Fibrinogen and Bleeding in Adult Cardiac Surgery: A Review of the Literature
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Search Results
3.2. Physiology of Fibrinogen
3.3. Mechanisms of Plasma Fibrinogen Disorder and Preservation during Cardiac Surgery
3.4. Kinetics of Plasma Fibrinogen in Cardiac Surgery
3.5. Fibrinogen Deficiency Diagnosis
3.6. Association between Fibrinogen Plasma Concentration and Bleeding
3.7. Fibrinogen Supplementation
3.7.1. Sources of Fibrinogen
3.7.2. Timing for Fibrinogen Supplementation
3.7.3. Trigger, Target Values, and Dosage
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
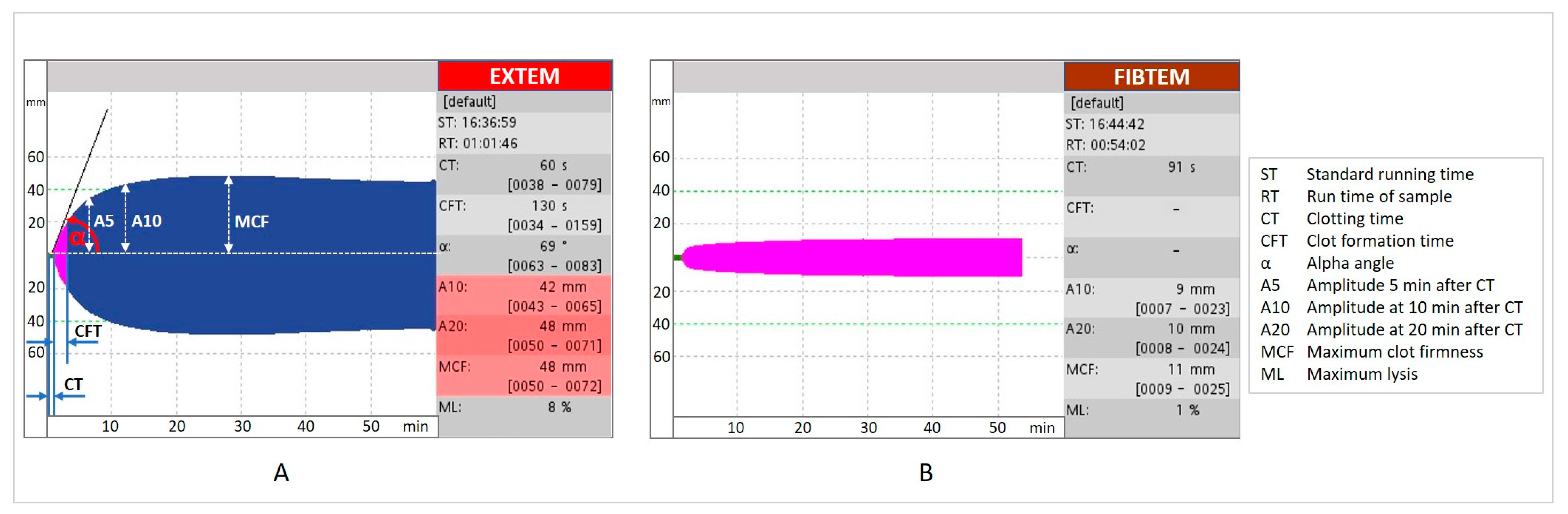
Abbreviations
| A5 | amplitude at 5 min |
| A10 | amplitude at 10 min |
| A20 | amplitude at 20 min |
| CABG | coronary artery bypass graft |
| CPB | cardiopulmonary bypass |
| CRP | C-reactive protein |
| CT | clotting time |
| CFT | clot formation time |
| DHCA | deep hypothermic circulatory arrest |
| EACA | Ɛ-aminocaproic acid |
| EACTA | European Association of Cardiothoracic Anaesthesiology |
| EACTS | European Association for Cardiothoracic Surgery |
| ESA | European Society of Anaesthesiology |
| EXTEM | extrinsic thromboelastometry |
| FC | fibrinogen concentrate |
| FDPs | fibrin degradation products |
| FF | functional fibrinogen |
| FFP | fresh–frozen plasma |
| FIBTEM | fibrin-based thromboelastometry test |
| GP | glycoprotein |
| HES | hydroxyethyl starch |
| ICU | intensive care unit |
| IL | interleukin |
| MA | maximum amplitude |
| MCF | maximum clot firmness |
| ML | maximum lysis |
| MiECC | minimally invasive extracorporeal circulation |
| mini-CPB | miniaturised CPB circuit |
| NPV | negative predictive value |
| PPV | positive predictive value |
| RBCs | red blood cells |
| RCT | Randomised Clinical Trial |
| REPLACE | Randomised Double-Blinded Placebo-Controlled Trial of Fibrinogen Concentrate Supplementation After Complex Cardiac Surgery |
| ROTEM® | rotational thromboelastometry |
| TEG® | thromboelastography |
| TF | tissue factor |
| t-PA | tissue plasminogen activator |
| TXA | Tranexamic acid |
| UDPB | Universal Definition of Perioperative Bleeding |
| UFH | unfractionated heparin |
| u-PA | urokinase plasminogen activator |
| VETs | viscoelastic tests |
| XIIIa | activated factor XIII |
References
- Sucker, C.; Zotz, R.B. The Cell-Based Coagulation Model. In Perioperative Hemostasis: Coagulation for Anesthesiologists; Marcucci, C.E., Schoettker, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–11. ISBN 978-3-642-55004-1. [Google Scholar]
- Kattula, S.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen and Fibrin in Hemostasis and Thrombosis. ATVB 2017, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momeni, M.; Carlier, C.; Baele, P.; Watremez, C.; Van Dyck, M.; Matta, A.; Kahn, D.; Rennotte, M.-T.; Glineur, D.; de Kerchove, L.; et al. Fibrinogen Concentration Significantly Decreases After On-Pump Versus Off-Pump Coronary Artery Bypass Surgery: A Systematic Point-of-Care ROTEM Analysis. J. Cardiothorac. Vasc. Anesth. 2013, 27, 5–11. [Google Scholar] [CrossRef] [Green Version]
- Erdoes, G.; Dietrich, W.; Stucki, M.P.; Merz, T.M.; Angelillo-Scherrer, A.; Nagler, M.; Carrel, T.; Eberle, B. Short-Term Recovery Pattern of Plasma Fibrinogen after Cardiac Surgery: A Prospective Observational Study. PLoS ONE 2018, 13, e0201647. [Google Scholar] [CrossRef]
- Roy, S.; Saha, K.; Mukherjee, K.; Dutta, S.; Mukhopadhyay, D.; Das, I.; Raychaudhuri, G. Activation of Coagulation and Fibrinolysis During Coronary Artery Bypass Grafting: A Comparison Between On-Pump and Off-Pump Techniques. Indian J. Hematol. Blood Transfus. 2014, 30, 333–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanna, J.M.; Keenan, J.E.; Wang, H.; Andersen, N.D.; Gaca, J.G.; Lombard, F.W.; Welsby, I.J.; Hughes, G.C. Use of Human Fibrinogen Concentrate during Proximal Aortic Reconstruction with Deep Hypothermic Circulatory Arrest. J. Thorac. Cardiovasc. Surg. 2016, 151, 376–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raspé, C.; Besch, M.; Charitos, E.I.; Flöther, L.; Bucher, M.; Rückert, F.; Treede, H. Rotational Thromboelastometry for Assessing Bleeding Complications and Factor XIII Deficiency in Cardiac Surgery Patients. Clin. Appl. Thromb. Hemost. 2018, 24, 136S–144S. [Google Scholar] [CrossRef] [Green Version]
- Sniecinski, R.M.; Chandler, W.L. Activation of the Hemostatic System During Cardiopulmonary Bypass. Anesth. Analg. 2011, 113, 1319–1333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pillai, R.C.; Fraser, J.F.; Ziegenfuss, M.; Bhaskar, B. The Influence of Circulating Levels of Fibrinogen and Perioperative Coagulation Parameters on Predicting Postoperative Blood Loss in Cardiac Surgery: A Prospective Observational Study: Role of Fibrinogen in Perioperative Bleeding, a Prospective Observational Study. J. Card. Surg. 2014, 29, 189–195. [Google Scholar] [CrossRef]
- Kwapisz, M.M.; Kent, B.; DiQuinzio, C.; LeGare, J.; Garnett, S.; Swyer, W.; Whynot, S.; Mingo, H.; Scheffler, M. The Prophylactic Use of Fibrinogen Concentrate in High-risk Cardiac Surgery. Acta Anaesthesiol. Scand. 2020, 64, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Nishi, T.; Mutsuga, M.; Akita, T.; Narita, Y.; Fujimoto, K.; Tokuda, Y.; Terazawa, S.; Ito, H.; Nishiwaki, K.; Usui, A. The Incidence and Risk Factors of Hypofibrinogenemia in Cardiovascular Surgery. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 335–341. [Google Scholar] [CrossRef]
- Tanaka, K.A.; Esper, S.; Bolliger, D. Perioperative Factor Concentrate Therapy. Br. J. Anaesth. 2013, 111, i35–i49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranucci, M. Hemostatic and Thrombotic Issues in Cardiac Surgery. Semin. Thromb. Hemost. 2015, 41, 084–090. [Google Scholar] [CrossRef] [PubMed]
- Gielen, C.L.I.; Grimbergen, J.; Klautz, R.J.M.; Koopman, J.; Quax, P.H.A. Fibrinogen Reduction and Coagulation in Cardiac Surgery: An Investigational Study. Blood Coagul. Fibrinolysis 2015, 26, 613–620. [Google Scholar] [CrossRef]
- Levy, J.H.; Welsby, I.; Goodnough, L.T. Fibrinogen as a Therapeutic Target for Bleeding: A Review of Critical Levels and Replacement Therapy: Fibrinogen: A Therapeutic Target for Bleeding. Transfusion 2014, 54, 1389–1405. [Google Scholar] [CrossRef] [PubMed]
- Perelman, I.; Saidenberg, E.; Tinmouth, A.; Fergusson, D. Trends and Outcomes in Multicomponent Blood Transfusion: An 11-year Cohort Study of a Large Multisite Academic Center. Transfusion 2019, 59, 1971–1987. [Google Scholar] [CrossRef] [PubMed]
