Abstract
We report a patient with COVID-19 requiring hospitalization for two weeks, complicated by multiple segmental pulmonary embolisms for which dabigatran was initiated. After clearing the infection, the patient remained asymptomatic for 5 months. He was then readmitted with a spontaneous haemothorax, most likely related to the use of dabigatran, which progressed to a pleural empyema with a trapped lung. The patient underwent a video assisted thoracoscopy (VATS) with decortication. Because of focal abnormalities, biopsies for histopathology were taken from the lung parenchyma. These showed an organizing pneumonia with progression towards fibrosis and arteries with intimal fibrosis. So far, no histopathological reports exist on late pulmonary changes after a COVID-19 infection. The unusual combined presence of microvascular damage and interstitial fibrosis may reflect a pathophysiological concept in which early endothelial damage by SARS-CoV-2 can lead to a chronic state of microvascular damage, low grade inflammation, and early progression towards pulmonary fibrosis.
1. Introduction
Since the outbreak of the SARS-CoV-2 pandemic in late 2019 [1], at least 143 million people have been infected and so far 3 million patients have died [2]. In less than one year, efforts from the worldwide scientific community have led to over 40,000 publications on prevention, diagnostics, pathophysiology and treatment of this disease. The growing body of knowledge is reflected in continuous adaptation of national and international guidelines, including those of the World Health Organization [3]. Because of the novelty of this disease, the number of reports on long term effects in previously infected patients and on long term pathophysiologic mechanisms is limited. Especially histologic reporting so far has been limited to autopsy reports of patients deceased from a COVID-19 infection [4,5,6,7,8,9]. These studies give insight in the mechanisms of lung injury during an active infection. But as yet, we have little idea of the extent of chronic pathology in survivors, let alone those with milder infections not requiring hospitalization.
We report a case of a patient who presented with a pleural empyema five months after his initial COVID-19 infection. During the surgical procedure removing his empyema, histology of the lung parenchyma was obtained, which showed ongoing damage probably related to his initial infection and its complications.
2. Case Presentation
Initial Admission, April 2020
A healthy, non-smoking, 75-year old man without reported exposure to dust or asbestos presented to a local hospital with nonproductive cough and watery diarrhea for three weeks. At home, the patient had experienced increasing shortness of breath, limiting his ability to walk.
