Compression Neuropathies of the Upper Extremity: A Review
Abstract
:1. Introduction
2. Compression Syndromes
2.1. Median Nerve
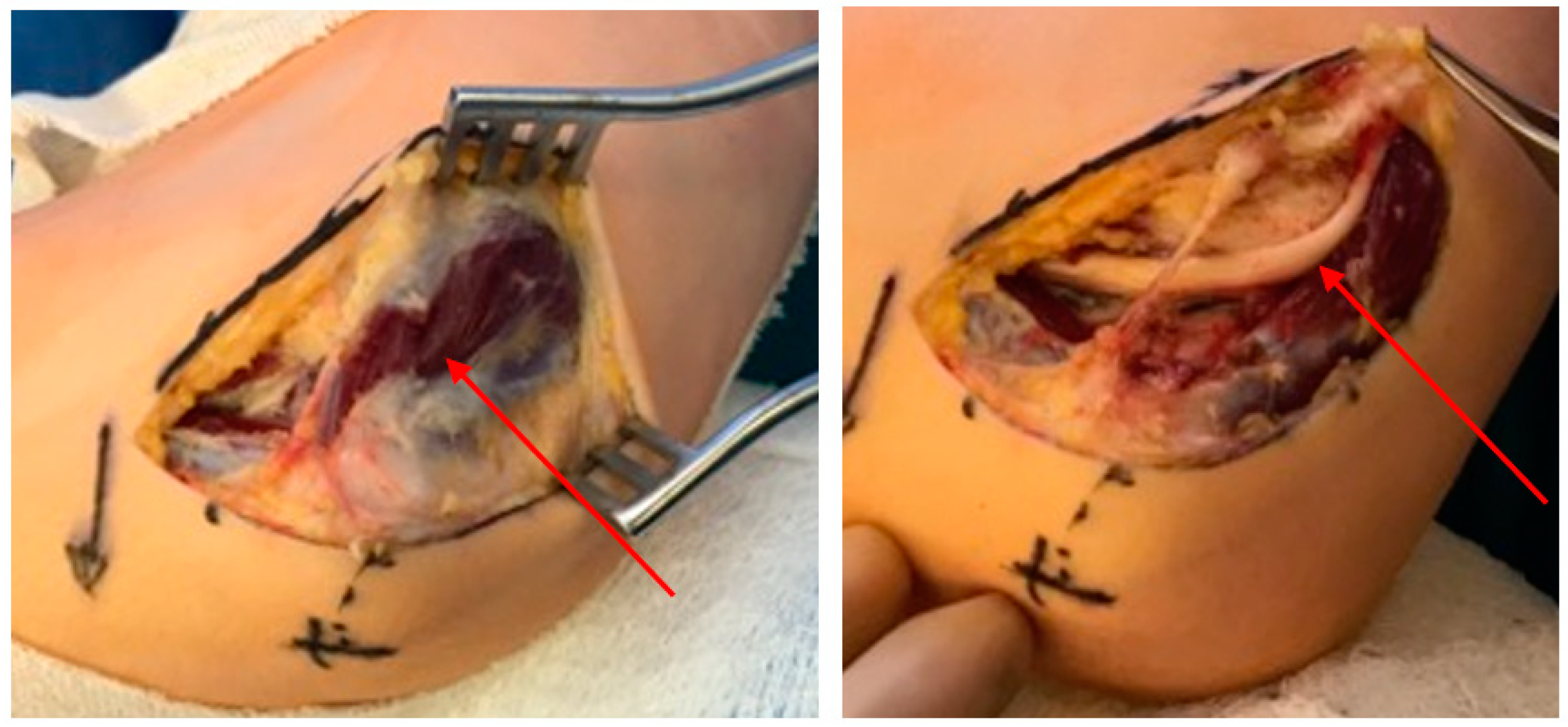
2.1.1. Carpal Tunnel Syndrome
2.1.2. Diagnosis and Assessment
2.1.3. Treatment
2.1.4. Open vs. Endoscopic
2.1.5. Etiology
2.1.6. Diagnosis and Physical Examination
2.1.7. Treatment
2.2. Pronator Syndrome
2.2.1. Etiology
2.2.2. Diagnosis and Physical Examination
2.2.3. Treatment
2.3. Ligament of Struthers Syndrome
Etiology
3. Ulnar Nerve
3.1. Cubital Tunnel Syndrome
3.1.1. Etiology
3.1.2. Anatomy
3.1.3. Diagnosis
3.1.4. Treatment
3.2. Guyon’s Canal
3.2.1. Etiology
3.2.2. Diagnosis
3.2.3. Treatment
4. Radial Nerve
4.1. Radial Tunnel Syndrome
4.1.1. Etiology and Anatomy
4.1.2. Diagnosis
4.1.3. Treatment
| Radial Nerve | |
|---|---|
| Radial Tunnel Syndrome | |
| Compression Site | Proposed compression of posterior interosseous nerve in proximal forearm, with multiple potential sites for compression. |
| Sensation Deficit | No sensory deficits. |
| Symptoms | Pain along dorsoradial proximal forearm, may worsen with pronation and supination. |
| Diagnosis | Thorough social and occupational history. MRI may demonstrate soft tissue changes. |
| Wartenberg Syndrome | |
| Compression of radial sensory nerve in forearm between fascial layers of the brachioradialis and extensor carpi radialis longus. May cause pain in dorsum of thumb, index finger, and radial half of middle finger. | |
| Physcial Examination Techniques | |
| Tinel Test | Elicted through tapping over the flexor retinaculum which creates paresthesias. Indicative of carpal tunnel syndrome (sensitivity 62%, specificity 93%) [68]. Typically negative in pronator syndrome. |
| Phalen Test | Produced through 90 degree wrist flexion with resultant paresthesias. Indicative of carpal tunnel syndrome (sensitivity 85%, specificity 90%) [68]. Typically negative in pronator syndrome. |
| Kiloh-Nevin Test | Evident through inability to make ‘ok’ sign with thumb and index finger. Indicative of anterior interosseous syndrome. |
| Froment’s Sign | Inability to maintain pinch grip, indicative of cubital tunnel syndrome. |
4.1.4. Wartenberg Syndrome
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, R.; Steward, O. Chronic nerve compression induces concurrent apoptosis and proliferation of Schwann cells. J. Comp. Neurol. 2003, 461, 174–186. [Google Scholar] [CrossRef]
- Mackinnon, S.E.; Dellon, A.L.; Hudson, A.R.; Hunter, D.A. Chronic human nerve compression–a histological assessment. Neuropathol. Appl. Neurobiol. 1986, 12, 547–565. [Google Scholar] [CrossRef] [PubMed]
- Menorca, R.M.G.; Fussell, T.S.; Elfar, J.C. Nerve physiology: Mechanisms of injury and recovery. Hand Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef] [Green Version]
- Kilgour, E.; Kosny, A.; McKenzie, D.; Collie, A. Interactions between injured workers and insurers in workers’ compensation systems: A systematic review of qualitative research literature. J. Occup. Rehabil. 2015, 25, 160–181. [Google Scholar] [CrossRef]