- Dyke, C.; Aronson, S.; Dietrich, W.; Hofmann, A.; Karkouti, K.; Levi, M.; Murphy, G.J.; Sellke, F.W.; Shore-Lesserson, L.; von Heymann, C.; et al. Universal Definition of Perioperative Bleeding in Adult Cardiac Surgery. J. Thorac. Cardiovasc. Surg. 2014, 147, 1458–1463.e1. [Google Scholar] [CrossRef] [Green Version]
- Greiff, G.; Pleym, H.; Stenseth, R.; Berg, K.S.; Wahba, A.; Videm, V. Prediction of Bleeding After Cardiac Surgery: Comparison of Model Performances: A Prospective Observational Study. J. Cardiothorac. Vasc. Anesth. 2015, 29, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Bartoszko, J.; Wijeysundera, D.N.; Karkouti, K.; on behalf of the Transfusion Avoidance in Cardiac Surgery Study Investigators; Callum, J.; Rao, V.; Crowther, M.; Grocott, H.P.; Pinto, R.; Scales, D.C.; et al. Comparison of Two Major Perioperative Bleeding Scores for Cardiac Surgery Trials. Anesthesiology 2018, 129, 1092–1100. [Google Scholar] [CrossRef]
- Ternström, L.; Hyllner, M.; Backlund, E.; Schersten, H.; Jeppsson, A. A Structured Blood Conservation Programme Reduces Transfusions and Costs in Cardiac Surgery. Interact. Cardiovasc. Thorac. Surg. 2014, 19, 788–794. [Google Scholar] [CrossRef] [Green Version]
- Mazer, C.D.; Whitlock, R.P.; Fergusson, D.A.; Belley-Cote, E.; Connolly, K.; Khanykin, B.; Gregory, A.J.; de Médicis, É.; Carrier, F.M.; McGuinness, S.; et al. Six-Month Outcomes after Restrictive or Liberal Transfusion for Cardiac Surgery. N. Engl. J. Med. 2018, 379, 1224–1233. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Clayton, T.C.; Mehran, R.; Dangas, G.; Parise, H.; Fahy, M.; Pocock, S.J. Impact of Major Bleeding and Blood Transfusions after Cardiac Surgery: Analysis from the Acute Catheterization and Urgent Intervention Triage StrategY (ACUITY) Trial. Am. Heart J. 2012, 163, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Biancari, F.; Mikkola, R.; Heikkinen, J.; Lahtinen, J.; Airaksinen, K.E.J.; Juvonen, T. Estimating the Risk of Complications Related to Re-Exploration for Bleeding after Adult Cardiac Surgery: A Systematic Review and Meta-Analysis. Eur. J. Cardio-Thorac. Surg. 2011, 41, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Canadyova, J.; Zmeko, D.; Mokracek, A. Re-Exploration for Bleeding or Tamponade after Cardiac Operation. Interact. Cardiovasc. Thorac. Surg. 2012, 14, 704–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fröjd, V.; Jeppsson, A. Reexploration for Bleeding and Its Association With Mortality After Cardiac Surgery. Ann. Thorac. Surg. 2016, 102, 109–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vivacqua, A.; Koch, C.G.; Yousuf, A.M.; Nowicki, E.R.; Houghtaling, P.L.; Blackstone, E.H.; Sabik, J.F. Morbidity of Bleeding After Cardiac Surgery: Is It Blood Transfusion, Reoperation for Bleeding, or Both? Ann. Thorac. Surg. 2011, 91, 1780–1790. [Google Scholar] [CrossRef]
- Essa, Y.; Zeynalov, N.; Sandhaus, T.; Hofmann, M.; Lehmann, T.; Doenst, T. Low Fibrinogen Is Associated with Increased Bleeding-Related Re-Exploration after Cardiac Surgery. Thorac. Cardiovasc. Surg. 2018, 66, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, M.; Baryshnikova, E.; Castelvecchio, S.; Pelissero, G. Major Bleeding, Transfusions, and Anemia: The Deadly Triad of Cardiac Surgery. Ann. Thorac. Surg. 2013, 96, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Göbel, K.; Eichler, S.; Wiendl, H.; Chavakis, T.; Kleinschnitz, C.; Meuth, S.G. The Coagulation Factors Fibrinogen, Thrombin, and Factor XII in Inflammatory Disorders—A Systematic Review. Front. Immunol. 2018, 9, 1731. [Google Scholar] [CrossRef] [Green Version]
- Fish, R.J.; Neerman-Arbez, M. Fibrinogen Gene Regulation. Thromb. Haemost. 2012, 108, 419–426. [Google Scholar] [CrossRef]
- Tabakcı, M.M.; Gerin, F.; Sunbul, M.; Toprak, C.; Durmuş, H.İ.; Demir, S.; Arslantaş, U.; Cerşit, S.; Batgerel, U.; Kargın, R. Relation of Plasma Fibrinogen Level With the Presence, Severity, and Complexity of Coronary Artery Disease. Clin. Appl. Thromb. Hemost. 2017, 23, 638–644. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.-F.; Li, X.-L.; Luo, S.-H.; Guo, Y.-L.; Zhu, C.-G.; Qing, P.; Wu, N.-Q.; Li, J.-J. Association of Fibrinogen with Severity of Stable Coronary Artery Disease in Patients with Type 2 Diabetic Mellitus. Dis. Markers 2014, 2014, 485687. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yu, T.; Sun, Z.; Li, Z.; He, D.; Sun, Z. Comparison of Prognostic Significance between Serum Fibrinogen and Global Registry of Acute Coronary Events Score for Prognosis of Patients with Non-ST-Elevation Acute Coronary Syndromes Undergoing Percutaneous Coronary Intervention. Coron. Artery Dis. 2020, 31, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.; Hoffman, M.; Monroe, D. A Cell-Based Model of Thrombin Generation. Semin. Thromb. Hemost. 2006, 32, 32–38. [Google Scholar] [CrossRef]
- Weisel, J.W.; Litvinov, R.I. Fibrin Formation, Structure and Properties. In Fibrous Proteins: Structures and Mechanisms; Parry, D.A.D., Squire, J.M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 82, pp. 405–456. ISBN 978-3-319-49672-6. [Google Scholar]
- McMichael, M. New Models of Hemostasis. Top. Companion Anim. Med. 2012, 27, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Urano, T.; Suzuki, Y.; Iwaki, T.; Sano, H.; Honkura, N.; Castellino, F.J. Recognition of Plasminogen Activator Inhibitor Type 1 as the Primary Regulator of Fibrinolysis. Curr. Drug Targets 2019, 20, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Longstaff, C.; Kolev, K. Basic Mechanisms and Regulation of Fibrinolysis. J. Thromb. Haemost. 2015, 13, S98–S105. [Google Scholar] [CrossRef] [Green Version]
- Hoppe, B. Fibrinogen and Factor XIII at the Intersection of Coagulation, Fibrinolysis and Inflammation. Thromb. Haemost. 2014, 112, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Gielen, C.L.I.; Brand, A.; van Heerde, W.L.; Stijnen, T.; Klautz, R.J.M.; Eikenboom, J. Hemostatic Alterations during Coronary Artery Bypass Grafting. Thromb. Res. 2016, 140, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Esper, S.A.; Subramaniam, K.; Tanaka, K.A. Pathophysiology of Cardiopulmonary Bypass: Current Strategies for the Prevention and Treatment of Anemia, Coagulopathy, and Organ Dysfunction. Semin. Cardiothorac. Vasc. Anesth. 2014, 18, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Bethlehem, I.; Wierda, K.; Visser, C.; Jekel, L.; Koopmans, M.; Kuiper, M.A. Influence of Two Colloidal Extracorporeal Primes on Coagulation of Cardiac Surgical Patients: A Prospectively Randomized Open-Label Pilot Trial. J. Extra Corpor. Technol. 2014, 46, 293–299. [Google Scholar]