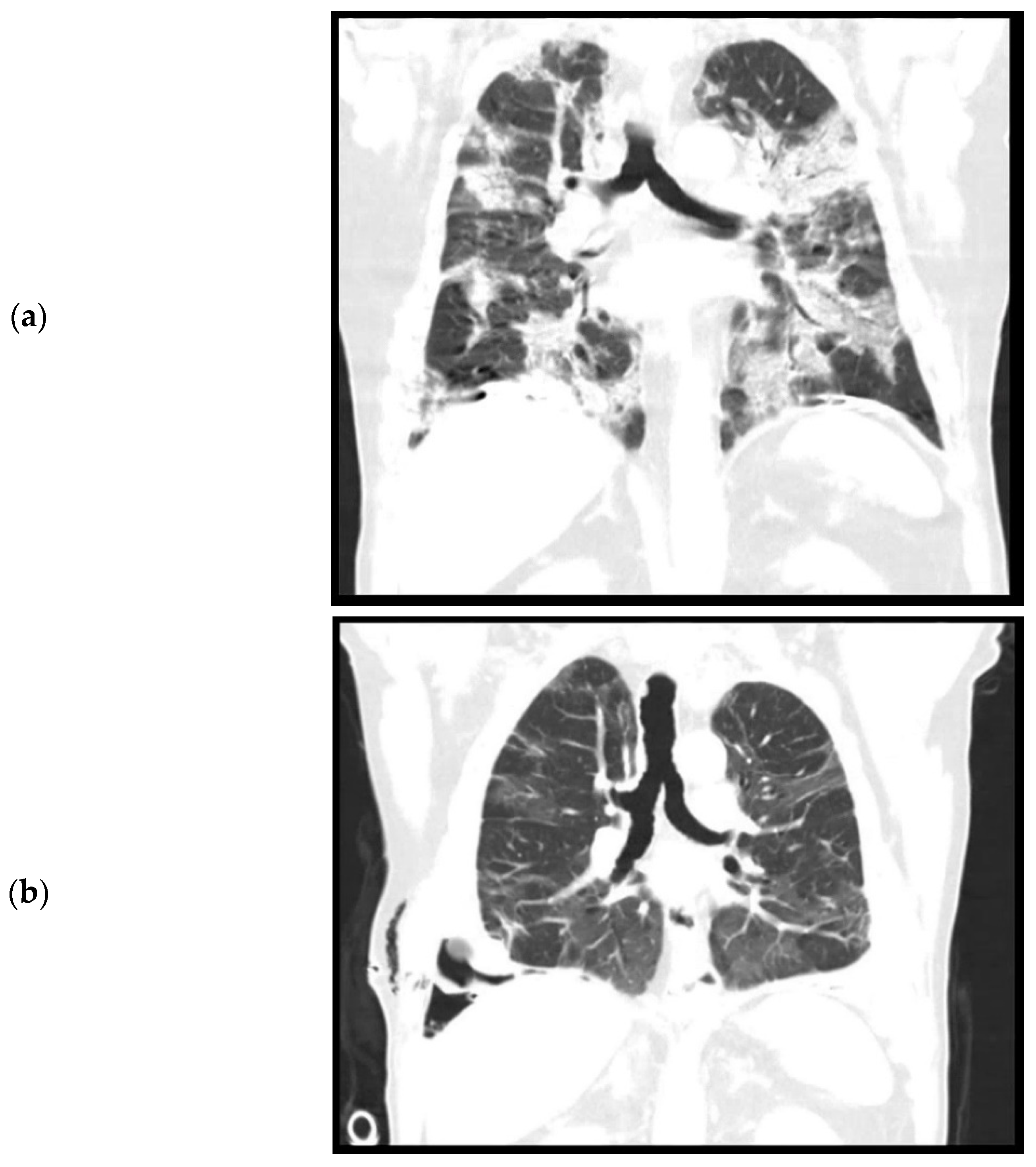
During admission, the patient did not have a fever. Oxygen saturation was 97% with 6 L oxygen. Laboratory results showed a white count of 16.6 × 109/L (reference: 4.0–10.0 × 109/L) and a C reactive protein (CRP) of 139 (reference < 5.0). Chest X-ray and chest CT performed on the day of admission showed bilateral consolidations and ground glass opacities without pleural effusion, suspicious for a COVID-19 infection (Figure 1a). Furthermore, pleural plaques were present on the right and left hemidiaphragm without evidence of round atelectasis or focal ischemia of the lung parenchyma. Multiple Polymerase Chain Reaction (PCR) for SARS-CoV-2 from nasopharyngeal swabs were negative, but after a few days a SARS-CoV-2 rectal swab was positive. This test was never repeated. The patient was admitted for oxygen support through a nasal cannula and cefuroxime and azithromycin were administered to prevent a bacterial superinfection. Furthermore, prophylactic low molecular weight heparin (nadroparine, 2850IE once daily) was started. The patient did not require mechanical ventilation. On the eleventh day of admission, a CT angiography of the chest was performed because of ongoing oxygen support. This showed a decrease in consolidations, but new bilateral segmental pulmonary embolisms, for which dabigatran was initiated. No coagulation parameters were tested during the admission. The patient could be weaned off oxygen support and after two weeks of admission, was discharged to a nursing home for further recovery. From there, eventually patient was discharged home in good condition.
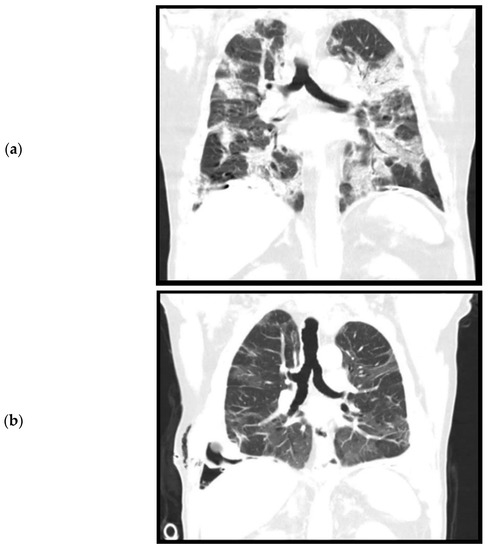
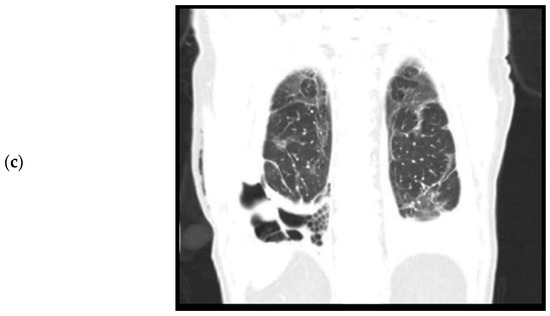
Figure 1.
Computed tomography, coronal plane. (a). April 2020. Level of trachea bifurcation. Bilateral consolidations and ground glass opacities during active SARS-CoV-2 infection. (b). September 2020. Level of trachea bifurcation. Bilateral residual ground glass opacities. (c). September 2020. Level of thoracic vertebrae. Right sided loculated pleural effusion, pleural empyema, and trapped lung.
Second Admission, September 2020
Five months later the patient was seen in the local hospital because new shortness of breath. He did not have a fever or other symptoms. A chest X-ray showed a right-sided pleural effusion and a chest tube was placed which drained 900 mL of dark bloody fluid. In the absence of a recent trauma and with the use of dabigatran, a spontaneous haemothorax was suspected and the dabigatran was discontinued. Amoxicillin was started.
