Ectopic Laryngeal Ossification after Bone Morphogenetic Protein-2
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Case Descriptions
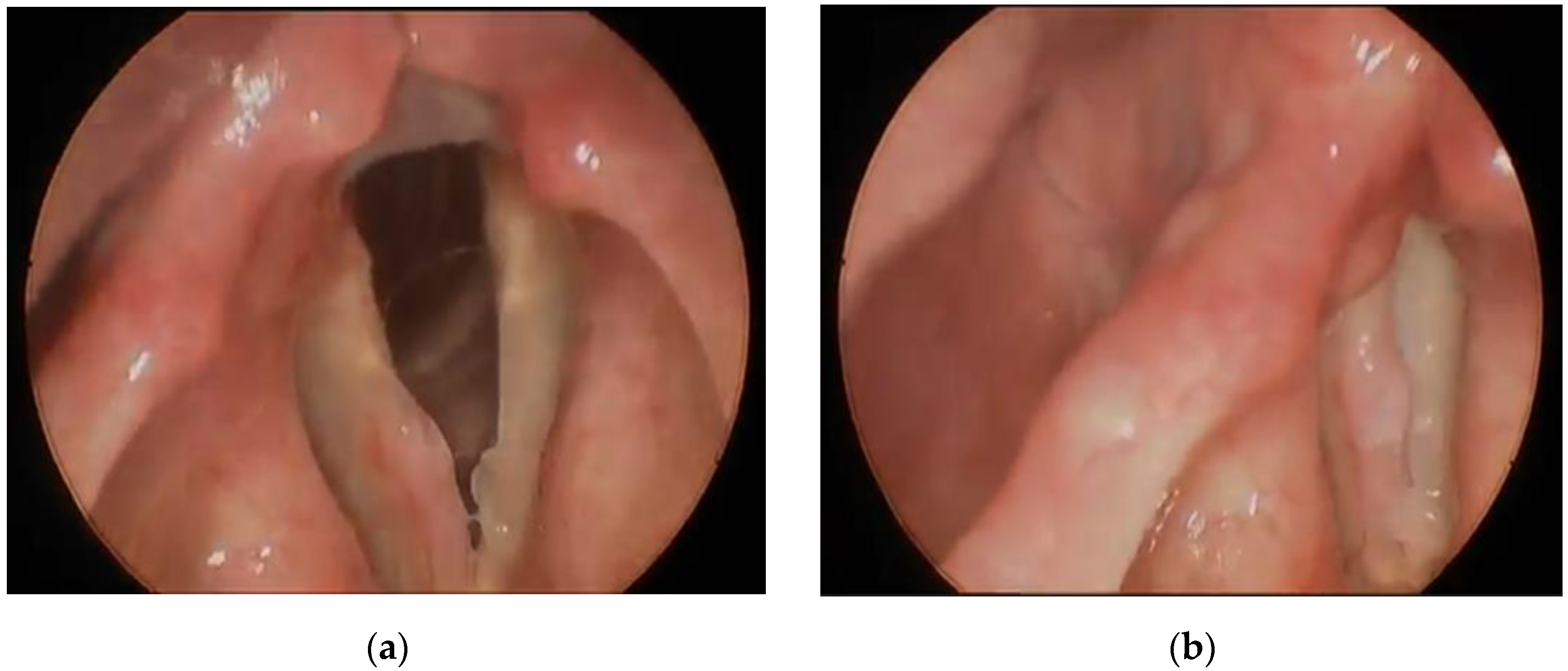
3.1.1. Case 1
3.1.2. Case 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- James, A.W.; Lachaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Hammoudeh, J.A.; Fahradyan, A.; Gould, D.J.; Liang, F.; Imahiyerobo, T.; Urbinelli, L.; Nguyen, J.T.; Magee, W.; Yen, S.; Urata, M.M. A Comparative Analysis of Recombinant Human Bone Morphogenetic Protein-2 with a Demineralized Bone Matrix versus Iliac Crest Bone Graft for Secondary Alveolar Bone Grafts in Patients with Cleft Lip and Palate: Review of 501 Cases. Plast. Reconstr. Surg. 2017, 140, 318e–325e. [Google Scholar] [CrossRef]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef]
- Fineberg, S.J.; Ahmadinia, K.; Oglesby, M.; Patel, A.A.; Singh, K. Hospital Outcomes and Complications of Anterior and Posterior Cervical Fusion with Bone Morphogenetic Protein. Spine 2013, 38, 1304–1309. [Google Scholar] [CrossRef]
- Burkus, J.K.; Dryer, R.F.; Arnold, P.M.; Foley, K.T. Clinical and Radiographic Outcomes in Patients Undergoing Single-level Anterior Cervical Arthrodesis: A Prospective Trial Comparing Allograft to a Reduced Dose of rhBMP-2. Clin. Spine Surg. 2017, 30, E1321–E1332. [Google Scholar] [CrossRef]
- Scheyer, E.T.; Lipton, D.; McGuire, M.K.; Calahan, B.G.; Demetter, R.S.; Mealey, B.L. Histologic Evaluation of rhBMP-2 in an Extraction Site Model in the Esthetic Zone: A Series of 16 Cases Preparing for Implant Placement. Int. J. Periodontics Restor. Dent. 2020, 40, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Sweeny, L.; Lancaster, W.P.; Dean, N.R.; Magnuson, J.S.; Carroll, W.R.; Louis, P.J.; Rosenthal, E.L. Use of recombinant bone morphogenetic protein 2 in free flap reconstruction for osteonecrosis of the mandible. J. Oral Maxillofac. Surg. 2012, 70, 1991–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, D.G. Public Health Notifications (Medical Devices)—FDA Public Health Notification: Life-threatening Com-plications Associated with Recombinant Human Bone Morphogenetic Protein in Cervical Spine Fusion. FDA. 2008. Available online: http://www.tccortho.com/pdf/FDAPublic%20Health%20Note.pdf (accessed on 6 August 2021).