- Lee, D.H.; Claussen, G.C.; Oh, S. Clinical nerve conduction and needle electromyography studies. J. Am. Acad. Orthop. Surg. 2004, 12, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, P.J.; Watts, A.C.; Duckworth, A.D.; McEachan, J.E. Socioeconomic deprivation and the epidemiology of carpal tunnel syndrome. J. Hand Surg. Eur. Vol. 2012, 37, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Mondelli, M.; Giannini, F.; Giacchi, M. Carpal tunnel syndrome incidence in a general population. Neurology 2002, 58, 289–294. [Google Scholar] [CrossRef]
- Shimizu, A.; Ikeda, M.; Kobayashi, Y.; Saito, I.; Mochida, J. Carpal tunnel syndrome with wrist trigger caused by hypertrophied lumbrical muscle and tenosynovitis. Case Rep. Orthop. 2015, 2015, 705237. [Google Scholar] [CrossRef] [Green Version]
- Genova, A.; Dix, O.; Saefan, A.; Thakur, M.; Hassan, A. Carpal tunnel syndrome: A review of literature. Cureus 2020, 12, e7333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosier, B.A.; Hughes, T.B. Recurrent carpal tunnel syndrome. Hand Clin. 2013, 29, 427–434. [Google Scholar] [CrossRef]
- Ly-Pen, D.; Andréu, J.-L.; Millán, I.; de Blas, G.; Sánchez-Olaso, A. Comparison of surgical decompression and local steroid injection in the treatment of carpal tunnel syndrome: 2-year clinical results from a randomized trial. Rheumatology 2012, 51, 1447–1454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Q.; MacDermid, J.C. Is surgical intervention more effective than non-surgical treatment for carpal tunnel syndrome? A systematic review. J. Orthop. Surg. Res. 2011, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Kim, P.-T.; Lee, H.-J.; Kim, T.-G.; Jeon, I.-H. Current approaches for carpal tunnel syndrome. Clin. Orthop. Surg. 2014, 6, 253–257. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, V.V.; de Oliveira, S.B.; Fernandes, M.d.C.B.; Saraiva, R.Â. Incidence of regional pain syndrome after carpal tunnel release: Is there a correlation with the anesthetic technique? Braz. J. Anesthesiol. 2011, 61, 425–433. [Google Scholar] [CrossRef] [Green Version]
- Neubrech, F.; Gentzsch, T.; Kotsougiani, D.; Bickert, B.; Kneser, U.; Harhaus, L. Prevalence and co-prevalence of complex regional pain syndrome (CRPS) and carpal tunnel syndrome (CTS) in hand rehabilitation. Handchir. Mikrochir. Plast. Chir. 2016, 48, 136–142. [Google Scholar] [CrossRef]
- Hubbard, Z.S.; Law, T.Y.; Rosas, S.; Jernigan, S.C.; Chim, H. Economic benefit of carpal tunnel release in the Medicare patient population. Neurosurg. Focus 2018, 44, E16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrier, V.J.M.M.; Poelstra, R.; Selles, R.W.; Slijper, H.P.; Amadio, P.C.; Hovius, S.E.R.; Porsius, J.T. Better patient-reported experiences with health care are associated with improved clinical outcome after carpal tunnel release surgery. Plast. Reconstr. Surg. 2019, 143, 1677–1684. [Google Scholar] [CrossRef]
- Kaltenborn, A.; Frey-Wille, S.; Hoffmann, S.; Wille, J.; Schulze, C.; Settje, A.; Vogt, P.M.; Gutcke, A.; Ruettermann, M. The risk of complications after carpal tunnel release in patients taking acetylsalicylic acid as platelet inhibition: A multicenter propensity score-matched study. Plast. Reconstr. Surg. 2020, 145, 360e–367e. [Google Scholar] [CrossRef] [PubMed]