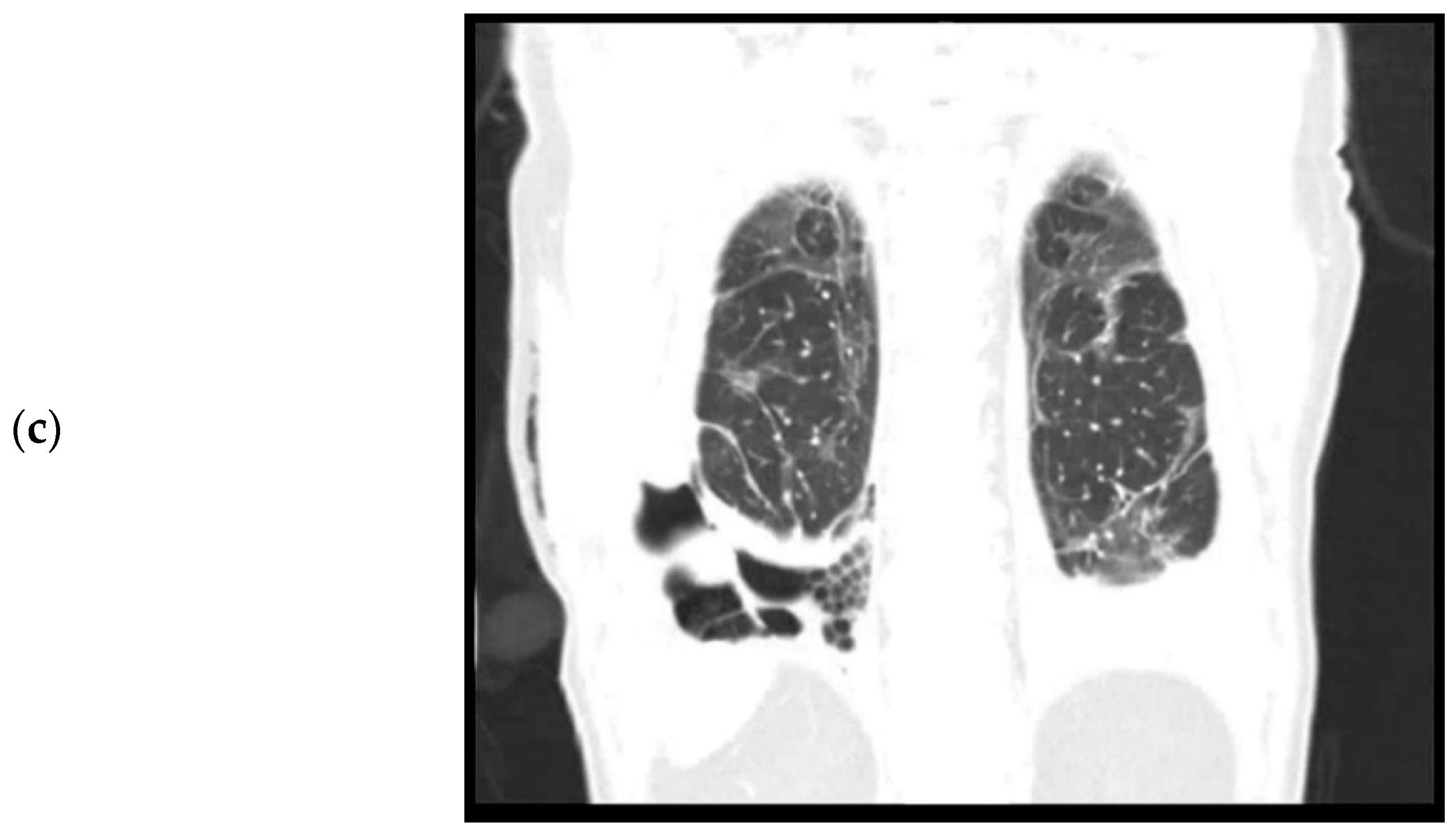
The patient was re-admitted for observation. Laboratory results showed a white count of 10.7 × 109/L and a CRP of 79. A culture of the pleural fluid was negative for bacteria. A chest CT performed after drainage showed bilateral residual ground glass opacities after the initial COVID-19 infection (Figure 1b) and a right-sided loculated pleural effusion (Figure 1c), suspicious for a thoracic empyema. The patient was referred to our hospital, a tertiary care center for thoracic surgery, for pleural decortication.
On admission in our hospital, he was in relatively good clinical condition and without a fever. A nasopharyngeal and rectal swab for SARS-CoV-2 were negative.
2.1. Operative Findings
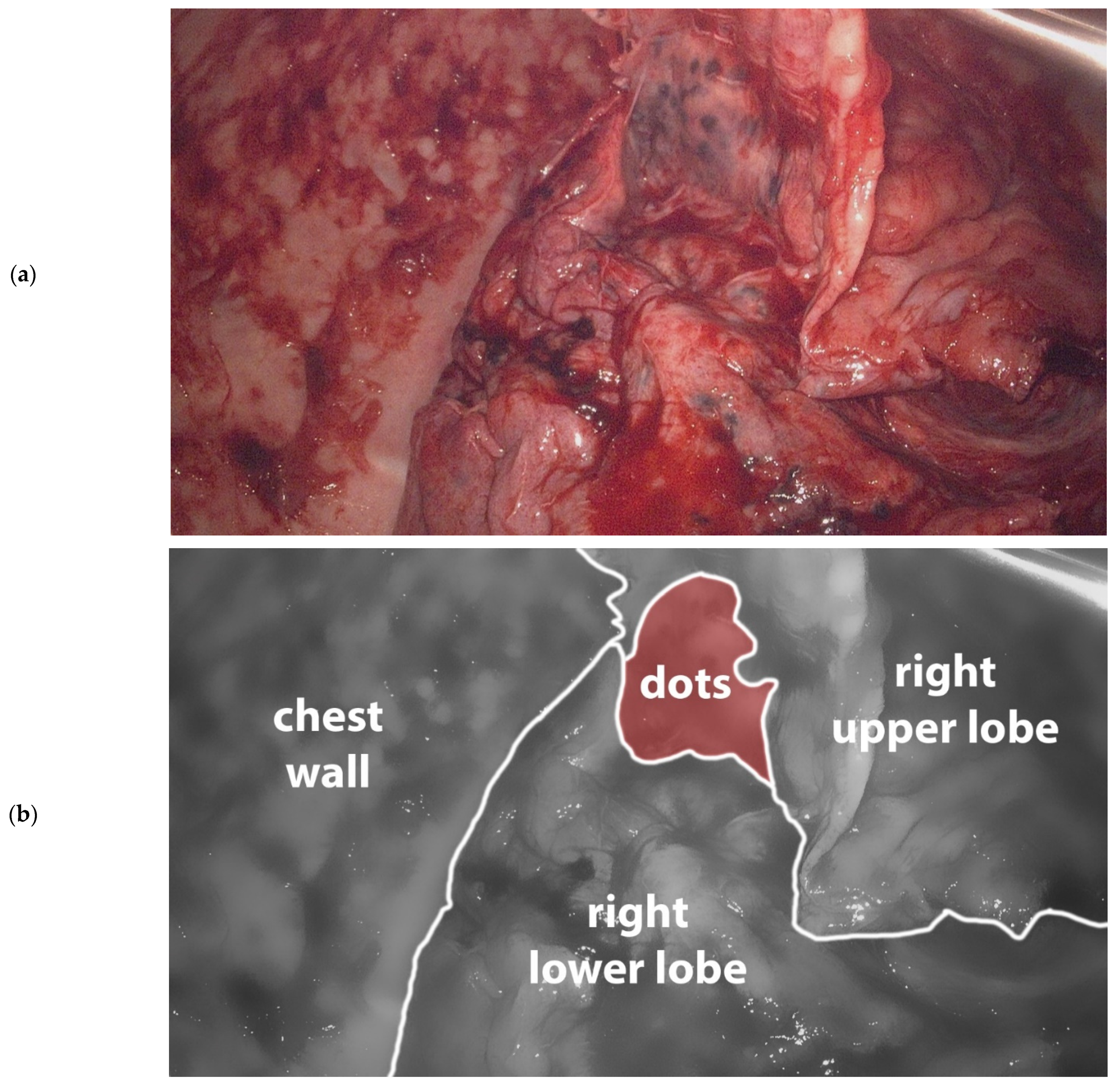
The next day, the patient underwent a video assisted thoracoscopy (VATS). The right pleural space was filled with dense fibrin and organized hematoma which were removed and sent for culture. The complete lung was trapped in a 2 mm thick, dense fibrous sheet, which was carefully peeled off the lung. This revealed a lung, covered with 2–3 mm black dots suspicious for anthracosilicosis (Figure 2). The diaphragm was covered by a calcified plate, making it almost completely immobile. The adjacent lung parenchyma was not involved in this process, and no signs of consolidation or pulmonary infarction were seen. Pleural fragments and a parenchymal wedge resection were sent for pathological evaluation. Despite a near-complete decortication of the visceral pleura, the lung could not fully expand. In combination with the diaphragmatic stiffness this led to a residual pneumothorax at the end of the procedure. Two silicone chest tubes were placed and put to 15 cm H2O