- Hakim, D.N.; Pelly, T.; Kulendran, M.; Caris, J.A. Benign tumours of the bone: A review. J. Bone Oncol. 2015, 4, 37–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eller, R.; Sillers, M. Common Fibro-osseous Lesions of the Paranasal Sinuses. Otolaryngol. Clin. N. Am. 2006, 39, 585–600. [Google Scholar] [CrossRef]
- Wang, W.; Kong, L.; Dong, R.; Zhao, H.; Ma, Y.; Lu, Y. Osteoma in the upper cervical spine with spinal cord compression. Eur. Spine J. 2006, 15, 616–620. [Google Scholar] [CrossRef] [Green Version]
- Angelillo, M.; Mazzone, S.; Costa, G.; Barillari, U. The first case of osteoma in the false vocal fold. Auris Nasus Larynx 2009, 36, 235–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marioni, G.; Altavilla, G.; Marino, F.; Marchese-Ragona, R.; Lelli-Mami, G.; Staffieri, A. Squamous cell carcinoma of the larynx with osteosarcoma-like stromal metaplasia. Acta Oto-Laryngol. 2004, 124, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Aurora, N.; Hashmi, I.; Misra, S.; Aydin, N. A rare presentation: A case report of osseous metaplasia and mature bone formation in a follicular adenoma of the thyroid. Int. J. Surg. Case Rep. 2017, 37, 83–86. [Google Scholar] [CrossRef]
- Mupparapu, M.; Vuppalapati, A. Ossification of laryngeal cartilages on lateral cephalometric radiographs. Angle Orthod. 2005, 75, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Yaremchuk, K.L.; Toma, M.S.; Somers, M.L.; Peterson, E. Acute airway obstruction in cervical spinal procedures with bone morphogenetic proteins. Laryngoscope 2010, 120, 1954–1957. [Google Scholar] [CrossRef]
- Zadegan, S.; Abedi, A.; Jazayeri, S.B.; Bonaki, H.N.; Vaccaro, A.R.; Rahimi-Movaghar, V. Bone Morphogenetic Proteins in Anterior Cervical Fusion: A Systematic Review and Meta-Analysis. World Neurosurg. 2017, 104, 752–787. [Google Scholar] [CrossRef]
- Benglis, D.; Wang, M.Y.; Levi, A.D. A Comprehensive Review of the Safety Profile of Bone Morphogenetic Protein in Spine Surgery. Oper. Neurosurg. 2008, 62, ONS423–ONS431. [Google Scholar] [CrossRef]
- Fernandes, J.d.Q.; de Lima, V.N.; Bonardi, J.P.; Filho, O.M.; Queiroz, S.B.F. Bone regeneration with recombinant human bone morphogenetic protein 2: A systematic review. J. Maxillofac. Oral Surg. 2016, 17, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Ramly, E.P.; Alfonso, A.R.; Kantar, R.S.; Wang, M.M.; Siso, J.R.D.; Ibrahim, A.; Coelho, P.G.; Flores, R.L. Safety and Efficacy of Recombinant Human Bone Morphogenetic Protein-2 (rhBMP-2) in Craniofacial Surgery. Plast. Reconstr. Surg.-Glob. Open 2019, 7, e2347. [Google Scholar] [CrossRef]
- Faundez, A.; Tournier, C.; Garcia, M.; Aunoble, S.; Le Huec, J.-C. Bone morphogenetic protein use in spine surgery—complications and outcomes: A systematic review. Int. Orthop. 2016, 40, 1309–1319. [Google Scholar] [CrossRef]
- Mroz, T.E.; Wang, J.C.; Hashimoto, R.; Norvell, D.C. Complications Related to Osteobiologics Use in Spine Surgery: A systematic review. Spine 2010, 35, S86–S104. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.M.; Anderson, K.K.; Selim, A.; Dryer, R.F.; Burkus, J.K. Heterotopic ossification following single-level anterior cervical discectomy and fusion: Results from the prospective, multicenter, historically controlled trial comparing allograft to an optimized dose of rhBMP-2. J. Neurosurg. Spine 2016, 25, 292–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baskin, D.S.; Ryan, P.; Sonntag, V.K.H.; Westmark, R.M.; Widmayer, M.A. A Prospective, Randomized, Controlled Cervical Fusion Study Using Recombinant Human Bone Morphogenetic Protein-2 With the CORNERSTONE-SR™ Allograft Ring and the ATLANTIS™ Anterior Cervical Plate. Spine 2003, 28, 1219–1224. [Google Scholar] [CrossRef]
