Systematic Review of the Integrative Medicine Recommendations for Patients with Pancreatic Cancer
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Obesity
3.2. Stress
3.3. Vitamins
3.4. Cannabinoids
3.5. Green Tea
3.6. Curcumin
3.7. Melatonin
3.8. Nuts
3.9. Anti-Inflammatory, Ketogenic and Mediterranean Diets
3.10. Probiotics
4. Discussion
5. Conclusions
Funding
Institutionnel Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorski:, D.H.; Novella, S.P. Clinical trials of integrative medicine: Testing whether magic works? Trends Mol. Med. 2014, 20, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Chabot, J.A.; Tsai, W.-Y.; Fine, R.L.; Chen, C.; Kumah, C.K.; Antman, K.A.; Grann, V.R. Pancreatic Proteolytic Enzyme Therapy Compared With Gemcitabine-Based Chemotherapy for the Treatment of Pancreatic Cancer. J. Clin. Oncol. 2010, 28, 2058–2063. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Br. Med J. 2021, 372, 105906. [Google Scholar] [CrossRef]
- Gumbs, A.A. Obesity, Pancreatitis, and Pancreatic Cancer. Obes. Surg. 2008, 18, 1183–1187. [Google Scholar] [CrossRef]
- Gumbs, A.A.; Bessler, M.; Milone, L.; Schrope, B.; Chabot, J. Contribution of obesity to pancreatic carcinogenesis. Surg. Obes. Relat. Dis. 2008, 4, 186–193. [Google Scholar] [CrossRef]
- Navarrete-Muñoz, E.M.; Wark, A.P.; Romaguera, D.; Bhoo-Pathy, N.; Michaud, D.; Molina-Montes, E.; Tjonneland, A.; Olsen, A.; Overvad, K.; Boutron-Ruault, M.-C.; et al. Sweet-beverage consumption and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC). Am. J. Clin. Nutr. 2016, 104, 760–768. [Google Scholar] [CrossRef]
- Zheng, Z.; Zheng, R.; He, Y.; Sun, X.; Wang, N.; Chen, T.; Chen, W. Risk Factors for Pancreatic Cancer in China: A Multicenter Case-Control Study. J. Epidemiol. 2016, 26, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Andersen, D.K.; Korc, M.; Petersen, G.M.; Eibl, G.; Li, D.; Rickels, M.; Chari, S.T.; Abbruzzese, J.L. Diabetes, Pancreatogenic Diabetes, and Pancreatic Cancer. Diabetes 2017, 66, 1103–1110. [Google Scholar] [CrossRef] [Green Version]
- Carreras-Torres, R.; Johansson, M.; Gaborieau, V.; Haycock, P.C.; Wade, K.; Relton, C.L.; Martin, R.M.; Smith, G.D.; Brennan, P. The Role of Obesity, Type 2 Diabetes, and Metabolic Factors in Pancreatic Cancer: A Mendelian Randomization Study. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [Green Version]
- Gordon-Dseagu, V.; Devesa, S.S.; Goggins, M.; Stolzenberg-Solomon, R. Pancreatic cancer incidence trends: Evidence from the Surveillance, Epidemiology and End Results (SEER) population-based data. Int. J. Epidemiol. 2018, 47, 427–439. [Google Scholar] [CrossRef]
- Da Silva, M.; Weiderpass, E.; Licaj, I.; Lissner, L.; Rylander, C. Excess body weight, weight gain and obesity-related cancer risk in women in Norway: The Norwegian Women and Cancer study. Br. J. Cancer 2018, 119, 646–656. [Google Scholar] [CrossRef]
- Chung, K.M.; Singh, J.; Lawres, L.; Dorans, K.J.; Garcia, C.; Burkhardt, D.; Robbins, R.; Bhutkar, A.; Cardone, R.; Zhao, X.; et al. Endocrine-Exocrine Signaling Drives Obesity-Associated Pancreatic Ductal Adenocarcinoma. Cell 2020, 181, 832–847.e18. [Google Scholar] [CrossRef]