- Van der Linden, P.; Ickx, B.E. The Effects of Colloid Solutions on Hemostasis. Can. J. Anesth. 2006, 53, S30–S39. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.; Yu, B. Effects of Artificial Colloids on Haemostasis. Br. J. Hosp. Med. 2009, 70, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Stump, D.C.; Strauss, R.G.; Henriksen, R.A.; Petersen, R.E.; Saunders, R. Effects of Hydroxyethyl Starch on Blood Coagulation, Particularly Factor VIII. Transfusion 1985, 25, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Treib, J.; Haass, A.; Pindur, G. Coagulation Disorders Caused by Hydroxyethyl Starch. Thromb. Haemost. 1997, 78, 974–983. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, V.G. Colloids Decrease Clot Propagation and Strength: Role of Factor XIII-Fibrin Polymer and Thrombin-Fibrinogen Interactions. Acta Anaesthesiol. Scand. 2005, 49, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Schramko, A.; Suojaranta-Ylinen, R.; Kuitunen, A.; Raivio, P.; Kukkonen, S.; Niemi, T. Hydroxyethylstarch and Gelatin Solutions Impair Blood Coagulation after Cardiac Surgery: A Prospective Randomized Trial. Br. J. Anaesth. 2010, 104, 691–697. [Google Scholar] [CrossRef] [Green Version]
- Ghijselings, I.; Himpe, D.; Rex, S. Safety of Gelatin Solutions for the Priming of Cardiopulmonary Bypass in Cardiac Surgery: A Systematic Review and Meta-Analysis. Perfusion 2017, 32, 350–362. [Google Scholar] [CrossRef]
- Pagano, D.; Milojevic, M.; Meesters, M.I.; Benedetto, U.; Bolliger, D.; von Heymann, C.; Jeppsson, A.; Koster, A.; Osnabrugge, R.L.; Ranucci, M.; et al. 2017 EACTS/EACTA Guidelines on Patient Blood Management for Adult Cardiac Surgery. Eur. J. Cardio-Thorac. Surg. 2018, 53, 79–111. [Google Scholar] [CrossRef] [Green Version]
- Raphael, J.; Mazer, C.D.; Subramani, S.; Schroeder, A.; Abdalla, M.; Ferreira, R.; Roman, P.E.; Patel, N.; Welsby, I.; Greilich, P.E.; et al. Society of Cardiovascular Anesthesiologists Clinical Practice Improvement Advisory for Management of Perioperative Bleeding and Hemostasis in Cardiac Surgery Patients. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2887–2899. [Google Scholar] [CrossRef] [Green Version]
- Smith, B.B.; Nuttall, G.A.; Mauermann, W.J.; Schroeder, D.R.; Scott, P.D.; Smith, M.M. Coagulation Test Changes Associated with Acute Normovolemic Hemodilution in Cardiac Surgery. J. Card. Surg. 2020, 35, 1043–1050. [Google Scholar] [CrossRef]
- Tanaka, K.A.; Egan, K.; Szlam, F.; Ogawa, S.; Roback, J.D.; Sreeram, G.; Guyton, R.A.; Chen, E.P. Transfusion and Hematologic Variables after Fibrinogen or Platelet Transfusion in Valve Replacement Surgery: Preliminary Data of Purified Lyophilized Human Fibrinogen Concentrate versus Conventional Transfusion: Fibrinogen versus PLT Concentrate in Valve Replacement. Transfusion 2014, 54, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Yamamoto, K.; Kakinuma, A.; Nakata, Y.; Sawamura, S. Accelerated Activation of the Coagulation Pathway during Cardiopulmonary Bypass in Aortic Replacement Surgery: A Prospective Observational Study. J. Cardiothorac. Surg. 2015, 10, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morisaki, A.; Nakahira, A.; Sasaki, Y.; Hirai, H.; Okada, Y.; Suehiro, S.; Shibata, T. Is Elimination of Cardiotomy Suction Preferable in Aortic Valve Replacement? Assessment of Perioperative Coagulation, Fibrinolysis and Inflammation. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 507–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giri, H.; Panicker, S.R.; Cai, X.; Biswas, I.; Weiler, H.; Rezaie, A.R. Thrombomodulin Is Essential for Maintaining Quiescence in Vascular Endothelial Cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2022248118. [Google Scholar] [CrossRef]
- Esmon, C.T. The Normal Role of Activated Protein C in Maintaining Homeostasis and Its Relevance to Critical Illness. Crit. Care 2001, 5, S7. [Google Scholar] [CrossRef] [Green Version]
- Gorki, H.; Nakamura, J.; Kunert, A.; Hoenicka, M.; Liebold, A. Pericardial Fluids or Cardiopulmonary Bypass—Is There a Major Culprit for Changes in Coagulation and Inflammation? Thorac. Cardiovasc. Surg. 2020, 68, 219–222. [Google Scholar] [CrossRef]
- Gäbel, J.; Hakimi, C.S.; Westerberg, M.; Radulovic, V.; Jeppsson, A. Retransfusion of Cardiotomy Suction Blood Impairs Haemostasis: Ex Vivo and in Vivo Studies. Scand. Cardiovasc. J. 2013, 47, 368–376. [Google Scholar] [CrossRef]
- Paparella, D.; Whitlock, R. Safety of Salvaged Blood and Risk of Coagulopathy in Cardiac Surgery. Semin. Thromb. Hemost. 2016, 42, 166–171. [Google Scholar] [CrossRef]
- Adam, E.H.; Funke, M.; Zacharowski, K.; Meybohm, P.; Keller, H.; Weber, C.F. Impact of Intraoperative Cell Salvage on Blood Coagulation Factor Concentrations in Patients Undergoing Cardiac Surgery. Anesth. Analg. 2020, 130, 1389–1395. [Google Scholar] [CrossRef]
- Sharma, A.D.; Al-Achi, A.; Seccombe, J.F.; Hummel, R.; Preston, M.; Behrend, D. Does Incorporation of Thromboelastography Improve Bleeding Prediction Following Adult Cardiac Surgery? Blood Coagul. Fibrinolysis 2014, 25, 561–570. [Google Scholar] [CrossRef] [Green Version]
- Boyle, G.; Kuffel, A.; Parmar, K.; Gibson, K.; Smith, M.; Grehan, A.; Hunt, B.J.; Chambers, D.J. A Comparison of Haemostatic Biomarkers during Low-Risk Patients Undergoing Cardiopulmonary Bypass Using Either Conventional Centrifugal Cell Salvage or the HemoSep Device. Perfusion 2019, 34, 76–83. [Google Scholar] [CrossRef] [PubMed]
- McNair, E.; McKay, W.; Qureshi, A.M.; Rosin, M.; Gamble, J.; Dalshaug, G.; Mycyk, T.; Prasad, K. Outcomes and Biochemical Parameters Following Cardiac Surgery: Effects of Transfusion of Residual Blood Using Centrifugation and Multiple-Pass Hemoconcentration. J. Cardiothorac. Vasc. Anesth. 2013, 27, 1174–1180. [Google Scholar] [CrossRef]
