef]
- Manasa, P.; Lei, Z.J.; Ran, F. Biomass waste derived low cost activated carbon from carchorus olitorius (Jute fiber) as sustainable and novel electrode material. J. Energy Storage 2020, 30, 101494. [Google Scholar] [CrossRef]
- Raj, C.J.; Rajesh, M.; Manikandan, R.; Yu, K.H.; Anusha, J.; Ahn, J.H.; Kim, D.-W.; Park, S.Y.; Kim, B.C. High electrochemical capacitor performance of oxygen and nitrogen enriched activated carbon derived from the pyrolysis and activation of squid gladius chitin. J. Power Source 2018, 386, 66–76. [Google Scholar] [CrossRef]
- Na, R.; Wang, X.; Lu, N.; Huo, G.; Lin, H.; Wang, G. Novel egg white gel polymer electrolyte and a green solid-state supercapacitor derived from the egg and rice waste. Electrochim. Acta 2018, 274, 316–325. [Google Scholar] [CrossRef]
- Gong, C.; Wang, X.; Ma, D.; Chen, H.; Zhang, S.; Liao, Z. Microporous carbon from a biological waste-stiff silkworm for capacitive energy storage. Electrochim. Acta 2016, 220, 331–339. [Google Scholar] [CrossRef]
- Rawal, S.; Joshi, B.; Kumar, Y. Synthesis and characterization of activated carbon from the biomass of Saccharum bengalense for electrochemical supercapacitors. J. Energy Storage 2018, 20, 418–426. [Google Scholar] [CrossRef]
- Su, X.-L.; Li, S.-H.; Jiang, S.; Peng, Z.-K.; Guan, X.-X.; Zheng, X.-C. Superior capacitive behavior of porous activated carbon tubes derived from biomass waste-cotonier strobili fibers. Adv. Powder Technol. 2018, 29, 2097–2107. [Google Scholar] [CrossRef]
- Song, M.; Zhou, Y.; Ren, X.; Wan, J.; Du, Y.; Wu, G.; Ma, F. Biowaste-based porous carbon for supercapacitor: The influence of preparation processes on structure and performance. J. Colloid Interface Sci. 2019, 535, 276–286. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, K. Converting corncob to activated porous carbon for supercapacitor application. Nanomaterials 2018, 8, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitravinda, T.; Nanaji, K.; Anandan, S.; Jyothirmayi, A.; Chakravadhanula, V.S.K.; Sharma, C.S.; Rao, T.N. Facile synthesis of corn silk derived nanoporous carbon for an improved supercapacitor performance. J. Electrochem. Soc. 2018, 165, A3369. [Google Scholar] [CrossRef]
- Yin, L.; Chen, Y.; Zhao, X.; Hou, B.; Cao, B. 3-Dimensional hierarchical porous activated carbon derived from coconut fibers with high-rate performance for symmetric supercapacitors. Mater. Des. 2016, 111, 44–50. [Google Scholar] [CrossRef]
- Mutuma, B.K.; Sylla, N.F.; Bubu, A.; Ndiaye, N.M.; Santoro, C.; Brilloni, A.; Poli, F.; Manyala, N.; Soavi, F. Valorization of biodigestor plant waste in electrodes for supercapacitors and microbial fuel cells. Electrochim. Acta 2021, 391, 138960. [Google Scholar] [CrossRef]
- Dhakal, G.; Mohapatra, D.; Kim, Y.-I.; Lee, J.; Kim, W.K.; Shim, J.-J. High-performance supercapacitors fabricated with activated carbon derived from lotus calyx biowaste. Renew. Energy 2022, 189, 587–600. [Google Scholar] [CrossRef]
- Liang, T.; Hou, R.; Dou, Q.; Zhang, H.; Yan, X. The Applications of Water-in-Salt Electrolytes in Electrochemical Energy Storage Devices. Adv. Funct. Mater. 2021, 31, 2006749. [Google Scholar] [CrossRef]
- Wang, Z.; Yun, S.; Wang, X.; Wang, C.; Si, Y.; Zhang, Y.; Xu, H. Aloe peel-derived honeycomb-like bio-based carbon with controllable morphology and its superior electrochemical properties for new energy devices. Ceram. Int. 2019, 45, 4208–4218. [Google Scholar] [CrossRef]