suction. Postoperatively the patient had a significant air leak up to 500 mL/hour, fluid drainage was limited. Over the course of two weeks, the air leak gradually declined to 60 mL/hour. Overall, the patient did well, had unremarkable vitals, and was mobilizing. The white count was fluctuating between 14–18 × 109/L. On the 16th postoperative day, patient developed shortness of breath and a fever, and laboratory results showed an elevated white count (25 × 109/L) and CRP (343). Patient started broad spectrum antibiotics (piperacillin-tazobactam) for hospital acquired pneumonia and possible recurrent empyema. Chest tube fluid culture was positive for Staphylococcus Aureus. Computed tomography showed a partially expanded right lung and a recurrence of the loculated pleural effusion in the same location as before. Again, he was brought to the operating room for a VATS inspection and debridement. This time, the chest was filled with soft fibrin sheets which were easily removed. The chest tubes were replaced with two new chest tubes, which were removed on day one and day six after the second procedure. Serial chest X-rays showed a persistent pleural effusion and a right lung that never fully expanded. On the eighth postoperative day, patient was discharged to the referring hospital in good clinical condition. Eventually, he was discharged home on the 14th day after the second surgery, for a total of 44 days of admission.
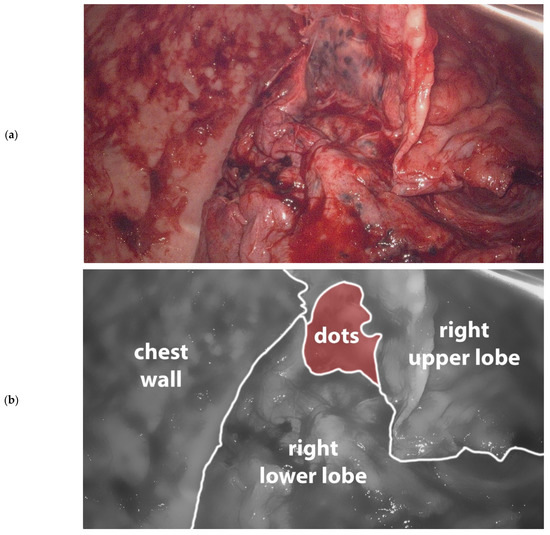
Figure 2.
(a). Image taken during primary VATS decortication of the right lung. Lung tissue is covered with 2–3 mm black dots consistent with anthrasilicosis. Camera port in 8th intercostal space anteriorly, camera is looking in cranial direction. (b). Schematic representation of (a).