- Colli, A.; Balduzzi, S.; Ruyra, X. The Hemobag: The Modern Ultrafiltration System for Patients Undergoing Cardiopulmonary by Pass. J. Cardiothorac. Surg. 2012, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Beckmann, S.; Lynn, P.; Miller, S.; Harris, R.; DiMarco, R.; Ross, J. Evaluation of Coagulation Factors and Platelet Function from an Off-Line Modified Ultrafiltration Technique for Post-Cardiopulmonary Bypass Circuit Blood Recovery. Perfusion 2013, 28, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Paparella, D.; Parolari, A.; Rotunno, C.; Vincent, J.; Myasoedova, V.; Guida, P.; De Palo, M.; Margari, V.; Devereaux, P.J.; Lamy, A.; et al. The Effects of Steroids on Coagulation Dysfunction Induced by Cardiopulmonary Bypass: A Steroids in Cardiac Surgery (SIRS) Trial Substudy. Semin. Thorac. Cardiovasc. Surg. 2017, 29, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Onorati, F.; Santini, F.; Raffin, F.; Menon, T.; Graziani, M.S.; Chiominto, B.; Milano, A.; Faggian, G.; Mazzucco, A. Clinical Evaluation of New Generation Oxygenators With Integrated Arterial Line Filters for Cardiopulmonary Bypass: Clinical Outcome with New Integrated Filter Oxygenators. Artif. Organs 2012, 36, 875–885. [Google Scholar] [CrossRef]
- Fluger, I.; Maderová, K.; Šimek, M.; Hájek, R.; Zapletalová, J.; Lonský, V. The Effect of a Cardiopulmonary Bypass System with Biocompatible Coating on Fibrinogen Levels Determined by the TEG—Functional Fibrinogen Method: Preliminary Results. Perfusion 2011, 26, 503–509. [Google Scholar] [CrossRef]
- Zindovic, I.; Sjögren, J.; Bjursten, H.; Ingemansson, R.; Ingimarsson, J.; Larsson, M.; Svensson, P.J.; Strandberg, K.; Wierup, P.; Nozohoor, S. The Coagulopathy of Acute Type A Aortic Dissection: A Prospective, Observational Study. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2746–2754. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Han, L.; Li, J.; Gong, M.; Zhang, H.; Guan, X. Consumption Coagulopathy in Acute Aortic Dissection: Principles of Management. J. Cardiothorac. Surg. 2017, 12, 50. [Google Scholar] [CrossRef]
- Guan, X.L.; Wang, X.L.; Liu, Y.Y.; Lan, F.; Gong, M.; Li, H.Y.; Liu, O.; Jiang, W.J.; Liu, Y.M.; Zhu, J.M.; et al. Changes in the Hemostatic System of Patients With Acute Aortic Dissection Undergoing Aortic Arch Surgery. Ann. Thorac. Surg. 2016, 101, 945–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozek-Langenecker, S.A.; Ahmed, A.B.; Afshari, A.; Albaladejo, P.; Aldecoa, C.; Barauskas, G.; De Robertis, E.; Faraoni, D.; Filipescu, D.C.; Fries, D.; et al. Management of Severe Perioperative Bleeding: Guidelines from the European Society of Anaesthesiology: First Update 2016. Eur. J. Anaesthesiol. EJA 2017, 34, 332–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y. Contact Pathway of Coagulation and Inflammation. Thromb. J. 2015, 13, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, N.J.; Gainer, J.V.; Murphey, L.J.; Vaughan, D.E. Bradykinin Stimulates Tissue Plasminogen Activator Release From Human Forearm Vasculature Through B2 Receptor–Dependent, NO Synthase–Independent, and Cyclooxygenase-Independent Pathway. Circulation 2000, 102, 2190–2196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaguer, J.M.; Yu, C.; Byrne, J.G.; Ball, S.K.; Petracek, M.R.; Brown, N.J.; Pretorius, M. Contribution of Endogenous Bradykinin to Fibrinolysis, Inflammation, and Blood Product Transfusion Following Cardiac Surgery: A Randomized Clinical Trial. Clin. Pharmacol. Ther. 2013, 93, 326–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Z.; Tian, G.; Ren, Y.; Sun, Z.; Lu, W.; Hou, X. Pharmacokinetic Model of Unfractionated Heparin during and after Cardiopulmonary Bypass in Cardiac Surgery. J. Transl. Med. 2015, 13, 45. [Google Scholar] [CrossRef] [Green Version]
- Boer, C.; Meesters, M.I.; Veerhoek, D.; Vonk, A.B.A. Anticoagulant and Side-Effects of Protamine in Cardiac Surgery: A Narrative Review. Br. J. Anaesth. 2018, 120, 914–927. [Google Scholar] [CrossRef] [Green Version]
- Kindo, M.; Hoang Minh, T.; Gerelli, S.; Perrier, S.; Meyer, N.; Schaeffer, M.; Bentz, J.; Announe, T.; Mommerot, A.; Collange, O.; et al. Plasma Fibrinogen Level on Admission to the Intensive Care Unit Is a Powerful Predictor of Postoperative Bleeding after Cardiac Surgery with Cardiopulmonary Bypass. Thromb. Res. 2014, 134, 360–368. [Google Scholar] [CrossRef]
- Vuylsteke, A.; Pagel, C.; Gerrard, C.; Reddy, B.; Nashef, S.; Aldam, P.; Utley, M. The Papworth Bleeding Risk Score: A Stratification Scheme for Identifying Cardiac Surgery Patients at Risk of Excessive Early Postoperative Bleeding. Eur. J. Cardio-Thorac. Surg. 2011, 39, 924–930. [Google Scholar] [CrossRef] [Green Version]
- Emeklibas, N.; Kammerer, I.; Bach, J.; Sack, F.-U.; Hellstern, P. Preoperative Hemostasis and Its Association with Bleeding and Blood Component Transfusion Requirements in Cardiopulmonary Bypass Surgery: Preoperative Hemostasis and Bleeding. Transfusion 2013, 53, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Bilecen, S.; de Groot, J.A.H.; Kalkman, C.J.; Spanjersberg, A.J.; Brandon Bravo Bruinsma, G.J.; Moons, K.G.M.; Nierich, A.P. Effect of Fibrinogen Concentrate on Intraoperative Blood Loss Among Patients With Intraoperative Bleeding During High-Risk Cardiac Surgery: A Randomized Clinical Trial. JAMA 2017, 317, 738. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, E.; Rubino, A.; Altemimi, B.; Collier, T.; Besser, M.W.; Klein, A.A. Validation of Viscoelastic Coagulation Tests during Cardiopulmonary Bypass. J. Thromb. Haemost. 2015, 13, 1207–1216. [Google Scholar] [CrossRef]
- Fabbro, M.; Gutsche, J.T.; Miano, T.A.; Augoustides, J.G.; Patel, P.A. Comparison of Thrombelastography-Derived Fibrinogen Values at Rewarming and Following Cardiopulmonary Bypass in Cardiac Surgery Patients. Anesth. Analg. 2016, 123, 570–577. [Google Scholar] [CrossRef]
- Erdoes, G.; Gerster, G.; Colucci, G.; Kaiser, H.; Alberio, L.; Eberle, B. Prediction of Post-Weaning Fibrinogen Status during Cardiopulmonary Bypass: An Observational Study in 110 Patients. PLoS ONE 2015, 10, e0126692. [Google Scholar] [CrossRef]
- Mace, H.; Lightfoot, N.; McCluskey, S.; Selby, R.; Roy, D.; Timoumi, T.; Karkouti, K. Validity of Thromboelastometry for Rapid Assessment of Fibrinogen Levels in Heparinized Samples During Cardiac Surgery: A Retrospective, Single-Center, Observational Study. J. Cardiothorac. Vasc. Anesth. 2016, 30, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Solomon, C.; Baryshnikova, E.; Schlimp, C.J.; Schöchl, H.; Asmis, L.M.; Ranucci, M. FIBTEM PLUS Provides an Improved Thromboelastometry Test for Measurement of Fibrin-Based Clot Quality in Cardiac Surgery Patients. Anesth. Analg. 2013, 117, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Schlimp, C.J.; Solomon, C.; Ranucci, M.; Hochleitner, G.; Redl, H.; Schöchl, H. The Effectiveness of Different Functional Fibrinogen Polymerization Assays in Eliminating Platelet Contribution to Clot Strength in Thromboelastometry. Anesth. Analg. 2014, 118, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kumar, S.; Tewari, P.; Pande, S.; Murari, M. Utility of Thromboelastography versus Routine Coagulation Tests for Assessment of Hypocoagulable State in Patients Undergoing Cardiac Bypass Surgery. Ann. Cardiac. Anaesth. 2018, 21, 151. [Google Scholar] [CrossRef]