- Surya, K.; Michael, M.S. Hierarchical porous activated carbon prepared from biowaste of lemon peel for electrochemical double layer capacitors. Biomass Bioenergy 2021, 152, 106175. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, Z.; Wang, Y.; Liu, Z.; Li, Z. Simple one-pot strategy for converting biowaste into valuable graphitized hierarchically porous biochar for high-efficiency capacitive storage. J. Energy Storage 2021, 44, 103259. [Google Scholar] [CrossRef]
- Quan, H.; Tao, W.; Wang, Y.; Chen, D. Enhanced supercapacitor performance of Camellia oleifera shell derived hierarchical porous carbon by carbon quantum dots. J. Energy Storage 2022, 55, 105573. [Google Scholar] [CrossRef]
- Vinayagam, M.; Suresh Babu, R.; Sivasamy, A.; Ferreira de Barros, A.L. Biomass-derived porous activated carbon from Syzygium cumini fruit shells and Chrysopogon zizanioides roots for high-energy density symmetric supercapacitors. Biomass Bioenergy 2020, 143, 105838. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, D.; Wang, S. Self-assembly of biomass derivatives into multiple heteroatom-doped 3D-interconnected porous carbon for advanced supercapacitors. Carbon 2022, 199, 258–267. [Google Scholar] [CrossRef]
- Xu, X.; Sielicki, K.; Min, J.; Li, J.; Hao, C.; Wen, X.; Chen, X.; Mijowska, E. One-step converting biowaste wolfberry fruits into hierarchical porous carbon and its application for high-performance supercapacitors. Renew. Energy 2022, 185, 187–195. [Google Scholar] [CrossRef]
- Cao, L.; Li, H.; Xu, Z.; Zhang, H.; Ding, L.; Wang, S.; Zhang, G.; Hou, H.; Xu, W.; Yang, F.; et al. Comparison of the heteroatoms-doped biomass-derived carbon prepared by one-step nitrogen-containing activator for high performance supercapacitor. Diamond. Relat. Mater. 2021, 114, 108316. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, H.; Tan, Z.; Cheng, X. Rice husk derived capacitive carbon prepared by one-step molten salt carbonization for supercapacitors. J. Energy Storage 2022, 55, 105437. [Google Scholar] [CrossRef]
- Rani, M.U.; Nanaji, K.; Rao, T.N.; Deshpande, A.S. Corn husk derived activated carbon with enhanced electrochemical performance for high-voltage supercapacitors. J. Power Source 2020, 471, 228387. [Google Scholar] [CrossRef]
- Elmouwahidi, A.; Bailón-García, E.; Pérez-Cadenas, A.F.; Maldonado-Hódar, F.J.; Carrasco-Marín, F. Activated carbons from KOH and H3PO4-activation of olive residues and its application as supercapacitor electrodes. Electrochim. Acta 2017, 229, 219–228. [Google Scholar] [CrossRef]
- Coal—Statistical Review of World Energy 2021-BP. Available online: https://www.bp.com/content/dam/bp/business-sites/en/global/corporate/pdfs/energy-economics/statistical-review/bp-stats-review-2021-full-report.pdf (accessed on 11 July 2023).
- Nalbandian, H.; House, P. Non-Fuel Uses of Coal; IEA Coal Research Center: London, UK, 2014. [Google Scholar]
- Voncken, J. The Origin and Classification of Coal. In Geology of Coal Deposits of South Limburg, The Netherlands; Springer: Berlin/Heidelberg, Germany, 2020; pp. 25–40. [Google Scholar]
- Zhao, X.-Y.; Huang, S.-S.; Cao, J.-P.; Xi, S.-C.; Wei, X.-Y.; Kamamoto, J.; Takarada, T. KOH activation of a HyperCoal to develop activated carbons for electric double-layer capacitors. J. Anal. Appl. Pyrolysis 2014, 105, 116–121. [Google Scholar] [CrossRef] [Green Version]
- Shi, M.; Xin, Y.; Chen, X.; Zou, K.; Jing, W.; Sun, J.; Chen, Y.; Liu, Y. Coal-derived porous activated carbon with ultrahigh specific surface area and excellent electrochemical performance for supercapacitors. J. Alloys Compd. 2021, 859, 157856. [Google Scholar] [CrossRef]
- Peng, Z.; Guo, Z.; Chu, W.; Wei, M. Facile synthesis of high-surface-area activated carbon from coal for supercapacitors and high CO2 sorption. RSC Adv. 2016, 6, 42019–42028. [Google Scholar] [CrossRef]