- Olaiya, O.R.; Alagabi, A.M.; Mbuagbaw, L.; McRae, M.H. Carpal tunnel release without a tourniquet: A systematic review and meta-analysis. Plast. Reconstr. Surg. 2020, 145, 737–744. [Google Scholar] [CrossRef]
- Chen, L.; Duan, X.; Huang, X.; Lv, J.; Peng, K.; Xiang, Z. Effectiveness and safety of endoscopic versus open carpal tunnel decompression. Arch. Orthop. Trauma Surg. 2014, 134, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Atroshi, I.; Hofer, M.; Larsson, G.-U.; Ranstam, J. Extended follow-up of a randomized clinical trial of open vs endoscopic release surgery for carpal tunnel syndrome. JAMA 2015, 314, 1399–1401. [Google Scholar] [CrossRef]
- Gould, D.; Kulber, D.; Kuschner, S.; Dellamaggiorra, R.; Cohen, M. Our surgical experience: Open versus endoscopic carpal tunnel surgery. J. Hand Surg. 2018, 43, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Karamanos, E.; Jillian, B.-Q.; Person, D. Endoscopic carpal tunnel release: Indications, technique, and outcomes. Orthop. Clin. North Am. 2020, 51, 361–368. [Google Scholar] [CrossRef]
- Michelotti, B.; Romanowsky, D.; Hauck, R.M. Prospective, randomized evaluation of endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome: An interim analysis. Ann. Plast. Surg. 2014, 73, S157–S160. [Google Scholar] [CrossRef]
- Vasen, A.P.; Kuntz, K.M.; Simmons, B.P.; Katz, J.N. Open versus endoscopic carpal tunnel release: A decision analysis. J. Hand Surg. 1999, 24, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Satteson, E.S.; Cunningham, T.C.; Gerard, J.; Person, D.W.; Tannan, S.C. Single surgeon series of outcomes of 897 consecutive endoscopic carpal tunnel releases stratified by disease severity. J. Plast. Reconstr. Aesthet. Surg. 2019, 72, 137–171. [Google Scholar] [CrossRef] [PubMed]
- Koehler, D.M.; Balakrishnan, R.; Lawler, E.A.; Shah, A.S. Endoscopic versus open carpal tunnel release: A detailed analysis using time-driven activity-based costing at an academic medical center. J. Hand Surg. 2019, 44, 62.e1–62.e9. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.C.; Walters, M.R.; Greenfield, M.L.V.H.; Chernew, M.E. Endoscopic versus open carpal tunnel release: A cost-effectiveness analysis. Plast. Reconstr. Surg. 1998, 102, 1089–1099. [Google Scholar] [CrossRef]
- Chatterjee, A.; McCarthy, J.E.; Montagne, S.A.; Leong, K.; Kerrigan, C.L. A cost, profit, and efficiency analysis of performing carpal tunnel surgery in the operating room versus the clinic setting in the United States. Ann. Plast. Surg. 2011, 66, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, M.R.; Lalonde, J.; Lalonde, D.H. A detailed cost and efficiency analysis of performing carpal tunnel surgery in the main operating room versus the ambulatory setting in Canada. Hand 2007, 2, 173–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas, C.R.; Long, T.C.; Kumar, A.R. Trainee operative autonomy in plastic surgery. Ann. Plast. Surg. 2020, 85, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Yee, A.; Padovano, W.M.; Rowe, A.G.; Hill, E.J.R.; Fox, I.K.; Moore, A.M.; Coert, J.H.; Mackinnon, S.E. The effect of surgical video on resident performance of carpal tunnel release: A cadaveric simulation-based, prospective, randomized, blinded pilot study. Plast. Reconstr. Surg. 2020, 145, 1455–1463. [Google Scholar] [CrossRef]