- Park, S.K.; Oh, C.-M.; Kim, M.-H.; Ha, E.; Choi, Y.-S.; Ryoo, J.-H. Metabolic syndrome, metabolic components, and their relation to the risk of pancreatic cancer. Cancer 2020, 126, 1979–1986. [Google Scholar] [CrossRef]
- Jiao, L.; Chen, L.; White, D.L.; Tinker, L.; Chlebowski, R.T.; Van Horn, L.V.; Richardson, P.; Lane, D.; Sangi-Haghpeykar, H.; El-Serag, H.B. Low-fat Dietary Pattern and Pancreatic Cancer Risk in the Women’s Health Initiative Dietary Modification Randomized Controlled Trial. J. Natl. Cancer Inst. 2018, 110, 49–56. [Google Scholar] [CrossRef]
- Levi, Z.; Rottenberg, Y.; Twig, G.; Katz, L.; Leiba, A.; Derazne, E.; Tzur, D.; Eizenstein, S.; Keinan-Boker, L.; Afek, A.; et al. Adolescent overweight and obesity and the risk for pancreatic cancer among men and women: A nationwide study of 1.79 million Israeli adolescents. Cancer 2019, 125, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Q.-L.; Wang, C.-F.; Tian, Y.-T.; Huang, H.; Zhang, S.-S.; Zhao, D.-B.; Ma, J.; Yuan, W.; Sun, Y.-M.; Che, X.; et al. Body mass index does not affect the survival of pancreatic cancer patients. World J. Gastroenterol. 2017, 23, 6287–6293. [Google Scholar] [CrossRef] [Green Version]
- Schauer, D.P.; Feigelson, H.S.; Koebnick, C.; Caan, B.; Weinmann, S.; Leonard, A.C.; Powers, J.D.; Yenumula, P.R.; Arterburn, D.E. Bariatric Surgery and the Risk of Cancer in a Large Multisite Cohort. Ann. Surg. 2019, 269, 95–101. [Google Scholar] [CrossRef]
- Naudin, S.; Viallon, V.; Hashim, D.; Freisling, H.; Jenab, M.; Weiderpass, E.; Perrier, F.; McKenzie, F.; Bueno-De-Mesquita, H.B.; Olsen, A.; et al. Healthy lifestyle and the risk of pancreatic cancer in the EPIC study. Eur. J. Epidemiol. 2019, 35, 1–12. [Google Scholar] [CrossRef]
- Incio, J.; Liu, H.; Suboj, P.; Chin, S.M.; Chen, I.X.; Pinter, M.; Ng, M.R.; Nia, H.T.; Grahovac, J.; Kao, S.; et al. Obesity-Induced Inflammation and Desmoplasia Promote Pancreatic Cancer Progression and Resistance to Chemotherapy. Cancer Discov. 2016, 6, 852–869. [Google Scholar] [CrossRef] [Green Version]
- Pratapwar, M.; Stenzel, A.E.; Joseph, J.M.; Fountzilas, C.; Etter, J.L.; Mongiovi, J.M.; Cannioto, R.; Moysich, K.B. Physical Inactivity and Pancreatic Cancer Mortality. J. Gastrointest. Cancer 2020, 51, 1088–1093. [Google Scholar] [CrossRef]
- De Boer, A.G.E.M.; Bruinvels, D.; Tytgat, K.M.A.J.; Schoorlemmer, A.; Klinkenbijl, J.H.G.; Frings-Dresen, M.H.W. Employment status and work-related problems of gastrointestinal cancer patients at diagnosis: A cross-sectional study. BMJ Open 2011, 1, e000190. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.S.; Choi, S.W.; Yun, S.S.; Koo, B.H.; Choi, I.S.; Kim, S.J.; Park, J.S.; Seok, J.-H.; Yoon, D.-S. Preliminary Findings on the Effectiveness of Meaning-Centered Psychotherapy in Patients with Pancreatobiliary Cancer. Yonsei Med. J. 2018, 59, 1107–1114. [Google Scholar] [CrossRef]
- Sato, N.; Hasegawa, Y.; Saito, A.; Motoi, F.; Ariake, K.; Katayose, Y.; Nakagawa, K.; Kawaguchi, K.; Fukudo, S.; Unno, M.; et al. Association between chronological depressive changes and physical symptoms in postoperative pancreatic cancer patients. Biopsychosoc. Med. 2018, 12, 13. [Google Scholar] [CrossRef]
