Confluent Small Bowel Lipomatosis: A Rare Cause of Recurrent Abdominal Pain
Abstract
:1. Introduction
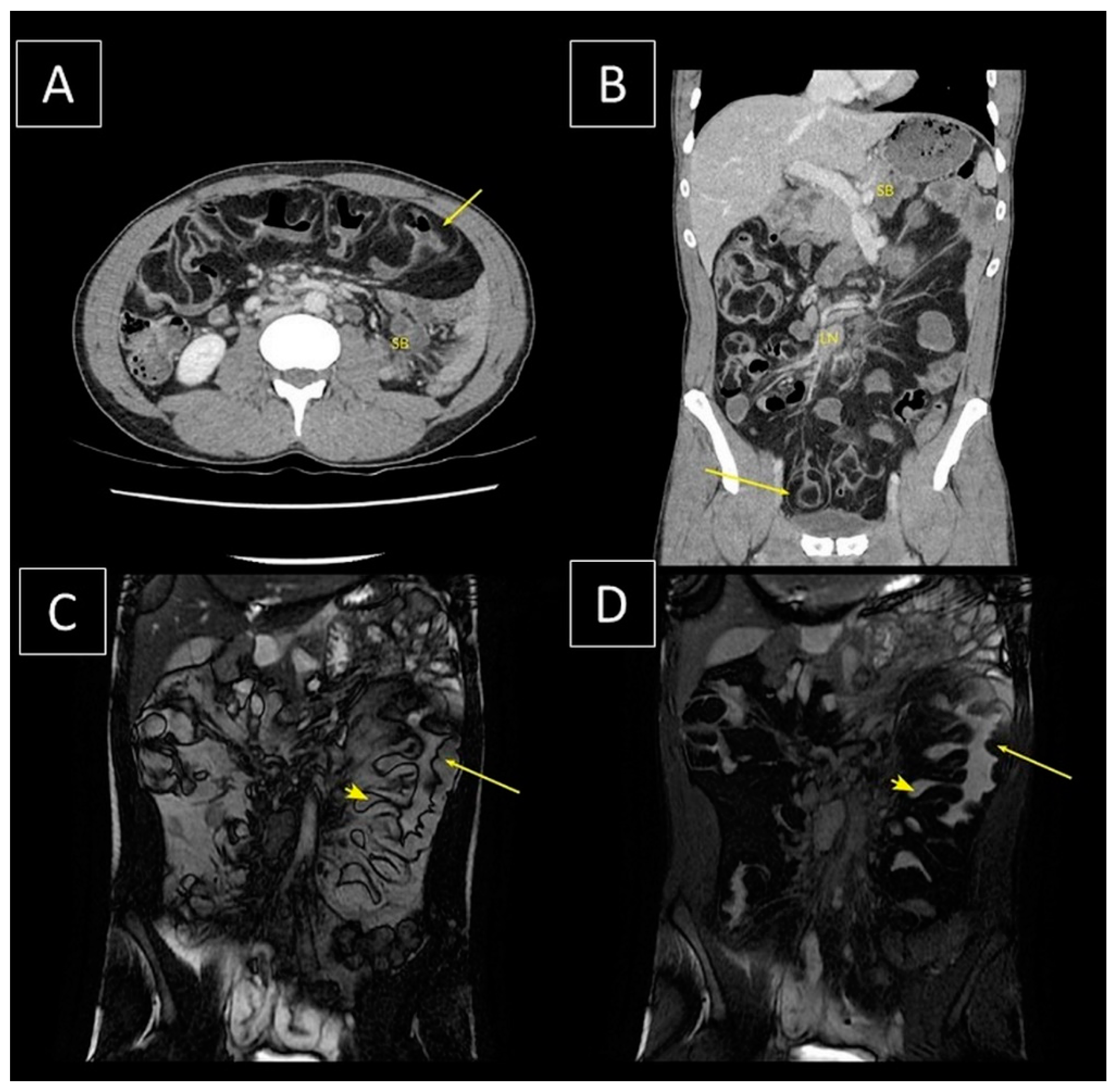
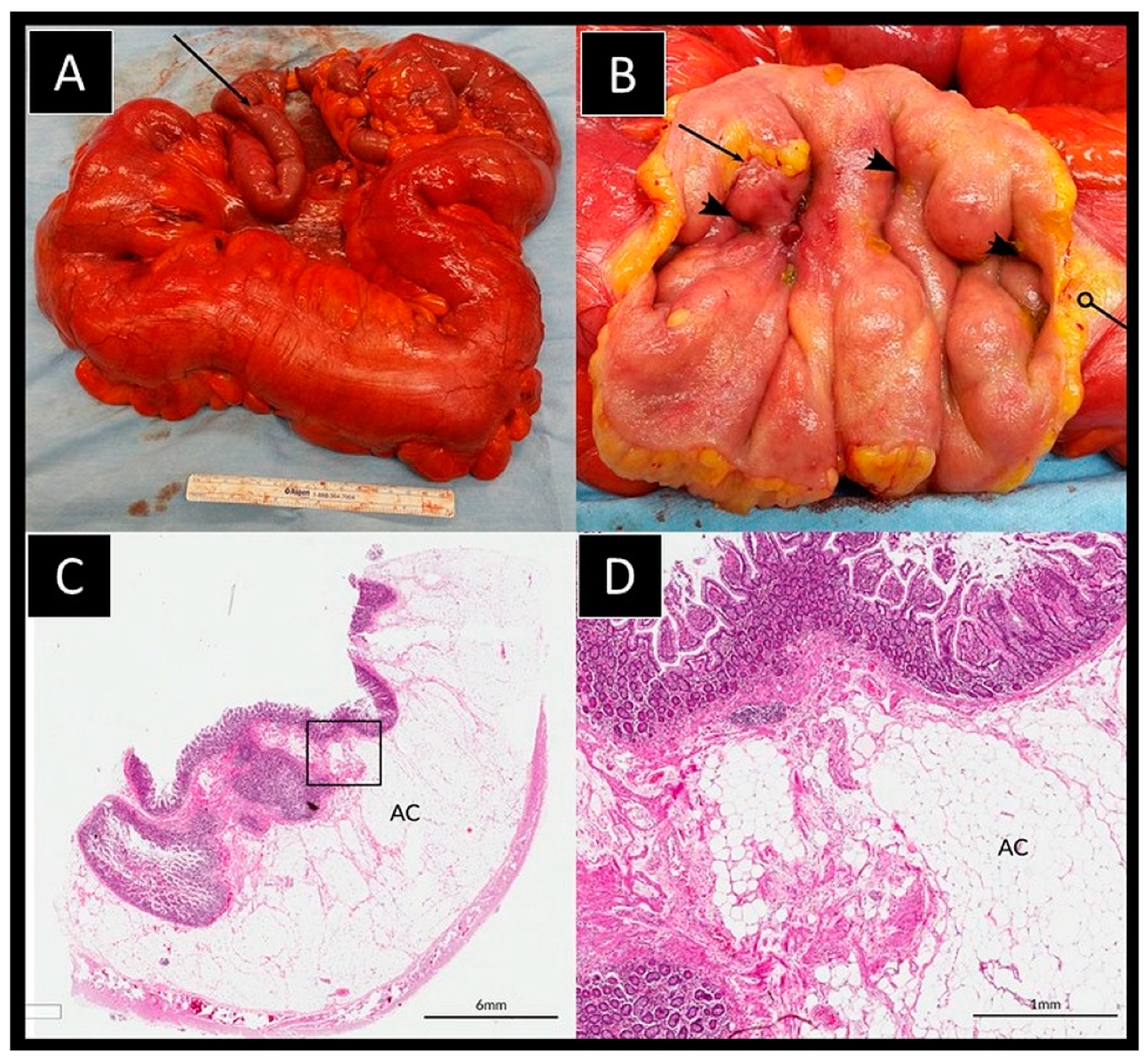
2. Case Details
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akagi, I.; Miyashita, M.; Hashimoto, M.; Makino, H.; Nomura, T.; Tajiri, T. Adult Intussusception Caused by an Intestinal Lipoma: Report of a Case. J. Nippon. Med. Sch. 2008, 75, 166–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, Y.; Priesack, W.; Sotje, G.; Brix, F.; Scheunemann, C. Hemorrhagic jejunal lipoma with intermittent intussusception. Eur. J. Radiol. 1996, 22, 123–125. [Google Scholar] [CrossRef]
- Eyselbergs, M.; Ceulemans, L.J.; De Bontridder, S.; Vanhoenacker, F.; Van Overbeke, L.; Quanten, I.; Jacomen, G.; Snoeckx, A. Ileocolic intussusception due to lipomatosis of the ileum: A common complication of a rare clinical entity. JBR-BTR 2014, 97, 36–38. [Google Scholar] [CrossRef] [Green Version]
- Gao, P.-J.; Chen, L.; Wang, F.-S.; Zhu, J.-Y. Ileo-colonic intussesception secondary to small-bowel lipomatosis: A case report. World J. Gastroenterol. 2014, 20, 2117–2119. [Google Scholar] [CrossRef]
- Lucas, L.C.; Fass, R.; Krouse, R.S. Laparoscopic Resection of a Small Bowel Lipoma with Incidental Intussusception. J. Soc. Laparoendosc. Surg. 2010, 14, 615–618. [Google Scholar] [CrossRef] [Green Version]
- McKay, R. Ileocecal Intussusception in an Adult: The Laparoscopic Approach. J. Soc. Laparosc. Surg. 2006, 10, 250–253. [Google Scholar]
- Moues, C.M.; Steenvoorde, P.; Viersma, J.H.; van Groningen, K.; de Bruine, J.F. Jejunal Intussusception of a Gastric Lipoma. Dig. Surg. 2002, 19, 418–420. [Google Scholar] [CrossRef]
- Rathore, M.A.; Andrabi, S.I.H.; Mansha, M. Adult Intussusception—A Surgical Dilemma. J. Ayub Med. Coll. Abbottabad 2006, 18, 18. [Google Scholar]
- Urbano, J.; Serantes, A.; Hernandez, L.; Turegano, F. Lipoma-induced jejunojejunal intussusception: US and CT diagnosis. Abdom. Imaging 1996, 21, 522–524. [Google Scholar] [CrossRef]