2.2. Pathology Findings
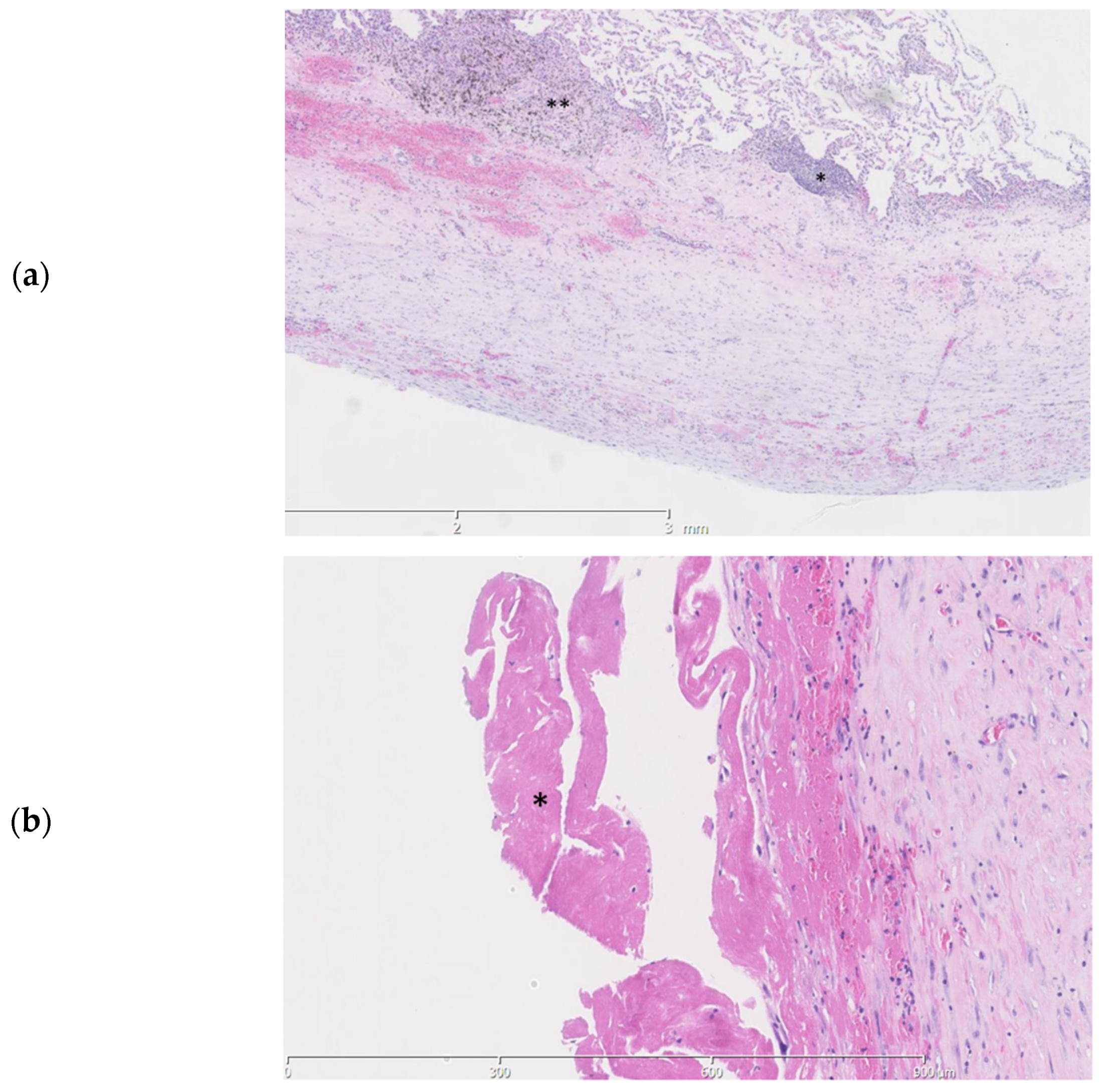
Pathologic examination of the pleural fragments showed thickening of the pleura with fibrinous exudate at the surface. The pleura contained a varying mononuclear inflammatory infiltrate, reactive blood vessels and some calcifications (Figure 3a,b).
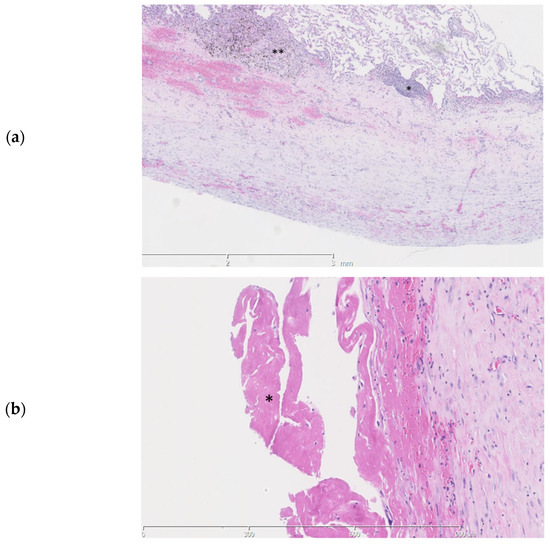
Figure 3.