- Hüttner, F.J.; Rooman, I.; Bouche, G.; Knebel, P.; Hüsing, J.; Mihaljevic, A.L.; Hackert, T.; Strobel, O.; Büchler, M.W.; Diener, M.K. Pancreatic resection with perioperative drug repurposing of propranolol and etodolac: Trial protocol of the phase-II randomised placebo controlled PROSPER trial. BMJ Open 2020, 10, e040406. [Google Scholar] [CrossRef]
- Ding, Y.; Mullapudi, B.; Torres, C.; Mascariñas, E.; Mancinelli, G.; Diaz, A.M.; McKinney, R.; Barron, M.; Schultz, M.; Heiferman, M.; et al. Omega-3 Fatty Acids Prevent Early Pancreatic Carcinogenesis via Repression of the AKT Pathway. Nutrients 2018, 10, 1289. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yang, A.L.; Chung, Y.T.; Zhang, W.; Liao, J.; Yang, G.-Y. Sulindac inhibits pancreatic carcinogenesis in LSL-KrasG12D-LSL-Trp53R172H-Pdx-1-Cre mice via suppressing aldo-keto reductase family 1B10 (AKR1B10). Carcinogenesis 2013, 34, 2090–2098. [Google Scholar] [CrossRef] [Green Version]
- Jeon, C.Y.; Pandol, S.J.; Wu, B.; Cook-Wiens, G.; Gottlieb, R.A.; Merz, C.N.B.; Goodman, M.T. The Association of Statin Use after Cancer Diagnosis with Survival in Pancreatic Cancer Patients: A SEER-Medicare Analysis. PLoS ONE 2015, 10, e0121783. [Google Scholar] [CrossRef] [Green Version]
- Archibugi, L.; Piciucchi, M.; Stigliano, S.; Valente, R.; Zerboni, G.; Barucca, V.; Milella, M.; Maisonneuve, P.; Fave, G.D.; Capurso, G. Exclusive and Combined Use of Statins and Aspirin and the Risk of Pancreatic Cancer: A Case-Control Study. Sci. Rep. 2017, 7, 13024. [Google Scholar] [CrossRef]
- Risch, H.A.; Lu, L.; Streicher, S.A.; Wang, J.; Zhang, W.; Ni, Q.; Kidd, M.S.; Yu, H.; Gao, Y.-T. Aspirin Use and Reduced Risk of Pancreatic Cancer. Cancer Epidemiol. Biomark. Prev. 2017, 26, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Li, Y.; Liu, L.; Jiang, Z.; Liu, G. Aspirin use and pancreatic cancer risk. Medicine 2019, 98, e18033. [Google Scholar] [CrossRef]
- Alkhushaym, N.; Almutairi, A.R.; AlThagafi, A.; Fallatah, S.B.; Oh, M.; Martin, J.R.; Babiker, H.M.; McBride, A.; Abraham, I. Exposure to proton pump inhibitors and risk of pancreatic cancer: A meta-analysis. Expert Opin. Drug Saf. 2020, 19, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Genkinger, J.M.; Wang, M.; Li, R.; Albanes, D.; Anderson, K.E.; Bernstein, L.; Brandt, P.V.D.; English, D.; Freudenheim, J.L.; Fuchs, C.S.; et al. Dairy products and pancreatic cancer risk: A pooled analysis of 14 cohort studies. Ann. Oncol. 2014, 25, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, M.; Risch, H.A.; Bosetti, C.; Anderson, K.E.; Petersen, G.M.; Bamlet, W.; Cotterchio, M.; Cleary, S.P.; Ibiebele, T.I.; La Vecchia, C.; et al. Vitamin D and pancreatic cancer: A pooled analysis from the Pancreatic Cancer Case—Control Consortium. Ann. Oncol. 2015, 26, 1776–1783. [Google Scholar] [CrossRef] [PubMed]