- Bora, M.; Tamuly, J.; Maria Benoy, S.; Hazarika, S.; Bhattacharjya, D.; Saikia, B.K. Highly scalable and environment-friendly conversion of low-grade coal to activated carbon for use as electrode material in symmetric supercapacitor. Fuel 2022, 329, 125385. [Google Scholar] [CrossRef]
- Dong, D.; Zhang, Y.; Xiao, Y.; Wang, T.; Wang, J.; Romero, C.E.; Pan, W.-P. High performance aqueous supercapacitor based on nitrogen-doped coal-based activated carbon electrode materials. J. Colloid Interface Sci. 2020, 580, 77–87. [Google Scholar] [CrossRef]
- Qin, B.; Wang, Q.; Zhang, X.; Xie, X.; Jin, L.E.; Cao, Q. One-pot synthesis of interconnected porous carbon derived from coal tar pitch and cellulose for high-performance supercapacitors. Electrochim. Acta 2018, 283, 655–663. [Google Scholar] [CrossRef]
- Cheng, J.; Lu, Z.; Zhao, X.; Chen, X.; Liu, Y. Green needle coke-derived porous carbon for high-performance symmetric supercapacitor. J. Power Source 2021, 494, 229770. [Google Scholar] [CrossRef]
- Liu, H.; Song, H.; Hou, W.; Chang, Y.; Zhang, Y.; Li, Y.; Zhao, Y.; Han, G. Coal tar pitch-based hierarchical porous carbons prepared in molten salt for supercapacitors. Mater. Chem. Phys. 2021, 265, 124491. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, X.; Huang, G.; Xing, B.; Fan, Y.; Zhang, C.; Cao, Y. Microporous carbon derived from anthracite as supercapacitor electrodes with commercial level mass loading. J. Energy Storage 2021, 43, 103200. [Google Scholar] [CrossRef]
- Dong, D.; Zhang, Y.; Wang, T.; Wang, J.; Romero, C.E.; Pan, W.-p. Enhancing the pore wettability of coal-based porous carbon as electrode materials for high performance supercapacitors. Mater. Chem. Phys. 2020, 252, 123381. [Google Scholar] [CrossRef]
- Yang, N.; Ji, L.; Fu, H.; Shen, Y.; Wang, M.; Liu, J.; Chang, L.; Lv, Y. Hierarchical porous carbon derived from coal-based carbon foam for high-performance supercapacitors. Chin. Chem. Lett. 2022, 33, 3961–3967. [Google Scholar] [CrossRef]
- Mastragostino, M.; Arbizzani, C.; Paraventi, R.; Zanelli, A. Polymer selection and cell design for electric-vehicle supercapacitors. J. Electrochem. Soc. 2000, 147, 407. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Sajjad, M.; Khan, M.I.; Cheng, F.; Lu, W. A review on selection criteria of aqueous electrolytes performance evaluation for advanced asymmetric supercapacitors. J. Energy Storage 2021, 40, 102729. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, K.; Chen, Y. Wide Voltage Aqueous Asymmetric Supercapacitors: Advances, Strategies, and Challenges. Adv. Funct. Mater. 2021, 32, 2108107. [Google Scholar] [CrossRef]
- Xu, C.; Yang, G.; Wu, D.; Yao, M.; Xing, C.; Zhang, J.; Zhang, H.; Li, F.; Feng, Y.; Qi, S. Roadmap on Ionic Liquid Electrolytes for Energy Storage Devices. Chem. Asian J. 2021, 16, 549–562. [Google Scholar] [CrossRef]
- Bhat, T.; Patil, P.; Rakhi, R. Recent trends in electrolytes for supercapacitors. J. Energy Storage 2022, 50, 104222. [Google Scholar] [CrossRef]
- Abdallah, T.; Lemordant, D.; Claude-Montigny, B. Are room temperature ionic liquids able to improve the safety of supercapacitors organic electrolytes without degrading the performances? J. Power Source 2012, 201, 353–359. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Keim, W. Ionic liquids—New “solutions” for transition metal catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Welton, T. Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef]