Histopathologic slides of the pleura. (a). Thickening of the pleura is seen with a subpleural mononuclear inflammatory infiltrate (*) and anthracosilicotic changes (**) (b). A fibrinous exsudate (*) is seen at the pleural surface.
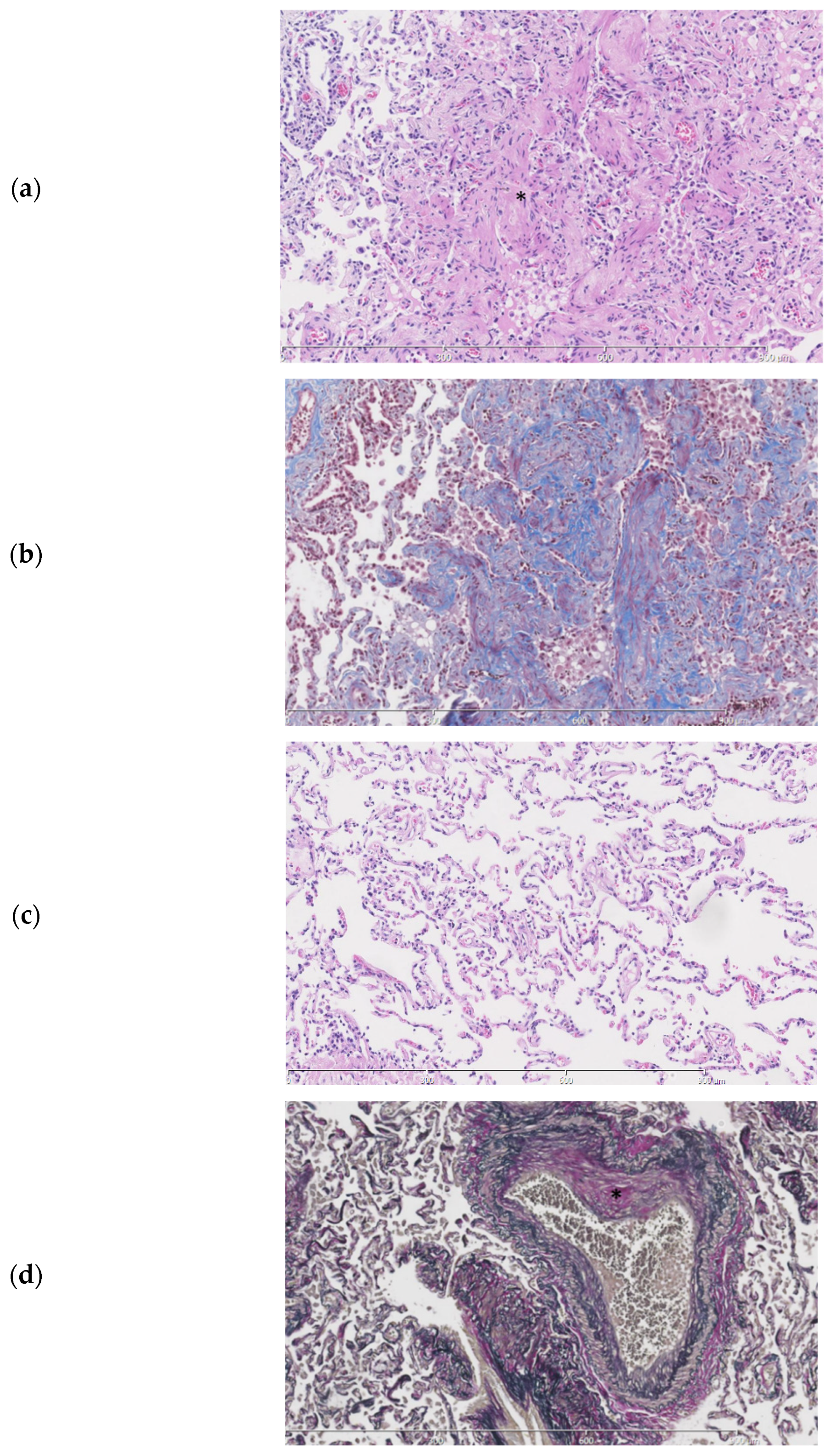
Examination of the parenchymal wedge resection showed an organizing pneumonia with progression towards fibrosis (Figure 4a,b). In more fibrotic areas bronchiolization of the alveolar epithelium was seen. Unaffected areas showed slender alveolar septa without fibrotic changes (Figure 4c). Predominantly subpleural histiocytes with anthracotic pigment were found. Birefringence showed silica crystals in these areas. The arteries showed irregular intimal fibrosis without new thrombi or signs of recanalization (Figure 4d). The alveolar tissue did not show any signs of ischemia or infarction. No abnormalities were found in the bronchi. As an underlying cause for the organizing pneumonia neither a clinical history nor additional histologic features of a collagen vascular disease, hypersensitivity pneumonitis or active inflammation/infection were present.
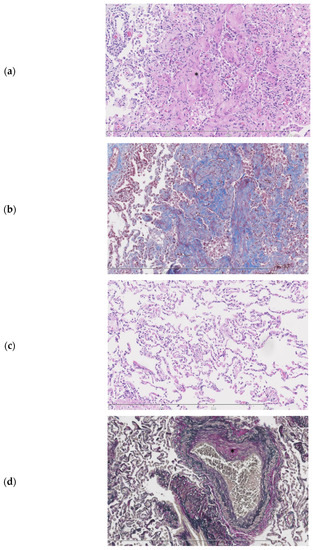
Figure 4.