- Springett, G.M.; Husain, K.; Neuger, A.; Centeno, B.; Chen, D.-T.; Hutchinson, T.Z.; Lush, R.M.; Sebti, S.; Malafa, M.P. A Phase I Safety, Pharmacokinetic, and Pharmacodynamic Presurgical Trial of Vitamin E δ-tocotrienol in Patients with Pancreatic Ductal Neoplasia. EBioMedicine 2015, 2, 1987–1995. [Google Scholar] [CrossRef] [Green Version]
- Francois, R.; Zhang, A.; Husain, K.; Wang, C.; Hutchinson, S.; Kongnyuy, M.; Batra, S.K.; Coppola, D.; Sebti, S.M.; Malafa, M.P. Vitamin E δ-tocotrienol sensitizes human pancreatic cancer cells to TRAIL-induced apoptosis through proteasome-mediated down-regulation of c-FLIPs. Cancer Cell Int. 2019, 19, 189. [Google Scholar] [CrossRef]
- Han, X.; Li, J.; Brasky, T.M.; Xun, P.; Stevens, J.; White, E.; Gammon, M.D.; He, K. Antioxidant intake and pancreatic cancer risk. Cancer 2012, 119, 1314–1320. [Google Scholar] [CrossRef] [Green Version]
- Monti, D.A.; Mitchell, E.; Bazzan, A.J.; Littman, S.; Zabrecky, G.; Yeo, C.J.; Pillai, M.V.; Newberg, A.B.; Deshmukh, S.; Levine, M. Phase I Evaluation of Intravenous Ascorbic Acid in Combination with Gemcitabine and Erlotinib in Patients with Metastatic Pancreatic Cancer. PLoS ONE 2012, 7, e29794. [Google Scholar] [CrossRef]
- Polireddy, K.; Dong, R.; Reed, G.; Yu, J.; Chen, P.; Williamson, S.; Violet, P.-C.; Pessetto, Z.; Godwin, A.K.; Fan, F.; et al. High Dose Parenteral Ascorbate Inhibited Pancreatic Cancer Growth and Metastasis: Mechanisms and a Phase I/IIa study. Sci. Rep. 2017, 7, 17188. [Google Scholar] [CrossRef] [Green Version]
- Michalski, C.W.; Oti, F.E.; Erkan, M.; Sauliunaite, D.; Bergmann, F.; Pacher, P.; Batkai, S.; Müller, M.W.; Giese, N.A.; Friess, H.; et al. Cannabinoids in pancreatic cancer: Correlation with survival and pain. Int. J. Cancer 2008, 122, 742–750. [Google Scholar] [CrossRef] [Green Version]
- Donadelli, M.; Dando, I.; Zaniboni, T.; Costanzo, C.; Pozza, E.D.; Scupoli, M.T.; Scarpa, A.; Zappavigna, S.; Marra, M.; Abbruzzese, A.; et al. Gemcitabine/cannabinoid combination triggers autophagy in pancreatic cancer cells through a ROS-mediated mechanism. Cell Death Dis. 2011, 2, e152. [Google Scholar] [CrossRef]
- Yasmin-Karim, S.; Moreau, M.; Mueller, R.; Sinha, N.; Dabney, R.; Herman, A.; Ngwa, W. Enhancing the Therapeutic Efficacy of Cancer Treatment With Cannabinoids. Front. Oncol. 2018, 8, 114. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Huynh, N.; Dumesny, C.; Wang, K.; He, H.; Nikfarjam, M. Cannabinoids Inhibited Pancreatic Cancer via P-21 Activated Kinase 1 Mediated Pathway. Int. J. Mol. Sci. 2020, 21, 8035. [Google Scholar] [CrossRef]
- Zeng, J.-L.; Li, Z.-H.; Wang, Z.-C.; Zhang, H.-L. Green Tea Consumption and Risk of Pancreatic Cancer: A Meta-analysis. Nutrients 2014, 6, 4640–4650. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Zhang, Q.; Peng, M.; Shen, Y.; Wan, P.; Xie, G. Relationship between tea consumption and pancreatic cancer risk. Eur. J. Cancer Prev. 2014, 23, 353–360. [Google Scholar] [CrossRef]