- Zdilla, M.J.; Pacurari, P.; Celuck, T.J.; Andrews, R.C.; Lambert, H.W. A Gantzer muscle arising from the brachialis and flexor digitorum superficialis: Embryological considerations and implications for median nerve entrapment. Anat. Sci. Int. 2019, 94, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Agarwal, K.K.; Parshuram, V.; Das, A.R. Gantzer muscles and their applied aspects: An exceptional finding. Singap. Med. J. 2013, 54, e102–e104. [Google Scholar] [CrossRef] [Green Version]
- Rodner, C.M.; Tinsley, B.A.; O’Malley, M.P. Pronator syndrome and anterior interosseous nerve syndrome. J. Am. Acad. Orthop. Surg. 2013, 21, 268–275. [Google Scholar] [CrossRef]
- Stutz, C.M. Neuralgic amyotrophy: Parsonage-Turner Syndrome. J. Hand Surg. 2010, 35, 2104–2106. [Google Scholar] [CrossRef] [PubMed]
- Van Alfen, N.; van Engelen, B.G.M. The clinical spectrum of neuralgic amyotrophy in 246 cases. Brain 2006, 129, 438–450. [Google Scholar] [CrossRef] [Green Version]
- Chi, Y.; Harness, N.G. Anterior interosseous nerve syndrome. J. Hand Surg. 2010, 35, 2078–2080. [Google Scholar] [CrossRef]
- Eversmann, W.W. Proximal median nerve compression. Hand Clin. 1992, 8, 307–315. [Google Scholar] [CrossRef]
- Tsai, P.; Steinberg, D.R. Median and radial nerve compression about the elbow. J. Bone Jt. Surg. 2008, 90, 420–428. [Google Scholar]
- Dang, A.C.; Rodner, C.M. Unusual compression neuropathies of the forearm, part II: Median nerve. J. Hand Surg. 2009, 34, 1915–1920. [Google Scholar] [CrossRef]
- Lubahn, J.D.; Cermak, M.B. Uncommon nerve compression syndromes of the upper extremity. J. Am. Acad. Orthop. Surg. 1998, 6, 378–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller-Breslow, A.; Terrono, A.; Millender, L.H. Nonoperative treatment of anterior interosseous nerve paralysis. J. Hand Surg. 1990, 15, 493–496. [Google Scholar] [CrossRef]
- Seki, M.; Nakamura, H.; Kono, H. Neurolysis is not required for young patients with a spontaneous palsy of the anterior interosseous nerve. J. Bone Jt. Surg. Br. 2006, 88, 1606–1609. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, F.; Daly, F.; Gagyor, I. Antiviral agents added to corticosteroids for early treatment of adults with acute idiopathic facial nerve paralysis (bell palsy). JAMA 2016, 316, 874–875. [Google Scholar] [CrossRef] [PubMed]
- Simon, C. Peripheral neuropathy. InnovAiT Educ. Inspir. Gen. Pract. 2009, 2, 538–545. [Google Scholar] [CrossRef] [Green Version]
- Hartz, C.R.; Linscheid, R.L.; Gramse, R.R.; Daube, J.R. The pronator teres syndrome: Compressive neuropathy of the median nerve. J. Bone Jt. Surg. 1981, 63, 885–890. [Google Scholar] [CrossRef] [Green Version]
- Olehnik, W.K.; Manske, P.R.; Szerzinski, J. Median nerve compression in the proximal forearm. J. Hand Surg. 1994, 19, 121–126. [Google Scholar] [CrossRef]