- Zissin, R. Enteroenteric intussusception secondary to a lipoma: CT diagnosis. Emerg. Radiol. 2004, 11, 107–109. [Google Scholar] [CrossRef]
- Young, T.-H.; Ho, P.; Tang, H.-S.; Hsu, C.-T.; Chao, Y.-C. A Rare Cause of Multiple Intussusceptions: Intense Segmentary Lipomatosis of The Ileum. Am. J. Gastroenterol. 1996, 91, 162–163. [Google Scholar] [PubMed]
- Tatsuguchi, A.; Fukuda, Y.; Moriyama, T.; Yamanaka, N. Lipomatosis Of The Small Intestine And Colon Associated With Intussusception In tTe Ileocecal Region. Gastrointest. Endosc. 1999, 49, 118–121. [Google Scholar] [CrossRef]
- Karthikeyan, V.S.; Dhanesekar, P.; Sistla, S.C.; Ali, M.S.; Balasubramaniam, G.; Rajkumar, N. Jejuno-jejunal Intussusception Secondary to Small-Bowel Lipomatosis: A Case Report. S. Afr. J. Sci. 2012, 50, 43–44. [Google Scholar]
- Lill, M.; Berkeley, B.; Cooper, G. Multiple Lipomatosis—A Rare Cause For Small Bowel Intussusception. N. Z. Med. J. 2007, 120. [Google Scholar]
- Suarez-Moreno, R.M.; Hernandez-Ramirez, D.A.; Madrazo-Navarro, M.; Salazal-Lozano, C.R.; Martinez-Gen, R. Multiple intestinal lipomatosis. Case Rep. Cir. Cir. 2010, 78, 163–165. [Google Scholar]
- Fang, S.-H.; Dong, D.-J.; Chen, F.-H.; Jin, M.; Zhong, B.-S. Small intestinal lipomas: Diagnostic value of multi-slice CT enterography. World J. Gastroenterol. 2010, 16, 2677–2681. [Google Scholar] [CrossRef]
- Weinberg, T.; Feldman, M., Sr. Lipomas of the gastrointestinal tract. Am. J. Clin. Pathol. 1955, 25, 272–281. [Google Scholar] [CrossRef]
- Kaczynski, J.; Hilton, J. Giant lipoma of the small bowel associated with peroforated ileal diverticulum. BMJ Case Rep. 2012, 2012, bcr1220115299. [Google Scholar] [CrossRef] [Green Version]
- Pezzoli, A.; Pennazio, M.; Fusetti, N.; Simone, L.; Zelante, A.; Cifala, V.; Sprujevnik, T.; Carella, A.; Gullini, S. Occult intestinal haemorrhage due to lipoma of the small bowel detected with the combined use of the new endoscopic techniques. A report of two cases. Dig. Liver Dis. 2008, 40, 306–309. [Google Scholar] [CrossRef]
- Bilgic, Y.; Hasan, B.A.; Yildirium, N.; Alatas, O.; Kanat, B.H.; Sahin, A. Familial Abdominal and Intestinal Lipomatosis Presenting with Upper GI Bleeding. Case Rep. Gastrointest. Med. 2015, 2015, 123723. [Google Scholar] [CrossRef]