- Bimonte, S.; Barbieri, A.; Palma, G.; Luciano, A.; Rea, D.; Arra, C. Curcumin Inhibits Tumor Growth and Angiogenesis in an Orthotopic Mouse Model of Human Pancreatic Cancer. BioMed Res. Int. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y.; et al. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2010, 68, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Ju, H.Q.; Li, H.; Tian, T.; Lu, Y.X.; Bal, L.; Chen, L.Z.; Sheng, H.; Mo, H.Y.; Zeng, J.B.; Deng, W.; et al. Melatonin overcomes gemcitabine resistance in pancreatic ductal adenocarcinoma by abrogating nuclear factor-jB activation. J. Pineal Res. 2016, 60, 27–38. [Google Scholar] [CrossRef]
- Bao, Y.; Hu, F.B.; Giovannucci, E.L.; Wolpin, B.M.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S. Nut consumption and risk of pancreatic cancer in women. Br. J. Cancer 2013, 109, 2911–2916. [Google Scholar] [CrossRef] [Green Version]
- Nieuwenhuis, L.; Brandt, P.A.V.D. Total Nut, Tree Nut, Peanut, and Peanut Butter Consumption and the Risk of Pancreatic Cancer in the Netherlands Cohort Study. Cancer Epidemiol. Biomark. Prev. 2018, 27, 274–284. [Google Scholar] [CrossRef] [Green Version]
- Obón-Santacana, M.; Luján-Barroso, L.; Freisling, H.; Naudin, S.; Boutron-Ruault, M.; Mancini, F.R.; Rebours, V.; Kühn, T.; Katzke, V.; Boeing, H.; et al. Consumption of nuts and seeds and pancreatic ductal adenocarcinoma risk in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2020, 146, 76–84. [Google Scholar] [CrossRef]
- Zheng, J.; Merchant, A.T.; Wirth, M.D.; Zhang, J.; Antwi, S.O.; Shoaibi, A.; Shivappa, N.; Stolzenberg-Solomon, R.Z.; Hebert, J.R.; Steck, S.E. Inflammatory potential of diet and risk of pancreatic cancer in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Int. J. Cancer 2018, 142, 2461–2470. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wirth, M.D.; Merchant, A.T.; Zhang, J.; Shivappa, N.; Stolzenberg-Solomon, R.Z.; Hebert, J.R.; Steck, S.E. Inflammatory Potential of Diet, Inflammation-Related Lifestyle Factors, and Risk of Pancreatic Cancer: Results from the NIH-AARP Diet and Health Study. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- Zahra, A.; Fath, M.; Opat, E.; Mapuskar, K.A.; Bhatia, S.K.; Rodman, S.; Iii, S.N.R.; Snyders, T.P.; Chenard, C.A.; Eichenberger-Gilmore, J.M.; et al. Consuming a Ketogenic Diet while Receiving Radiation and Chemotherapy for Locally Advanced Lung Cancer and Pancreatic Cancer: The University of Iowa Experience of Two Phase 1 Clinical Trials. Radiat. Res. 2017, 187, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Tognon, G.; Nilsson, L.M.; Lissner, L.; Johansson, I.; Hallmans, G.; Lindahl, B.; Winkvist, A. The Mediterranean Diet Score and Mortality Are Inversely Associated in Adults Living in the Subarctic Region. J. Nutr. 2012, 142, 1547–1553. [Google Scholar] [CrossRef] [Green Version]
- La Vecchia, C. Association between Mediterranean dietary patterns and cancer risk. Nutr. Rev. 2009, 67, S126–S129. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-M.; Chieng, W.-W.; Huang, S.-W.; Hsu, L.-J.; Jan, M.-S. The synergistic tumor growth-inhibitory effect of probiotic Lactobacillus on transgenic mouse model of pancreatic cancer treated with gemcitabine. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Campbell, T.; Parpia, B.; Chen, J. Diet, lifestyle, and the etiology of coronary artery disease: The Cornell China Study. Am. J. Cardiol. 1998, 82, 18–21. [Google Scholar] [CrossRef]