- Camerlinck, M.; Vanhoenacker, F.M.; Kiekens, G. Ultrasound demonstration of Struthers’ ligament. J. Clin. Ultrasound 2010, 38, 499–502. [Google Scholar] [CrossRef]
- Aydinlioglu, A.; Cirak, B.; Akpinar, F.; Tosun, N.; Dogan, A. Bilateral median nerve compression at the level of Struthers’ ligament. J. Neurosurg. 2000, 92, 693–696. [Google Scholar] [CrossRef]
- Shon, H.-C.; Park, J.-K.; Kim, D.-S.; Kang, S.-W.; Kim, K.-J.; Hong, S.-H. Supracondylar process syndrome: Two cases of median nerve neuropathy due to compression by the ligament of Struthers. J. Pain Res. 2018, 11, 803–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lordan, J.; Rauh, P.; Spinner, R.J. The clinical anatomy of the supracondylar spur and the ligament of Struthers. Clin. Anat. 2005, 18, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Boone, S.; Gelberman, R.H.; Calfee, R.P. The management of cubital tunnel syndrome. J. Hand Surg. 2015, 40, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Cammarata, M.J.; Hill, J.B.; Sharma, S. Ulnar nerve compression due to anconeus epitrochlearis: A case report and review of the literature. JBJS Case Connect. 2019, 9, e0189. [Google Scholar] [CrossRef] [PubMed]
- Nakashian, M.N.; Ireland, D.; Kane, P.M. Cubital tunnel syndrome: Current concepts. Curr. Rev. Musculoskelet. Med. 2020, 13, 520–524. [Google Scholar] [CrossRef]
- Dellon, A.L. Review of treatment results for ulnar nerve entrapment at the elbow. J. Hand Surg. 1989, 14, 688–700. [Google Scholar] [CrossRef]
- Caliandro, P.; La Torre, G.; Padua, R.; Giannini, F.; Padua, L. Treatment for ulnar neuropathy at the elbow. Cochrane Database Syst. Rev. 2016, 11, CD006839. [Google Scholar] [CrossRef]
- Dengler, J.; Dolen, U.; Patterson, J.M.M.; Davidge, K.M.; Kahn, L.C.; Yee, A.; Mackinnon, S.E. Supercharge end-to-side anterior interosseous-to-ulnar motor nerve transfer restores intrinsic function in cubital tunnel syndrome. Plast. Reconstr. Surg. 2020, 146, 808–818. [Google Scholar] [CrossRef]
- Strohl, A.B.; Zelouf, D.S. Ulnar tunnel syndrome, radial tunnel syndrome, anterior interosseous nerve syndrome, and pronator syndrome. J. Am. Acad. Orthop. Surg. 2017, 25, e1–e10. [Google Scholar] [CrossRef]
- Naam, N.H.; Nemani, S. Radial tunnel syndrome. Orthop. Clin. N. Am. 2012, 43, 529–536. [Google Scholar] [CrossRef]
- Van den Ende, K.I.; Steinmann, S.P. Radial tunnel syndrome. J. Hand Surg. 2010, 35, 1004–1006. [Google Scholar] [CrossRef] [PubMed]
- Van Rijn, R.M.; Huisstede, B.M.; Koes, B.W.; Burdorf, A. Associations between work-related factors and specific disorders at the elbow: A systematic literature review. Rheumatology 2009, 48, 528–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roquelaure, Y.; Raimbeau, G.; Saint-Cast, Y.; Martin, Y.H.; Pelier-Cady, M.C. Occupational risk factors for radial tunnel syndrome in factory workers. Chir. Main 2003, 22, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Hof, J.J.; Kliot, M.; Slimp, J.; Haynor, D.R. What’s new in MRI of peripheral nerve entrapment? Neurosurg. Clin. N. Am. 2008, 19, 583–595. [Google Scholar] [CrossRef]
- Sarhadi, N.S.; Korday, S.N.; Bainbridge, L.C. Radial tunnel syndrome: Diagnosis and management. J. Hand Surg. Br. 1998, 23, 617–619. [Google Scholar] [CrossRef]