- Ranucci, M.; Baryshnikova, E. Fibrinogen Supplementation after Cardiac Surgery: Insights from the Zero-Plasma Trial (ZEPLAST). Br. J. Anaesth. 2016, 116, 618–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shams Hakimi, C.; Singh, S.; Hesse, C.; Jeppsson, A. Effects of Fibrinogen and Platelet Transfusion on Coagulation and Platelet Function in Bleeding Cardiac Surgery Patients. Acta Anaesthesiol. Scand. 2019, 63, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Szlam, F.; Chen, E.P.; Nishimura, T.; Kim, H.; Roback, J.D.; Levy, J.H.; Tanaka, K.A. A Comparative Evaluation of Rotation Thromboelastometry and Standard Coagulation Tests in Hemodilution-Induced Coagulation Changes after Cardiac Surgery: THROMBOELASTOMETRY IN CPB SURGERY. Transfusion 2012, 52, 14–22. [Google Scholar] [CrossRef]
- Solomon, C.; Cadamuro, J.; Ziegler, B.; Schöchl, H.; Varvenne, M.; Sørensen, B.; Hochleitner, G.; Rahe-Meyer, N. A Comparison of Fibrinogen Measurement Methods with Fibrin Clot Elasticity Assessed by Thromboelastometry, before and after Administration of Fibrinogen Concentrate in Cardiac Surgery Patients: Comparing Fibrinogen Measurement Methods. Transfusion 2011, 51, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Olde Engberink, R.H.G.; Kuiper, G.J.A.J.M.; Wetzels, R.J.H.; Nelemans, P.J.; Lance, M.D.; Beckers, E.A.M.; Henskens, Y.M.C. Rapid and Correct Prediction of Thrombocytopenia and Hypofibrinogenemia With Rotational Thromboelastometry in Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2014, 28, 210–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, S.; Szlam, F.; Bolliger, D.; Nishimura, T.; Chen, E.P.; Tanaka, K.A. The Impact of Hematocrit on Fibrin Clot Formation Assessed by Rotational Thromboelastometry. Anesth. Analg. 2012, 115, 16–21. [Google Scholar] [CrossRef]
- Hans, G.A.; Hartstein, G.; Roediger, L.; Hubert, B.; Peters, P.; Senard, M. Impact of 6 % Hydroxyethyl Starch (HES) 130/0.4 on the Correlation between Standard Laboratory Tests and Thromboelastography (TEG®) after Cardiopulmonary Bypass. Thromb. Res. 2015, 135, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Solomon, C.; Rahe-Meyer, N.; Schöchl, H.; Ranucci, M.; Görlinger, K. Effect of Haematocrit on Fibrin-Based Clot Firmness in the FIBTEM Test. Blood Transfus. 2013, 11, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.S.; Middleton, P.; McCarthy, H.; Spiess, B.D. A Comparison of a New Ultrasound-Based Whole Blood Viscoelastic Test (SEER Sonorheometry) Versus Thromboelastography in Cardiac Surgery. Anesth. Analg. 2016, 123, 1400–1407. [Google Scholar] [CrossRef]
- Espinosa, A.; Stenseth, R.; Videm, V.; Pleym, H. Comparison of Three Point-of-Care Testing Devices to Detect Hemostatic Changes in Adult Elective Cardiac Surgery: A Prospective Observational Study. BMC Anesthesiol. 2014, 14, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groves, D.S.; Welsby, I.J.; Naik, B.I.; Tanaka, K.; Hauck, J.N.; Greenberg, C.S.; Winegar, D.A.; Viola, F. Multicenter Evaluation of the Quantra QPlus System in Adult Patients Undergoing Major Surgical Procedures. Anesth. Analg. 2020, 130, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Erdoes, G.; Schloer, H.; Eberle, B.; Nagler, M. Next Generation Viscoelasticity Assays in Cardiothoracic Surgery: Feasibility of the TEG6s System. PLoS ONE 2018, 13, e0209360. [Google Scholar] [CrossRef]
- Vasques, F.; Spiezia, L.; Manfrini, A.; Tarzia, V.; Fichera, D.; Simioni, P.; Gerosa, G.; Ori, C.; Di Gregorio, G. Thromboelastometry Guided Fibrinogen Replacement Therapy in Cardiac Surgery: A Retrospective Observational Study. J. Anesth. 2017, 31, 286–290. [Google Scholar] [CrossRef]
- Weber, C.F.; Görlinger, K.; Meininger, D.; Hermann, E.; Bingold, T.; Moritz, A.; Cohn, L.W.; Zacharowski, K. Point of Care Testing: A Prospective, Randomized Clinical Trial of Efficacy in Coagulopathic Cardiac Surgery Patients. Surv. Anesthesiol. 2013, 57, 167. [Google Scholar] [CrossRef]
- Kuiper, G.J.A.J.M.; van Egmond, L.T.; Henskens, Y.M.C.; Roekaerts, P.M.; Maessen, J.G.; ten Cate, H.; Buhre, W.F.; Lancé, M.D. Shifts of Transfusion Demand in Cardiac Surgery After Implementation of Rotational Thromboelastometry–Guided Transfusion Protocols: Analysis of the HEROES-CS (HEmostasis Registry of PatiEntS in Cardiac Surgery) Observational, Prospective Open Cohort Database. J. Cardiothorac. Vasc. Anesth. 2019, 33, 307–317. [Google Scholar] [CrossRef] [Green Version]
- Sarrais Polo, C.; Alonso Morenza, A.; Rey Picazo, J.; Álvarez Mercadal, L.; Beltrao Sial, R.; Aguilar Lloret, C. Thromboelastometry as Guidance for Blood Management in Patients Undergoing Cardiac Surgery. Rev. Española Anestesiol. Reanim. 2018, 65, 129–134. [Google Scholar] [CrossRef]
- Waldén, K.; Jeppsson, A.; Nasic, S.; Backlund, E.; Karlsson, M. Low Preoperative Fibrinogen Plasma Concentration Is Associated With Excessive Bleeding After Cardiac Operations. Ann. Thorac. Surg. 2014, 97, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Rafiq, S.; Johansson, P.I.; Kofoed, K.F.; Olsen, P.S.; Steinbrüchel, D.A. Preoperative Hemostatic Testing and the Risk of Postoperative Bleeding in Coronary Artery Bypass Surgery Patients. J. Card. Surg. 2016, 31, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Gielen, C.; Dekkers, O.; Stijnen, T.; Schoones, J.; Brand, A.; Klautz, R.; Eikenboom, J. The Effects of Pre- and Postoperative Fibrinogen Levels on Blood Loss after Cardiac Surgery: A Systematic Review and Meta-Analysis. Interact. Cardiovasc. Thorac. Surg. 2014, 18, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Charbonneau, H.; Pasquie, M.; Mayeur, N. Preoperative Plasma Fibrinogen Level and Transfusion in Cardiac Surgery: A Biphasic Correlation. Interact. Cardiovasc. Thorac. Surg. 2020, 31, 622–625. [Google Scholar] [CrossRef] [PubMed]