- Liu, H.; Yu, H. Ionic liquids for electrochemical energy storage devices applications. J. Mater. Sci. Technol. 2019, 35, 674–686. [Google Scholar] [CrossRef]
- Stettner, T.; Balducci, A. Protic ionic liquids in energy storage devices: Past, present and future perspective. Energy Storage Mater. 2021, 40, 402–414. [Google Scholar] [CrossRef]
- Galiński, M.; Lewandowski, A.; Stępniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. “Water-in-salt” electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 2015, 350, 938–943. [Google Scholar] [CrossRef]
- Eftekhari, A. High-energy aqueous lithium batteries. Adv. Energy Mater. 2018, 8, 1801156. [Google Scholar] [CrossRef]
- Bu, X.; Su, L.; Dou, Q.; Lei, S.; Yan, X. A low-cost “water-in-salt” electrolyte for a 2.3 V high-rate carbon-based supercapacitor. J. Mater. Chem. A 2019, 7, 7541–7547. [Google Scholar] [CrossRef]
- Lannelongue, P.; Bouchal, R.; Mourad, E.; Bodin, C.; Olarte, M.; Le Vot, S.; Favier, F.; Fontaine, O. “Water-in-Salt” for supercapacitors: A compromise between voltage, power density, energy density and stability. J. Electrochem. Soc. 2018, 165, A657. [Google Scholar] [CrossRef]
- Suo, L.; Han, F.; Fan, X.; Liu, H.; Xu, K.; Wang, C. “Water-in-Salt” electrolytes enable green and safe Li-ion batteries for large scale electric energy storage applications. J. Mater. Chem. A 2016, 4, 6639–6644. [Google Scholar] [CrossRef]
- Sun, W.; Suo, L.; Wang, F.; Eidson, N.; Yang, C.; Han, F.; Ma, Z.; Gao, T.; Zhu, M.; Wang, C. “Water-in-Salt” electrolyte enabled LiMn2O4/TiS2 Lithium-ion batteries. Electrochem. Commun. 2017, 82, 71–74. [Google Scholar] [CrossRef]
- Dou, Q.; Lei, S.; Wang, D.-W.; Zhang, Q.; Xiao, D.; Guo, H.; Wang, A.; Yang, H.; Li, Y.; Shi, S. Safe and high-rate supercapacitors based on an “acetonitrile/water in salt” hybrid electrolyte. Energy Environ. Sci. 2018, 11, 3212–3219. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Feldblyum, J.I.; Mackanic, D.G.; Lissel, F.; Michels, D.L.; Cui, Y.; Bao, Z. Concentrated mixed cation acetate “water-in-salt” solutions as green and low-cost high voltage electrolytes for aqueous batteries. Energy Environ. Sci. 2018, 11, 2876–2883. [Google Scholar] [CrossRef]
- Tian, Z.; Deng, W.; Wang, X.; Liu, C.; Li, C.; Chen, J.; Xue, M.; Li, R.; Pan, F. Superconcentrated aqueous electrolyte to enhance energy density for advanced supercapacitors. Funct. Mater. Lett. 2017, 10, 1750081. [Google Scholar] [CrossRef]
- Maier, R.M.; Gentry, T.J. Microorganisms and organic pollutants. In Environmental Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 377–413. [Google Scholar]
- El Halimi, M.S.; Poli, F.; Mancuso, N.; Olivieri, A.; Mattioli, E.J.; Calvaresi, M.; Chafik, T.; Zanelli, A.; Soavi, F. Circumneutral concentrated ammonium acetate solution as water-in-salt electrolyte. Electrochim. Acta 2021, 389, 138653. [Google Scholar] [CrossRef]




| Material | Carbon Nanotubes | Graphene | Activated Carbon |
|---|---|---|---|
 |  |  | |
| Conductivity | High | High | Structure dependent |
| Volumetric capacitance | Low | Moderate | High |
| Cost | High | Moderate | Low |
| Biowaste | SSA (m2 g−1) | Specific Capacitance (F/g) | Electrolyte for the Assembled Device | Energy Density (Wh kg−1) | Power Density (kW kg−1) | Cyclic Stability (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Lotus calyx | 798 | 223 (1 A/g) | 1 M Na2SO4 | 17.5 | 0.8 | 95.5 (10,000) | [94] |
| Stem pith of helianthus annuus | 1900.2 | 403.6 (0.5 A/g) | 6 M KOH | 5.8 | 17.3 | 94.5 (10,000) | [95] |
| Mangosteen peel | 2623 | 357 (1 A/g) | 1 M Li2SO4 | 17.28 | 0.401 | 80 (10,000) | [96] |