- Jung, H.-R.; Park, W.-H.; Choi, S.-O.; Kwon, K.-Y.; Lee, S.-S. Intestinal chondrolipoma: Uncommon cause of bowel obstruction. J. Pediatr. Sugery 2007, 42, E21–E23. [Google Scholar] [CrossRef] [PubMed]
- Kenkare, S.; Ainapurapu, B. Macrodactylia Fibrolipomatosis Presenting as a Small Bowel Obstruction. South. Med. J. 2010, 103, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Balmadrid, B.; Gluck, M. Chronic iron deficiancy anemia caused by small-bowel lipoma. Gastrointest. Endosc. 2014, 79, 678–679. [Google Scholar] [CrossRef] [PubMed]
- Blakeborough, A.; McWilliams, R.G.; Raja, U.; Robinson, P.J.A.; Reynolds, J.V.; Chapman, A.H. Pseudolipoma of inverted Meckel’s diverticulum: Clinical, radiological and pathological correlation. Eur. Radiol. 1997, 7, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Kitagawa, A.; Oka, Y.; Nakao, K. Multiple Lipomas Of The Ileum With Volvulous. Arch. Surg. 1977, 112, 1144–1145. [Google Scholar] [CrossRef]
- Neilson, D.; Wilkinson, N.; Magell, J. Case of Simultaneous Diveticulosis, Lipomatosis and Volvulus of The Small Intestine. Br. J. Surg. 1990, 77, 105. [Google Scholar] [CrossRef]
- Lee, B.J.; Park, J.-J.; Joo, M.K.; Kim, J.H.; Yeon, J.E.; Kim, J.S.; Chun, H.J.; Byun, K.S.; Choi, J.H.; Kim, C.D.; et al. A Case of Small-Bowel Intussusception Caused by Intestinal Lipomatosis: Preoperative Diagnosis and Reduction of Intussusception with Double-Balloon Enterospcopy. Gastrointest. Endosc. 2010, 71, 1329–1332. [Google Scholar] [CrossRef]
- Komagata, T.; Takebayashi, S.; Hirasawa, K.; Fukawa, T.; Arai, M. Extensive Lipomatosis Of The Small Bowel And Mesentery: CT and MRI Findings. Radiat. Med. 2007, 25, 480–483. [Google Scholar] [CrossRef]
- Mayo, C.W.; Pagtalunan, R.J.G.; Brown, D.J. Lipoma of the Alimentay Tract. Surgery 1963, 53, 598–603. [Google Scholar]
- Cojocari, N.; David, L. Acute Intestinal Infarction Due to Diffuse Jejunoileal and Mesenteric Lipomatosis in a 39-Year-Old Woman. Am. J. Case Rep. 2020, 21, e922830. [Google Scholar] [CrossRef] [Green Version]