- Roles, N.C.; Maudsley, R.H. Radial tunnel syndrome: Resistant tennis elbow as a nerve entrapment. J. Bone Jt. Surg. Br. 1972, 54, 499–508. [Google Scholar] [CrossRef]
- Loh, Y.C.; Lam, W.L.; Stanley, J.K.; Soames, R.W. A new clinical test for radial tunnel syndrome–the Rule-of-Nine test: A cadaveric study. J. Orthop. Surg. 2004, 12, 83–86. [Google Scholar] [CrossRef]
- Wiesman, I.M.; Novak, C.B.; Mackinnon, S.E.; Winograd, J.M. Sensitivity and specificity of clinical testing for carpal tunnel syndrome. Can. J. Plast. Surg. 2003, 11, 70–72. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.; Pierce, P.; Chiu, D.T.W. A fascial band implicated in Wartenberg syndrome. Plast. Reconstr. Surg. 2014, 133, 440e–442e. [Google Scholar] [CrossRef]
- Balakrishnan, C.; Bachusz, R.C.; Balakrishnan, A.; Elliot, D.; Careaga, D. Intraneural lipoma of the radial nerve presenting as Wartenberg syndrome: A case report and review of literature. Plast. Surg. 2009, 17, e39–e41. [Google Scholar]
- Gaspar, M.P.; Kane, P.M.; Vosbikian, M.M.; Ketonis, C.; Rekant, M.S. Neurolysis with amniotic membrane nerve wrapping for treatment of secondary wartenberg syndrome: A preliminary report. J. Hand Surg. Asian Pac. Vol. 2017, 22, 222–228. [Google Scholar] [CrossRef] [PubMed]



| Level | Treatment | Benefit |
|---|---|---|
| Initial Management | ||
| Baseline | Conservative management, including rest and splinting of affected extremity. Physical therapy may also aid in reduction of symptoms. | Decreases compression and irritation of affected nerve. |
| Medication if needed | Non-steroidal anti-inflammatory agents (NSAIDs) | Assists with pain management and decreases inflammation. |
| Optimization of comorbid conditions | Proper management of existing conditions which may include smoking cessation, weight loss, glycemic control, and regular exercise. | Holistically addresses the patient and may prevent the patient progressing to more involved and invasive treatments. |
| If Initial Management Fails | ||
| Medication escalation | Steroid injections. | Helps with pain management, inflammation, and diagnosis. |
| Final treatment | Surgery | Definitive decompression of nerve. |
| Median Nerve | |
|---|---|
| Carpal Tunnel Syndrome | |
| Compression Site | Carpal tunnel containing median nerve, four flexor digitorum superficialis (FDS) tendons, four flexor digitorum profundus (FDP) tendons, and the flexor policus longus (FPL) tendon. |
| Sensation Deficit | Intermittent, nocturnal paresthesia’s and dysesthesias in thumb, index finger, middle finger, and medial aspect of ring finger. |
| Diagnosis | Presence of sensory defects and thenar atrophy. Advanced disease may produce thenar atrophy. Positive Tinel and Phalen sign. Electromyography (EMG) can be used to confirm diagnosis. |
| Anterior Interosseous Syndrome | |
| Compression Site | Usually in forearm within fibrous arch of FDS, less often by Gantzer muscle (accessory FPL). |
| Sensation Deficit | No sensory deficits. |
| Symptoms | Motor weakness of FPL and FDP of index and middle fingers. |