- Selinger, E.; Kühn, T.; Procházková, M.; Anděl, M.; Gojda, J. Vitamin B12 Deficiency Is Prevalent Among Czech Vegans Who Do Not Use Vitamin B12 Supplements. Nutrients 2019, 11, 3019. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.; Han, J.; Hu, F.B.; Giovannucci, E.L.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S. Association of Nut Consumption with Total and Cause-Specific Mortality. N. Engl. J. Med. 2013, 369, 2001–2011. [Google Scholar] [CrossRef] [Green Version]
- Naudin, S.; Li, K.; Jaouen, T.; Assi, N.; Kyrø, C.; Tjønneland, A.; Overvad, K.; Boutron-Ruault, M.-C.; Rebours, V.; Védie, A.-L.; et al. Lifetime and baseline alcohol intakes and risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition study. Int. J. Cancer 2018, 143, 801–812. [Google Scholar] [CrossRef]
- Ferro, R.; Adamska, A.; Lattanzio, R.; Mavrommati, I.; Edling, C.E.; Arifin, S.A.; Fyffe, C.A.; Sala, G.; Sacchetto, L.; Chiorino, G.; et al. GPR55 signalling promotes proliferation of pancreatic cancer cells and tumour growth in mice, and its inhibition increases effects of gemcitabine. Oncogene 2018, 37, 6368–6382. [Google Scholar] [CrossRef]
- Kollarova, H.; Azeem, K.; Tomaskova, H.; Horakova, D.; Prochazka, V.; Martinek, A.; Shonova, O.; Sevcikova, J.; Sevcikova, V.; Janout, V. Is physical activity a protective factor against pancreatic cancer? Bratislavske Lekarske Listy 2014, 115, 474–478. [Google Scholar] [CrossRef] [Green Version]
- Saxe, G.A.; Hebert, J.R.; Carmody, J.F.; Kabat-Zinn, J.; Rosenzweig, P.H.; Jarzobski, D.; Reed, G.W.; Blute, R.D. Can diet in conjunction with stress reduction affect the rate of increase in prostate specific antigen after biochemical recurrence of prostate cancer? J. Urol. 2001, 166, 2202–2207. [Google Scholar] [CrossRef]
- Cramer, H.; Lauche, R.; Paul, A.; Dobos, G. Mindfulness-Based Stress Reduction for Breast Cancer—A Systematic Review and Meta-Analysis. Curr. Oncol. 2012, 19, 343–352. [Google Scholar] [CrossRef] [Green Version]
- Lengacher, C.A.; Reich, R.R.; Kip, K.E.; Barta, M.; Ramesar, S.; Paterson, C.L.; Moscoso, M.S.; Carranza, I.; Budhrani, P.H.; Kim, S.J.; et al. Influence of mindfulness-based stress reduction (MBSR) on telomerase activity in women with breast cancer (BC). Biol. Res. Nurs. 2014, 16, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Carlson, L.; Rouleau, C.R.; Garland, S. The impact of mindfulness-based interventions on symptom burden, positive psychological outcomes, and biomarkers in cancer patients. Cancer Manag. Res. 2015, 7, 121–131. [Google Scholar] [CrossRef] [Green Version]
| Section and Topic | Item # | Checklist Item | Reported (Yes/No) |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review. | Y |
| Background | |||
| Objectives | 2 | Provide an explicit statement of the main objective(s) or question(s) the review addresses. | Y |
| Methods | |||
| Eligibility criteria | 3 | Specify the inclusion and exclusion criteria for the review. | Y |
| Information sources | 4 | Specify the information sources (e.g. databases, registers) used to identify studies and the date when each was last searched. | |