Histopathologic slides of the lung. (a). Lung parenchyma showing a probable history of organizing pneumonia with progression towards fibrosis (*). (b). Fibrotic areas staining blue in a Masson trichome stain. (c). Normal lung from the same patient parenchyma for comparison. (d). Elastic van Gieson stain showing an artery with irregular intimal fibrosis (*) No signs of thrombosis or recanalization are seen.
3. Discussion
Since late 2019, approximately 143 million patients worldwide were infected with SARS-CoV-2, of whom 97.8% have survived [2]. Despite ongoing efforts in describing pathophysiological mechanisms in the acute setting, little is known about late effects in COVID-19 survivors. We report a patient who cleared a SARS-CoV-2 infection, and needed lung surgery 5 months later for a spontaneous haemothorax while using dabigatran. Lung biopsies taken at the time of surgery showed an organizing pneumonia with progression towards fibrosis and arteries with intimal fibrosis.
Since the SARS-CoV-2 virus emerged in the Wuhan province in China in late 2019, much has been elucidated about the mechanism of a COVID-19 infection. Similar to the 2002 SARS-CoV virus [10,11], the angiotensin-converting enzyme 2 (ACE-2) seems to play a crucial role in cell entry in both the nasal [12] and lung [13] epithelial cells. One of the most striking features of COVID-19 is the wide spectrum of symptoms that patients experience, from asymptomatic to severe respiratory disease with multi organ failure to death. Depending on definitions, it is estimated that 40–80% of patients remains asymptomatic during their infection [14,15]. For the group of symptomatic patients, 80% experience mild symptoms like a fever and cough. Approximately 20% of patients then progress to severe and critical disease which include acute respiratory distress syndrome and multi organ failure [16].
Special attention has been drawn to the hypercoagulable state patients experience during their infection [17]. In several series, a high number of both venous and arterial thrombotic complications were observed in critically ill patients with COVID-19 [18,19]. It has been hypothesized that severe, complicated cases of COVID-19 represent an endothelial disease [20]. During the cytokine storm of a severe infection, the inflammatory balance maintained by the endothelium is lost, ultimately leading to an inflammation induced coagulopathy.
With a multitude of clinical and imaging reports available, pathology reports are increasingly important to guide understanding pathophysiological mechanisms in COVID-19 patients. Several autopsy studies have reported high rates of deep venous thrombosis and pulmonary embolism [5,6]. Furthermore, endothelial injury and microvascular thrombosis with micro-angiopathy were found to be more present in patients who died from COVID-19 compared with other causes [4]. It is possible that patients with this pro-thrombotic state progress earlier from a (pre-)exudative stage of diffuse alveolar damage to an organizing and fibrotic phase [21]. This has been confirmed in several case report series in which patients were diagnosed, either radiographically or with transbronchial biopsy, with organizing pneumonia during their primary COVID-19 infection [22,23]. This finding is also supported by other autopsy studies reporting a spectrum of parenchymal histopathology patterns: from organizing pneumonia or acute fibrinous and organizing pneumonia to a pattern of diffuse alveolar damage, mostly in organizing stage [5,7]. A consensus on the role of steroids for the treatment of pulmonary fibrosis in COVID-19 yet has to be established in large clinical studies [24,25]. One study reports lung transplantation as the ultimate therapy in three patients with end-stage pulmonary fibrosis developed shortly after a COVID-19 infection [26]. Despite these reports on COVID-19 infection mechanisms, very little is known about late histopathologic changes in patients who cleared their COVID-19 infection. Histopathologic samples of healed lung tissue are crucial in elucidating these changes.
The current report reveals structural damage 5 months after a moderate COVID-19 infection with organizing pneumonia and microvascular fibrosis as late sequelae. It is unlikely that the anthracosilicosis found during surgery and in the histopathology specimen is related to the COVID-19 infection. Anthracosilicosis can be seen after inhalation of dust containing quartz and carbon, which is typically produced in mining. The current patient could not recall exposure to carbon dust.
Another finding on imaging and during surgery was the presence of isolated pleural plaques on the diaphragm. On repeated chest CT scans, no signs of round atelectasis, focal infarction, or focal organizing pneumonia were found. It is therefore unlikely that the histopathological features found on multiple parenchymal wedge biopsies can be explained by the presence of pleural plaques.
As the reported patient suffered from multiple events, including a COVID-19 infection, pulmonary embolism, a late bleeding complication likely from dabigatran and a subsequent empyema, it is hard to distinguish the effect of each event on final histopathology samples taken during surgery. However, the unusual combined presence of interstitial and microvascular damage may reflect a pathophysiological concept in which early endothelial damage by SARS-CoV-2 can lead to a chronic state of microvascular damage, low grade inflammation, and possibly susceptibility to progression of these phenomena after another pulmonary event. To our knowledge, this is one of the first reports on late effects in COVID-19 survivors.
With the overwhelming majority of the currently registered 77 million COVID-19 patients surviving their infection, a growing group of patients worldwide is susceptible to late structural lung damage including interstitial fibrosis. Careful reporting of histopathologic findings in COVID-19 survivors can elucidate long term mechanisms and may eventually lead to therapeutic strategies for patients with late symptoms after a COVID-19 infection.
Author Contributions
Conception and design: all authors; Acquisition of data: J.L.D., J.L.W., E.A.F.M.; Drafting of manuscript: J.L.D., R.A.S.H., E.A.F.M.; Revision and final approval of manuscript: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Shi, Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Map. Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 21 April 2021).