| Lemon peel | 744.78 | 152.14 (1 mV/s) | 0.5 M H2SO4 | 4.67 | 8.113 | 95.5 (10,000) | [97] |
| Idesia polycarpa fruit oil residue | 1537.1 | 350.4 (1 A/g) | 6 M KOH | 6.4 | 0.1 | 95.4 (10,000) | [98] |
| Camellia oleifera shell | 1750 | 259 (1 A/g) | 1 M H2SO4 | 8.61 | 0.477 | 94 (20,000) | [99] |
| Syzygium cumini | - | 253 (0.5 A/g) | 6 M KOH | 27.22 | 0.2 | 96.5 (5000) | [100] |
| Chrysopogon zizanioides | - | 294 (0.5 A/g) | 6 M KOH | 16.72 | 0.2 | 91.8 (5000) | [100] |
| Baobab fruit shells | 2700.65 | 332 (1 A/g) | 6 M KOH | 17.7 | 166.4 | 93 (10,000) | [101] |
| Waste wolfberry fruits | 1423 | 365 (0.2 A/g) | 1 M Li2SO4 | 23.2 | 0.225 | 96.4 (10,000) | [102] |
| Camellia pollen | 810 | 300 (1 A/g) | 6 M KOH | 14.3 | - | 84.5 (20,000) | [103] |
| Rice husk | 1183 | 163.1 (0.2 A/g) | 6 M KOH | 5.1 | 0.049 | 85 (6000) | [104] |
| Corn Husk | 1370 | 127 (1 A/g) | 6 M KOH | 4.4 | 0.248 | 90 (5000) | [105] |
| Olive Seed | 1700 | 224 (0.25 A/g) | 1 M H2SO4/ 1 M Na2SO4 | 3–5 | 20–30 | 91 (12,500) | [106] |
| Lignin residue of biodigester | 1879 | 114 (0.5 A/g) | 2.5 M KNO3 | 10 | 6.9 | 84.5 (15,000) | [93] |
| Materials | SSA (m2 g−1) | Specific Capacitance (F g−1) | Electrolyte for the Assembled Device | Energy Density (Wh kg−1) | Power Density (W kg−1) | Cyclic Stability (%) | Ref. |
|---|---|---|---|---|---|---|---|
| Sub-bituminous coal | 1021 | 227 (0.5 A/g) | 6 M KOH | 25 | 12.952 | 82 (10,000) | [113] |
| Anthracite | 3550.7 | 433 (0.5 A/g) | 6 M KOH | 38.9 | 1000 | 99 (10,000) | [111] |
| Coal | 2129 | 323 (0.5 A/g) | 6 M KOH | 10 | 250 | 93.7 (10,000) | [114] |
| Coal tar pitch | 3305 | 308 (1 A/g) | 1 M Na2SO4 | 21.9 | 461.6 | - | [115] |
| Coal tar pitch | 3305 | 308 (1 A/g) | 6 M KOH | 8.92 | 254.9 | - | [115] |
| Coal-based green needle coke | 807.69 | 274.9 (1 A/g) | 6 M KOH | 20.51 | 1031.42 | 98.5 (5000) | [116] |
| Coal tar pitch | 2984 | 320 (0.1 A/g) | 6 M KOH | 10.6 | 50.1 | 94 (10,000) | [117] |
| Anthracite | 2947 | 282 (0.5 A/g) | 6 M KOH | 9.75 | 124.65 | - | [118] |
| Coal | 2168 | 215 (20 A/g) | 6 M KOH | 7.64 | 50 | 91.9 (5000) | [119] |
| Bituminous coal | 3472.41 | 487 (1 A/g) | 6 M KOH | 249.6 | 10.34 | 96 (10,000) | [120] |
| Electrolyte | Examples | ESW (V) | k (S cm−1); | Other Characteristics |
|---|---|---|---|---|
| Aqueous | H2SO4, KOH, Na2SO4, NH4Cl | ~1.2 | High | Cheap, safe, low environmental impact. |
| Organic | Organic salts (e.g., Et4NBF4) in Acetonitrile, propylene carbonate | ~3–3.5 | Moderate | Flammable, toxic, require low water content (<5 ppm). |
| Ionic liquid | Imidazolium, pyrrolidinium salts | ~4.5 | Low | Low flammability, costly. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Halimi, M.S.; Zanelli, A.; Soavi, F.; Chafik, T. Building towards Supercapacitors with Safer Electrolytes and Carbon Electrodes from Natural Resources. World 2023, 4, 431-449. https://doi.org/10.3390/world4030027
El Halimi MS, Zanelli A, Soavi F, Chafik T. Building towards Supercapacitors with Safer Electrolytes and Carbon Electrodes from Natural Resources. World. 2023; 4(3):431-449. https://doi.org/10.3390/world4030027
Chicago/Turabian StyleEl Halimi, Mohammad Said, Alberto Zanelli, Francesca Soavi, and Tarik Chafik. 2023. "Building towards Supercapacitors with Safer Electrolytes and Carbon Electrodes from Natural Resources" World 4, no. 3: 431-449. https://doi.org/10.3390/world4030027
APA StyleEl Halimi, M. S., Zanelli, A., Soavi, F., & Chafik, T. (2023). Building towards Supercapacitors with Safer Electrolytes and Carbon Electrodes from Natural Resources. World, 4(3), 431-449. https://doi.org/10.3390/world4030027