| Risk of bias | 5 | Specify the methods used to assess risk of bias in the included studies. | Y |
| Synthesis of results | 6 | Specify the methods used to present and synthesise results. | Y |
| Results | |||
| Included studies | 7 | Give the total number of included studies and participants and summarise relevant characteristics of studies. | Y |
| 8 | Present results for main outcomes, preferably indicating the number of included studies and participants for each. If meta-analysis was done, report the summary estimate and confidence/credible interval. If comparing groups, indicate the direction of the effect (i.e. which group is favoured). | Y | |
| Discussion | |||
| Limitations of evidence | 9 | Provide a brief summary of the limitations of the evidence included in the review (e.g. study risk of bias, inconsistency and imprecision). | Y |
| Interpretation | 10 | Provide a general interpretation of the results and important implications. | Y |
| Other | |||
| Funding | 11 | Specify the primary source of funding for the review. | Y |
| Registration | 12 | Provide the register name and registration number. | pending |
| Section and Topic | Item # | Checklist Item | Location Where Item Is Reported |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review. | p.1 |
| Abstract | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | Figure 2 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | p.4 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | p. 4–5 |
| Methods | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | p. 5–6 |
| Information sources | 6 | Specify all databases, registers, websites, organisations, reference lists and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | p. 5–6 |
| Search strategy | 7 | Present the full search strategies for all databases, registers and websites, including any filters and limits used. | p. 5–6 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | p. 5–6 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | p. 5–6 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g. for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | p. 5–6 |
| 10b | List and define all other variables for which data were sought (e.g. participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | p. 5–6 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | p. 5–6 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g. risk ratio, mean difference) used in the synthesis or presentation of results. | p. 5–6 |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g. tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | p. 5–6 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | p. 5–6 | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | p. 5–6 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | p. 5–6 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g. subgroup analysis, meta-regression). | p. 5–6 | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | p. 5–6 | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | p. 5–6 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | p. 5–6 |
| Results | |||
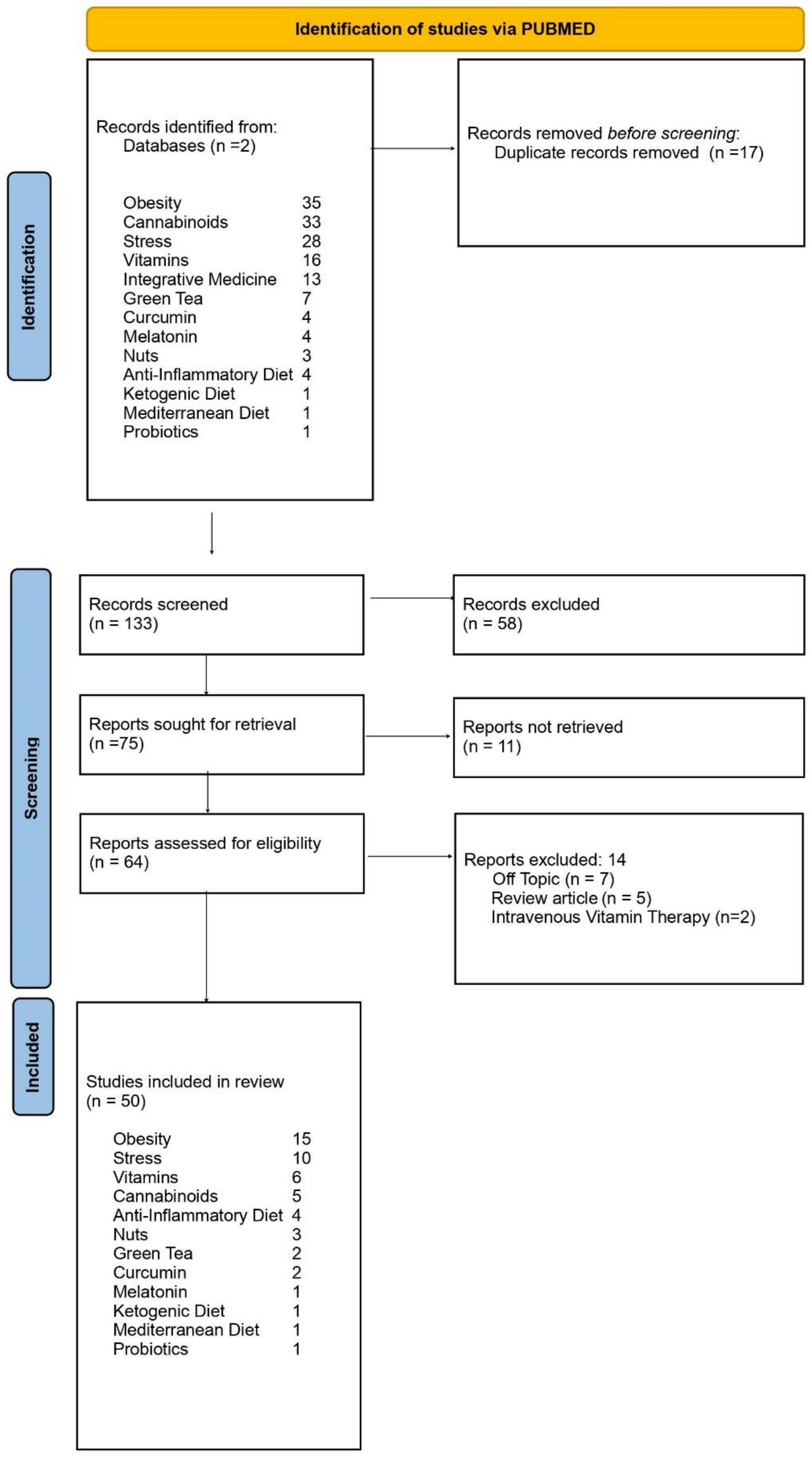