- Mazur, P.; Natorska, J.; Sobczyk, D.; Gawęda, B.; Bartuś, K.; Filip, G.; Kapelak, B.; Undas, A. Plasma Fibrin Clot Properties Affect Blood Loss after Surgical Aortic Valve Replacement for Aortic Stenosis. Eur. J. Cardio-Thorac. Surg. 2019, 55, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.J.; Lei, W.H.; Hu, P.; Wu, B.B.; Chen, J.X.; Ni, Y.M.; Lai, E.Y.; Han, F.; Chen, J.H.; Yang, Y. Preoperative Serum Fibrinogen Is Associated With Acute Kidney Injury after Cardiac Valve Replacement Surgery. Sci. Rep. 2020, 10, 6403. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Vuylsteke, A.; Gerrard, C.; Besser, M.; Baglin, T. Postoperative Fibrinogen Level Is Associated with Postoperative Bleeding Following Cardiothoracic Surgery and the Effect of Fibrinogen Replacement Therapy Remains Uncertain. J. Thromb. Haemost. 2013, 11, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, S.; Suzuki, Y.; Sato, T.; Kikura, M.; Katoh, T.; Sato, S. Four-Group Classification Based on Fibrinogen Level and Fibrin Polymerization Associated With Postoperative Bleeding in Cardiac Surgery. Clin. Appl. Thromb. Hemost. 2016, 22, 648–655. [Google Scholar] [CrossRef]
- Karkouti, K.; Callum, J.; Crowther, M.A.; McCluskey, S.A.; Pendergrast, J.; Tait, G.; Yau, T.M.; Beattie, W.S. The Relationship Between Fibrinogen Levels After Cardiopulmonary Bypass and Large Volume Red Cell Transfusion in Cardiac Surgery: An Observational Study. Anesth. Analg. 2013, 117, 14–22. [Google Scholar] [CrossRef]
- Ranucci, M.; Pistuddi, V.; Baryshnikova, E.; Colella, D.; Bianchi, P. Fibrinogen Levels After Cardiac Surgical Procedures: Association With Postoperative Bleeding, Trigger Values, and Target Values. Ann. Thorac. Surg. 2016, 102, 78–85. [Google Scholar] [CrossRef] [Green Version]
- Ranucci, M.; Jeppsson, A.; Baryshnikova, E. Pre-Operative Fibrinogen Supplementation in Cardiac Surgery Patients: An Evaluation of Different Trigger Values: Pre-Operative Fibrinogen Supplementation. Acta Anaesthesiol. Scand. 2015, 59, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Shim, J.-K.; Song, J.W.; Kim, E.-K.; Kwak, Y.-L. Impact of Preoperative Fibrinogen Concentration on Postoperative Outcome in Patients Who Received Dual Antiplatelet Therapy in Proximity to Off-Pump Coronary Bypass Surgery. Circ. J. 2014, 78, 1661–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranucci, M.; Baryshnikova, E.; Crapelli, G.B.; Rahe-Meyer, N.; Menicanti, L.; Frigiola, A.; the Surgical Clinical Outcome REsearch (SCORE) Group. Randomized, Double-Blinded, Placebo-Controlled Trial of Fibrinogen Concentrate Supplementation After Complex Cardiac Surgery. JAHA 2015, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Zhang, W.; Chen, N.; Wang, L.; Wang, S.; Yu, Y.; Ao, H. Can Preoperative C-Reactive Protein Predict Bleeding After On-Pump Coronary Artery Bypass Grafting? Ann. Thorac. Surg. 2020, 109, 541–546. [Google Scholar] [CrossRef]
- Theusinger, O.M.; Baulig, W.; Seifert, B.; Emmert, M.Y.; Spahn, D.R.; Asmis, L.M. Relative Concentrations of Haemostatic Factors and Cytokines in Solvent/Detergent-Treated and Fresh-Frozen Plasma. Br. J. Anaesth. 2011, 106, 505–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, J.H.; Szlam, F.; Tanaka, K.A.; Sniecienski, R.M. Fibrinogen and Hemostasis: A Primary Hemostatic Target for the Management of Acquired Bleeding. Anesth. Analg. 2012, 114, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Stanworth, S.; Baglin, T. Cryoprecipitate: An Outmoded Treatment?: Cryoprecipitate: An Outmoded Treatment? Transfus. Med. 2012, 22, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, S.M.; Kim, C.S.; Cho, H.S.; Lee, J.-H.; Lee, C.H.; Kim, E.; Sung, K.; Solomon, C.; Kang, J.; et al. Fibrinogen Recovery and Changes in Fibrin-Based Clot Firmness after Cryoprecipitate Administration in Patients Undergoing Aortic Surgery Involving Deep Hypothermic Circulatory Arrest: Fibrinogen Recovery with Cryoprecipitate. Transfusion 2014, 54, 1379–1387. [Google Scholar] [CrossRef]
- Callum, J.; Farkouh, M.E.; Scales, D.C.; Heddle, N.M.; Crowther, M.; Rao, V.; Hucke, H.-P.; Carroll, J.; Grewal, D.; Brar, S.; et al. Effect of Fibrinogen Concentrate vs Cryoprecipitate on Blood Component Transfusion After Cardiac Surgery: The FIBRES Randomized Clinical Trial. JAMA 2019, 322, 1966. [Google Scholar] [CrossRef]
- Waldén, K.; Jeppsson, A.; Nasic, S.; Karlsson, M. Fibrinogen Concentrate to Cardiac Surgery Patients with Ongoing Bleeding Does Not Increase the Risk of Thromboembolic Complications or Death. Thromb. Haemost. 2020, 120, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Fassl, J.; Lurati Buse, G.; Filipovic, M.; Reuthebuch, O.; Hampl, K.; Seeberger, M.D.; Bolliger, D. Perioperative Administration of Fibrinogen Does Not Increase Adverse Cardiac and Thromboembolic Events after Cardiac Surgery. Br. J. Anaesth. 2015, 114, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colin-Bracamontes, I.; Pérez-Calatayud, Á.A.; Carrillo-Esper, R.; Rodríguez-Ayala, E.; Padilla-Molina, M.; Posadas-Nava, A.; Olvera-Vázquez, S.; Hernández-Salgado, L. Observational Safety Study of Clottafact® Fibrinogen Concentrate: Real-World Data in Mexico. Clin. Drug Investig. 2020, 40, 485–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, T.; Miyata, S.; Usui, A.; Nishiwaki, K.; Tanaka, H.; Okita, Y.; Katori, N.; Shimizu, H.; Sasaki, H.; Ohnishi, Y.; et al. Safety of Fibrinogen Concentrate and Cryoprecipitate in Cardiovascular Surgery: Multicenter Database Study. J. Cardiothorac. Vasc. Anesth. 2019, 33, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Jeppsson, A.; Waldén, K.; Roman-Emanuel, C.; Thimour-Bergström, L.; Karlsson, M. Preoperative Supplementation with Fibrinogen Concentrate in Cardiac Surgery: A Randomized Controlled Study. Br. J. Anaesth. 2016, 116, 208–214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadeghi, M.; Atefyekta, R.; Azimaraghi, O.; Marashi, S.M.; Aghajani, Y.; Ghadimi, F.; Spahn, D.R.; Movafegh, A. A Randomized, Double Blind Trial of Prophylactic Fibrinogen to Reduce Bleeding in Cardiac Surgery. Braz. J. Anesthesiol. 2014, 64, 253–257. [Google Scholar] [CrossRef] [Green Version]