- Boakye, M.; Mummaneni, P.V.; Garrett, M.; Rodts, G.; Haid, R. Anterior cervical discectomy and fusion involving a polyetheretherketone spacer and bone morphogenetic protein. J. Neurosurg. Spine 2005, 2, 521–525. [Google Scholar] [CrossRef]
- Komai, Y.; Morimoto, S.; Saito, K.; Urushibara, M.; Sakai, K.; Ikeda, S. Possible involvement of bone morphogenetic protein 2 in heterotopic ossification in metastatic lesion from urothelial carcinoma of bladder. Int. J. Urol. 2006, 13, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.C.; Alves, G.G.; Zambuzzi, W.F.; Sogayar, M.C.; Granjeiro, J.M. Bone Morphogenetic Proteins: Structure, biological function and therapeutic applications. Arch. Biochem. Biophys. 2014, 561, 64–73. [Google Scholar] [CrossRef]
- FDA. InFuse Bone Graft/LT-Cage Lumbar Tampered Fusion Device. Summary of Safety and Effectiveness Data Premarket Approval Application P000058. In FDA; 2002. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf/P000058b.pdf (accessed on 17 October 2020).
- Hofstetter, C.P.; Hofer, A.S.; Levi, A.D. Exploratory meta-analysis on dose-related efficacy and morbidity of bone morphogenetic protein in spinal arthrodesis surgery. J. Neurosurg. Spine 2016, 24, 457–475. [Google Scholar] [CrossRef] [Green Version]
- Um, I.-W.; Ku, J.-K.; Kim, Y.-K.; Lee, B.-K.; Leem, D.H. Histological Review of Demineralized Dentin Matrix as a Carrier of rhBMP-2. Tissue Eng. Part B Rev. 2020, 26, 284–293. [Google Scholar] [CrossRef]
- Durham, E.L.; Howie, R.N.; Hall, S.; Larson, N.; Oakes, B.; Houck, R.; Grey, Z.; Steed, M.; LaRue, A.C.; Muise-Helmericks, R.; et al. Optimizing bone wound healing using BMP2 with absorbable collagen sponge and Talymed nanofiber scaffold. J. Transl. Med. 2018, 16, 321. [Google Scholar] [CrossRef]
- Smith, D.M.; Cooper, G.M.; Mooney, M.P.; Marra, K.G.; Losee, J.E. Bone Morphogenetic Protein 2 Therapy for Craniofacial Surgery. J. Craniofacial Surg. 2008, 19, 1244–1259. [Google Scholar] [CrossRef]
- Sasikumar, K.P.; Elavarasu, S.; Gadagi, J.S. The application of bone morphogenetic proteins to periodontal and peri-implant tissue regeneration: A literature review. J. Pharm. Bioallied Sci. 2012, 4, S427–S430. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Zhou, T.; Chen, H.; Li, C.; Jiang, Z.; Lao, L.; Kahn, S.A.; Duarte, M.E.L.; Zhao, J.; Daubs, M.D.; et al. Bone morphogenetic protein-2 promotes osteosarcoma growth by promoting epithelial-mesenchymal transition (EMT) through the Wnt/β-catenin signaling pathway. J. Orthop. Res. 2019, 37, 1638–1648. [Google Scholar] [CrossRef] [PubMed]
- Bumpous, J.M.; Jacono, A.A.; Sclafani, A.; Van De Water, T.; McCormick, S.; Frenz, D. Metaplastic Bone Formation in Nasal Polyps with Histologic Presence of Transforming Growth Factor β-1 (TGFβ-1) and Bone Morphogenetic Proteins (BMPs). Otolaryngol. Neck Surg. 2001, 125, 96–97. [Google Scholar] [CrossRef] [PubMed]
- Toyran, S.; Lin, A.Y.; Edward, D.P. Expression of growth differentiation factor-5 and bone morphogenic protein-7 in intraocular osseous metaplasia. Br. J. Ophthalmol. 2005, 89, 885–890. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wong, K.; Damrose, E.; Long, J. Ectopic Laryngeal Ossification after Bone Morphogenetic Protein-2. Surgeries 2021, 2, 384-390. https://doi.org/10.3390/surgeries2040038
Wong K, Damrose E, Long J. Ectopic Laryngeal Ossification after Bone Morphogenetic Protein-2. Surgeries. 2021; 2(4):384-390. https://doi.org/10.3390/surgeries2040038
Chicago/Turabian StyleWong, Kirsten, Edward Damrose, and Jennifer Long. 2021. "Ectopic Laryngeal Ossification after Bone Morphogenetic Protein-2" Surgeries 2, no. 4: 384-390. https://doi.org/10.3390/surgeries2040038