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | p. 6–16 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | Figure 1 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | p. 6–16 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | p. 6–16 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g. confidence/credible interval), ideally using structured tables or plots. | p. 6–16 |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | p. 6–16 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g. confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | p. 6–16 | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | p. 6–16 | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | p. 6–16 | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | p. 6–16 |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | p. 6–16 |
| Discussion | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | p.16 |
| 23b | Discuss any limitations of the evidence included in the review. | p. 16 | |
| 23c | Discuss any limitations of the review processes used. | p. 16 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | p. 16–24 | |
| Other Information | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | pending |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | Prospero | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | NA | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | p. 3 |
| Competing interests | 26 | Declare any competing interests of review authors. | NA |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | Pending Prospero Registration |
| Key Words | In Vitro | Animal Studies | Clinical Trials | RCTs | Meta-Analyses | Case Report |
|---|---|---|---|---|---|---|
| Obesity | x | x | ||||
| Stress | x | x | x | x (ongoing) | x | |
| Vitamins | x | x | x | |||
| Cannabinoids | x | |||||
| Anti-Inflammatory Diet | x | x | ||||
| Nuts | x | |||||
| Green Tea | x * | x * | x | |||
| Curcumin | x | x | x | |||
| Melatonin | x | x | x * | |||
| Ketogenic Diet | x ^ | x ^ | ||||
| Mediterranean Diet | x | |||||
| Probiotics | x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gumbs, A.A.; Gogol, M.; Spolverato, G.; Taher, H.; Chouillard, E.K. Systematic Review of the Integrative Medicine Recommendations for Patients with Pancreatic Cancer. Surgeries 2021, 2, 216-230. https://doi.org/10.3390/surgeries2020022
Gumbs AA, Gogol M, Spolverato G, Taher H, Chouillard EK. Systematic Review of the Integrative Medicine Recommendations for Patients with Pancreatic Cancer. Surgeries. 2021; 2(2):216-230. https://doi.org/10.3390/surgeries2020022
Chicago/Turabian StyleGumbs, Andrew A., Manana Gogol, Gaya Spolverato, Hebatallah Taher, and Elie K. Chouillard. 2021. "Systematic Review of the Integrative Medicine Recommendations for Patients with Pancreatic Cancer" Surgeries 2, no. 2: 216-230. https://doi.org/10.3390/surgeries2020022
APA StyleGumbs, A. A., Gogol, M., Spolverato, G., Taher, H., & Chouillard, E. K. (2021). Systematic Review of the Integrative Medicine Recommendations for Patients with Pancreatic Cancer. Surgeries, 2(2), 216-230. https://doi.org/10.3390/surgeries2020022