- Technical Guidance. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance (accessed on 21 April 2021).
- Wang, X.; Tu, Y.; Huang, B.; Li, Y.; Li, Y.; Zhang, S.; Luo, H. Pulmonary vascular endothelial injury and acute pulmonary hypertension caused by COVID-19: The fundamental cause of refractory hypoxemia? Cardiovasc. Diagn. Ther. 2020, 10, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Menter, T.; Haslbauer, J.D.; Nienhold, R.; Savic, S.; Hopfer, H.; Deigendesch, N.; Tzankov, A. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology 2020, 77, 198–209. [Google Scholar] [CrossRef]
- Wichmann, D.; Sperhake, J.P.; Lütgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients with COVID-19. Ann. Intern. Med. 2020, 173, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Flikweert, A.W.; Grootenboers, M.J.; Yick, D.C.; du Mée, A.W.; van der Meer, N.J.; Rettig, T.C.; Kant, M.K. Late histopathologic characteristics of critically ill COVID-19 patients: Different phenotypes without evidence of invasive aspergillosis, a case series. J. Crit. Care 2020, 59, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Aiolfi, A.; Bruni, B.; Biraghi, T.; Montisci, A.; Miceli, A.; Baronio, B.; Khor, D.; Cirri, S.; Donatelli, F.; Clemente, C.; et al. Late histological findings in symptomatic COVID-19 patients: A case report. Medicine 2020, 99, e21046. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Sungnak, W.; Huang, N.; Becavin, C.; Berg, M.; Queen, R.; Litvinukova, M.; Talavera-Lopez, C.; Maatz, H.; Reichart, D.; Sampaziotis, F.; et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020, 26, 681–687. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis ML, C.; Lely, A.T.; Navis, G.V.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Sutton, D.; Fuchs, K.; D’Alton, M.; Goffman, D. Universal Screening for SARS-CoV-2 in Women Admitted for Delivery. N. Engl. J. Med. 2020, 382, 2163–2164. [Google Scholar] [CrossRef]
- Oran, D.P.; Topol, E.J. Prevalence of Asymptomatic SARS-CoV-2 Infection: A Narrative Review. Ann. Intern. Med. 2020, 173, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Endeman, H.; van der Zee, P.; van Genderen, M.E.; van den Akker, J.P.C.; Gommers, D. Progressive respiratory failure in COVID-19: A hypothesis. Lancet Infect. Dis. 2020, 20, 1365. [Google Scholar] [CrossRef]
- Klok, F.A.; Kruip, M.J.H.A.; van der Meer, N.J.M.; Arbous, M.S.; Gommers, D.A.M.P.J.; Kant, K.M.; Kaptein, F.H.J.; van Paassen, J.; Stals, M.A.M.; Huisman, M.V. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020, 191, 145–147. [Google Scholar] [CrossRef]
- Bilaloglu, S.; Aphinyanaphongs, Y.; Jones, S.; Iturrate, E.; Hochman, J.; Berger, J.S. Thrombosis in Hospitalized Patients With COVID-19 in a New York City Health System. JAMA 2020, 324, 799–801. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Lüscher, T. COVID-19 is, in the end, an endothelial disease. Eur. Heart J. 2020, 41, 3038–3044. [Google Scholar] [CrossRef] [PubMed]
- Schwensen, H.F.; Borreschmidt, L.K.; Storgaard, M.; Redsted, S.; Christensen, S.; Madsen, L.B. Fatal pulmonary fibrosis: A post-COVID-19 autopsy case. J. Clin. Pathol. 2020, 74. [Google Scholar] [CrossRef]
- Vadász, I.; Husain-Syed, F.; Dorfmüller, P.; Roller, F.C.; Tello, K.; Hecker, M.; E Morty, R.; Gattenlöhner, S.; Walmrath, H.-D.; Grimminger, F.; et al. Severe organising pneumonia following COVID-19. Thorax 2021, 76, 201–204. [Google Scholar] [CrossRef]
- Simões, J.P.; Ferreira, A.R.A.; Almeida, P.M.; Trigueiros, F.; Braz, A.; Inácio, J.R.; Medeiros, F.C.; Braz, S.; de Lacerda, A.P. Organizing pneumonia and COVID-19: A report of two cases. Respir. Med. Case Rep. 2021, 32, 101359. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.P.; Mulani, J. Corticosteroids for COVID-19: The search for an optimum duration of therapy. Lancet Respir. Med. 2021, 9, e8. [Google Scholar] [CrossRef]
- Myall, K.J.; Mukherjee, B.; Castanheira, A.M.; Lam, J.L.; Benedetti, G.; Mak, S.M.; Preston, R.; Thillai, M.; Dewar, A.; Molyneaux, P.L.; et al. Persistent Post–COVID-19 Interstitial Lung Disease. An Observational Study of Corticosteroid Treatment. Ann. Am. Thorac. Soc. 2021, 18, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Y.; Qiao, K.; Liu, F.; Wu, B.; Xu, X.; Jiao, G.-Q.; Lu, R.-G.; Li, H.-X.; Zhao, J.; Huang, J.-A.; et al. Lung transplantation as therapeutic option in acute respiratory distress syndrome for coronavirus disease 2019-related pulmonary fibrosis. Chin. Med J. 2020, 133, 1390–1396. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).