- Rahe-Meyer, N.; Levy, J.H.; Mazer, C.D.; Schramko, A.; Klein, A.A.; Brat, R.; Okita, Y.; Ueda, Y.; Schmidt, D.S.; Ranganath, R.; et al. Randomized Evaluation of Fibrinogen vs Placebo in Complex Cardiovascular Surgery (REPLACE): A Double-Blind Phase III Study of Haemostatic Therapy. Br. J. Anaesth. 2016, 117, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Bilecen, S.; Peelen, L.M.; Kalkman, C.J.; Spanjersberg, A.J.; Moons, K.G.M.; Nierich, A.P. Fibrinogen Concentrate Therapy in Complex Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2013, 27, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, M.; Ternström, L.; Hyllner, M.; Baghaei, F.; Skrtic, S.; Jeppsson, A. Prophylactic Fibrinogen Infusion in Cardiac Surgery Patients: Effects on Biomarkers of Coagulation, Fibrinolysis, and Platelet Function. Clin. Appl. Thromb. Hemost. 2011, 17, 396–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahe-Meyer, N.; Solomon, C.; Hanke, A.; Schmidt, D.S.; Knoerzer, D.; Hochleitner, G.; Sørensen, B.; Hagl, C.; Pichlmaier, M. Effects of Fibrinogen Concentrate as First-Line Therapy during Major Aortic Replacement Surgery: A Randomized, Placebo-Controlled Trial. Anesthesiology 2013, 118, 40–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupu, I.-M.; Rebaine, Z.; Lhotel, L.; Watremez, C.; Eeckhoudt, S.; Van Dyck, M.; Momeni, M. A Low-Dose Human Fibrinogen Is Not Effective in Decreasing Postoperative Bleeding and Transfusion Requirements during Cardiac Surgery in Case of Concomitant Clinical Bleeding and Low FIBTEM Values: A Retrospective Matched Study. Ann. Card. Anaesth. 2018, 21, 262. [Google Scholar] [CrossRef] [PubMed]
- Kozek-Langenecker, S.A.; Afshari, A.; Albaladejo, P.; Santullano, C.A.A.; De Robertis, E.; Filipescu, D.C.; Fries, D.; Görlinger, K.; Haas, T.; Imberger, G.; et al. Management of Severe Perioperative Bleeding: Guidelines from the European Society of Anaesthesiology. Eur. J. Anaesthesiol. EJA 2013, 30, 270–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wikkelsø, A.; Lunde, J.; Johansen, M.; Stensballe, J.; Wetterslev, J.; Møller, A.M.; Afshari, A. Fibrinogen Concentrate in Bleeding Patients. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [Green Version]
- Rahe-Meyer, N.; Levy, J.H.; Mazer, C.D.; Schramko, A.; Klein, A.A.; Brat, R.; Okita, Y.; Ueda, Y.; Schmidt, D.S.; Gill, R. Randomized Evaluation of Fibrinogen versus Placebo in Complex Cardiovascular Surgery: Post Hoc Analysis and Interpretation of Phase III Results. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 566–574. [Google Scholar] [CrossRef]
- Li, J.-Y.; Gong, J.; Zhu, F.; Moodie, J.; Newitt, A.; Uruthiramoorthy, L.; Cheng, D.; Martin, J. Fibrinogen Concentrate in Cardiovascular Surgery: A Meta-Analysis of Randomized Controlled Trials. Anesth. Analg. 2018, 127, 612–621. [Google Scholar] [CrossRef]
- Ranucci, M.; Baryshnikova, E.; Ranucci, M.; Silvetti, S. Fibrinogen Levels Compensation of Thrombocytopenia-Induced Bleeding Following Cardiac Surgery. Int. J. Cardiol. 2017, 249, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Haensig, M.; Kempfert, J.; Kempfert, P.-M.; Girdauskas, E.; Borger, M.A.; Lehmann, S. Thrombelastometry Guided Blood-Component Therapy after Cardiac Surgery: A Randomized Study. BMC Anesthesiol. 2019, 19, 201. [Google Scholar] [CrossRef] [Green Version]
- Scolletta, S.; Simioni, P.; Campagnolo, V.; Celiento, M.; Fontanari, P.; Guadagnucci, A.; Guarracino, F.; Haxhiademi, D.; Paniccia, R.; Simeone, F.; et al. Patient Blood Management in Cardiac Surgery: The “Granducato Algorithm”. Int. J. Cardiol. 2019, 289, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Pajares, A.; Larrea, L.; Zarragoikoetexea, I.; Tur, A.; Vicente, R.; Argente, P. Patient Blood Management in Cardiac Surgery: Results. Rev. Española Anestesiol. Reanim. 2018, 65, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, M.; Baryshnikova, E.; Pistuddi, V.; Menicanti, L.; Frigiola, A. The Effectiveness of 10 Years of Interventions to Control Postoperative Bleeding in Adult Cardiac Surgery. Interact. Cardiovasc. Thorac. Surg. 2016, 24, 196–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birla, R.; Nawaytou, O.; Shaw, M.; Jackson, A.; Mills, K.; Kuduvalli, M.; Field, M.; Agarwal, S. Reducing Blood Transfusion in Aortic Surgery: A Novel Approach. Ann. Thorac. Surg. 2019, 108, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, D.; Zavatti, L.; Gabbieri, D.; Pedulli, M.; Giordano, G.; Meli, M. Point-of-Care-Based Protocol with First-Line Therapy with Coagulation Factor Concentrates Is Associated with Decrease Allogenic Blood Transfusion and Costs in Cardiovascular Surgery: An Italian Single-Center Experience. Minerva Anestesiol. 2016, 82, 12. [Google Scholar]

| Author (Year) | Type of Study | Type of Surgery | Number of Patients | Time Point of Fibrinogen Determination | Method | Fibrinogen Concentration | Associated Outcome |
|---|---|---|---|---|---|---|---|
| Waldén (2015) [106] | Prospective observational | CABG ± valve, valve, adult congenital, arrhythmia operations | 1954 | Preoperative | Clauss | <2.5 g/L | Excessive bleeding >1000 mL/12 h |
| Rafiq (2016) [107] | Prospective, observational | Elective, urgent CABG | 170 | Preoperative | Clauss | <2.5 g/L | Excessive bleeding >1000 mL/18 h |
| Fröjd (2016) [25] | Retrospective, observational | Cardiac surgery with CPB | 5345 | Preoperative | Clauss | Low | Re-exploration for bleeding |
| High | Mortality | ||||||
| Gielen (2014) [108] | Review and meta-analysis | Mixed cardiac surgery procedures | 5972 | Preoperative; Postoperative | Clauss, Clauss-like or not reported | Low | Blood loos |
| Kindo (2014) [79] | Prospective, observational | CABG ± valve, valve ± thoracic aorta surgery | 1956 | Preoperative; Postoperative (on admission to the ICU) | Clauss | Postoperative 2.1 ± 0.8; Cut-off 2.2 g/L | Excessive bleeding |
| Charbonneau (2020) [109] | Retrospective, observational | Elective cardiac surgery | 476 | Preoperative | Clauss | <1.5 g/L; >3 g/L; Fibrinogen gradient > 1.1 g/L | RBC transfusion |
| Mazur (2019) [110] | Prospective, observational | Aortic valve replacement, Bentall procedure | 77 | Preoperative | Clauss | 2.4 g/L (1.88–2.61) | Postoperative blood loss ≥600 mL after 12 h |
| Clot lysis time | 91 min (69–102) | ||||||
| Yang (2020) [111] | Retrospective, observational | Valve replacement surgery ± others | 3459 | Preoperative | Clauss | High | Acute kidney injury |
| Essa (2018) [27] | Retrospective, observational | Elective, urgent, emergency, CABG ± valve, valve, heart transplantations | 2403 | Preoperative; Postoperative (on admission to the ICU) | Clauss | Preoperative 2.8 ± 0.9; Postoperative 2.0 ± 0.6 | Bleeding, re-exploration |
| Pillai (2014) [9] | Prospective, observational | CABG ± valve, valve | 250 | Postoperative (post CPB) | Clauss | 2.95 ± 1.1 | Blood loss/24 h, blood transfusions |
| Yang (2013) [112] | Retrospective, observational | Cardiothoracic surgery | 391 | Postoperative (on admission to the ICU) | Clauss | Low | Chest drain blood loss within 1 h of measurement ≥ 3 mL/kg/h |