| Diagnosis | Positive Tinel sign, “Kiloh-Nevin Sign”, pinch maneuver. EMG with sharp waves, fibrillations, and abnormal latencies across affected muscles |
| Pronator Syndrome | |
| Compression Site | Between two heads of the pronator teres muscles or proximal arch of the FDS. |
| Symptoms | Volar forearm pain and paresthesia in median nerve distribution. |
| Diagnosis | Pain on compression of proximal volar forearm. EMG studies rule out other syndromes. |
| Ligament of Struthers Syndrome | |
| Caused by rare anatomical accessory fibrous band between the supracondylar process of the humerus and the medial humeral epicondyle. Causes pain, weakness, and sensory defects in median nerve distribution. | |
| Carpal Tunnel Release Surgery Cost Findings | |
|---|---|
| Author: | Finding: |
| Chung et al. (1998) [28] | Endoscopic release is cost effective baring low rate of median nerve damage. |
| Koehler et al. (2019) [27] | Open release incurs a lower cost than endoscopic. |
| Vasen et al. (1999) [25] | Endoscopic release is cost effect with low complication rate. |
| Chatterjee et al. (2011) [29] | Release surgery performed in the clinic has less cost than compared to release in OR. |
| Ulnar Nerve | |
|---|---|
| Cubital Tunnel Syndrome | |
| Compression Site | Compression within cubital tunnel, most often at level of Osborne’s ligament. |
| Sensation Deficit | Dorsal ulnar hand. |
| Symptoms | Grip weakness of ring and small finger FDP. |
| Diagnosis | Positive Froment’s and Wartenberg sign. EMG shows denervation of ulnar innervated muscles with prolonged latencies in cubital tunnel. |
| Complications | Injury to the medial antebrachial cutaneous nerve is common, causing remnant paresthesia and pain over olecranon. |
| Guyon’s Canal | |
| Compression Site | Compression at Guyon’s canal which lies between the volar carpal ligament and the transverse carpal ligament. |
| Sensation Deficit | Small finger and ulnar half of ring finger. |
| Symptoms | Dependent on compression zone. Zone 1: paresthesia and intrinsic muscle deficits. Zone 2: only motor deficits. Zone 3: only sensory deficits. |
| Diagnosis | First interosseous atrophy, inability to cross fingers, positive Wartenberg, Duchenne, and Jeanne sign. Thorough social and occupational history. EMG demonstrates prolonged latencies at the wrist. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boyd, C.J.; Singh, N.P.; Robin, J.X.; Sharma, S. Compression Neuropathies of the Upper Extremity: A Review. Surgeries 2021, 2, 320-334. https://doi.org/10.3390/surgeries2030032
Boyd CJ, Singh NP, Robin JX, Sharma S. Compression Neuropathies of the Upper Extremity: A Review. Surgeries. 2021; 2(3):320-334. https://doi.org/10.3390/surgeries2030032
Chicago/Turabian StyleBoyd, Carter J., Nikhi P. Singh, Joseph X. Robin, and Sheel Sharma. 2021. "Compression Neuropathies of the Upper Extremity: A Review" Surgeries 2, no. 3: 320-334. https://doi.org/10.3390/surgeries2030032
APA StyleBoyd, C. J., Singh, N. P., Robin, J. X., & Sharma, S. (2021). Compression Neuropathies of the Upper Extremity: A Review. Surgeries, 2(3), 320-334. https://doi.org/10.3390/surgeries2030032