| Kawashima (2016) [113] | Retrospective | Cardiac surgery procedures | 215 | Postoperative (at the rewarming of CPB) | Clauss FIBTEM A10 | <1.5 g/L and FIBTEM A10 < 6 mm; ≥1.5 g/L and FIBTEM A10 < 6 mm | Chest tube drainage within the first 24 h |
| Kindo (2014) [79] | Prospective, observational | CABG ± valve, valve ± thoracic aorta | 1956 | Postoperative (on admission to the ICU) | Clauss | 2.1 ± 0.8 Cut-off 2.2 g/L | Chest tube drainage within the first 24 h |
| Karkouti (2013) [114] | Retrospective observational | Cardiac surgery procedures | 4606 | Postoperative (post CPB) | Clauss | <2.0 g/L | Transfusion volume |
| Ranucci (2016) [115] | Retrospective, observational | Cardiac surgery procedures | 2800 | Postoperative (on admission to the ICU) | Clauss | <2.2 g/L | Chest drain blood loss > 1000 mL/12 h |
| Author (Year) | Type of Study | Type of Surgery | Number of Patients | Timing of Intervention | Dose of Fibrinogen/Control Drug ± Target Dose | Associated Outcome (FC Group) |
|---|---|---|---|---|---|---|
| Karlsson (2011) [133] | Prospective, randomised | Elective CABG and preoperative fibrinogen level ≤ 3.8 g/L | FC (n = 10); Control (n = 10) | Preoperative | 2 g | No or minimal changes in biomarkers reflecting coagulation, fibrinolysis, and platelet function |
| Sadeghi (2014) [130] | Prospective, randomised, double-blind | Elective CABG | FC (n = 30); Placebo (n = 30) | Preoperative | 1 g | Less postoperative bleeding |
| Jeppsson (2015) [129] | Prospective, randomised, double-blind | Elective low risk CABG | FC (n = 24); Placebo (n = 24) | Preoperative if plasma fibrinogen concentration ≤ 3.8 g/L | 2 g | No significant difference in blood loss, number of transfusions of blood products, proportion of transfused subjects; Higher postoperative fibrinogen |
| Rahe-Meyer (2013) [134] | Prospective, randomised, double-blind | Elective thoracic or thoracoabdominal aortic replacement surgery | FC (n = 29); Placebo (n = 32) | After removal from CPB and surgical haemostasis when 5 min bleeding mass is 60–250 g | 8 g (6–9) g; Target FIBTEM MCF 22 mm | Less transfusion of blood products; Total avoidance of transfusion; Higher fibrinogen at the end of surgery |
| Rahe-Meyer (2016) [131] (REPLACE study) | Multinational, multicentre, prospective, randomised, double-blind | Elective aortic surgery | FC (n = 7); Placebo (n = 74) | After removal from CPB and surgical haemostasis when 5 min bleeding mass is 60–250 g | 8 g (6–9) g; Target FIBTEM MCF 22 mm | More blood product transfusion; Fewer patients avoided transfusion; Increased plasma fibrinogen concentration and fibrin-based clot strength |
| Ranucci (2015) [118] (ZEPLAST study) | Prospective, randomised, double-blind | Cardiac surgery with CPB > 90 min and one of: age > 65 years, non-elective surgery, serum creatinine level > 1.36 mg/dl, redo surgery | FC (n = 58); Placebo (n = 58) | After protamine administration | 4 g (3–6) g; Target FIBTEM MCF 22 mm | Lower rate of blood products transfusion; Less postoperative bleeding |
| Bilecen (2017) [82] | Prospective, randomised, double-blind | Elective high-risk cardiac surgery | FC (n = 60); Placebo (n = 60) | After CPB when 5 min intraoperative blood volume is 60–250 ml | Mean dose 3.1 g/L; Target Clauss fibrinogen 2.5 g/L | No difference in intraoperative blood loss; No difference in blood transfusions |
| Kwapisz (2020) [10] | Prospective, randomised, double-blind | Elective high-risk cardiac surgery and preoperative fibrinogen ≤ 3.8 g/L | FC (n = 31); Placebo (n = 31) | After heparin reversal | Mean dose 3.2 g/L; Target FIBTEM MCF ≥ 15 mm | Less cumulative blood product units transfusion but not statistically significant; No difference in number of transfused patients; A trend toward less blood drainage; Higher FIBTEM MCF; Higher fibrinogen plasma levels at the end of surgery |
| Bilecen (2013) [132] | Prospective, nonrandomised | Complex cardiac surgery | FC (n = 264); Conventional therapy (n = 811) | During surgery, in case the initial haemostatic management was not effective, and a surgical source of bleeding was excluded | 2 g (2 to 3 g) | No difference in postoperative blood loss and transfusion of blood products |
| Yang (2013) [112] | Retrospective, observational | Cardiac surgery procedures | FC (n = 8); Cryoprecipitate (n = 76) | ICU within 12 h of completion of surgery | FC 46.28 ± 33.64 mg/kg; Cryoprecipitate 4.76 ± 2.94 mL/kg | Not significantly reduced bleeding rate after FC or cryoprecipitate infusion |
| Tanaka (2014) [53] | Prospective, randomised, open-label | Elective cardiac surgery with CPB | FC (n = 10); Platelets (n = 10) | After CPB if bleeding is moderate (controllable with applied pressure) or severe (multiple diffuse bleeding sites) | FC 4 g; 1 unit of platelets | Fewer platelet and cryoprecipitate transfusions; Higher FIBTEM MCF |
| Hanna 2016 [6] | Prospective, pilot, off-label | High-risk proximal aortic reconstruction with DHCA | FC (n = 22); Propensity-matched cohort (n = 22) | After separation from CPB | 70 mg/kg | Rapidly raised fibrinogen levels; Reduced transfusion requirement |
| Lupu (2018) [135] | Retrospective | Elective or emergency cardiac surgery | FC (n = 73); Propensity-matched cohort (n = 73) | If bleeding persisted after protamine administration and FIBTEM MCF < 6 mm, not first-line therapy | 1 g | Higher bleeding and more transfusion of blood products |
| Hakimi (2019) [91] | Prospective, observational | Cardiac surgery procedures | FC (n = 16); Platelet concentrate (n = 12); FC and platelet concentrate (n = 14) | Ongoing bleeding after surgery and FIBTEM MCF ≤ 10 mm | FC 2 (1–3) g; Platelet concentrate 2 (1–3) units; FC 2 (1–4) g and platelet concentrate 2 (1–3) units | Higher FIBTEM MCF; Lower bleeding volume after FC or FC and platelet concentrate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikić, V. Fibrinogen and Bleeding in Adult Cardiac Surgery: A Review of the Literature. Surgeries 2021, 2, 409-436. https://doi.org/10.3390/surgeries2040041
Ikić V. Fibrinogen and Bleeding in Adult Cardiac Surgery: A Review of the Literature. Surgeries. 2021; 2(4):409-436. https://doi.org/10.3390/surgeries2040041
Chicago/Turabian StyleIkić, Višnja. 2021. "Fibrinogen and Bleeding in Adult Cardiac Surgery: A Review of the Literature" Surgeries 2, no. 4: 409-436. https://doi.org/10.3390/surgeries2040041