- Gates, L.K.J.; Keate, R.F.; Smalley, J.J.J.; Richardson, J. Macrodactylia Fibrolipomatosis Complicated by Multiple Small Bowel Lipomas and Intussusception. J. Clin. Gastroenterol. 1996, 23, 241–242. [Google Scholar] [CrossRef] [PubMed]
- Mazziotti, S.; Salamone, I.; Vinci, S.; Pandolfo, A. Macrodactylia Fibrolipomatosis Associated with Multiple small-Bowel Lipomas. Am. J. Roentgenol. 2006, 186, 1195–1196. [Google Scholar] [CrossRef] [PubMed]
- Grudzińska, E.; Mrowiec, S.; Pilch-Kowalczyk, J.; Ciupińska, M.; Kusnierz, K. Small Intestinal Intussusception Due to Complicated Giant Jejunal Diverticulosis. Medicina 2021, 57, 116. [Google Scholar] [CrossRef] [PubMed]
- Yakabe, S.; Muranaka, T.; Sumii, T.; Takeshita, M.; Yamashita, T.; Tsuruta, S.; Saku, M.; Yoshida, K. Jejunal lipomatosis with diverticulosis: Report of a case. Surg. Today 1998, 28, 846–849. [Google Scholar] [CrossRef]
- Amzallag-Bellenger, E.; Oudjit, A.; Ruiz, A.; Cadiot, G.; Soyer, P.A.; Hoeffel, C.C. Effectiveness of MR Enterography for the Assessment of Small-Bowel Diseases beyond Crohn Disease. RadioGraphics 2012, 32, 1423–1444. [Google Scholar] [CrossRef]
- Farkas, N.; Wong, J.; Bethel, J.; Monib, S.; Frampton, A.; Thomson, S. A systematic review of symptomatic small bowel lipomas of the jejunum and ileum. Ann. Med. Surg. 2020, 58, 52–67. [Google Scholar] [CrossRef]
- Pillai, R.K.; Surti, U.; Swerdlow, S.H. Follicular lymphoma-like B cells of uncertain significance (in situ follicular lymphoma) may infrequently progress, but precedes follicular lymphoma, is associated with other overt lymphomas and mimics follicular lymphoma in flow cytometric studies. Haematologica 2013, 98, 1571–1580. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanijaun, S.; Cheluvappa, R.; Selvendran, S.; Pang, T. Confluent Small Bowel Lipomatosis: A Rare Cause of Recurrent Abdominal Pain. Surgeries 2022, 3, 11-17. https://doi.org/10.3390/surgeries3010003
Khanijaun S, Cheluvappa R, Selvendran S, Pang T. Confluent Small Bowel Lipomatosis: A Rare Cause of Recurrent Abdominal Pain. Surgeries. 2022; 3(1):11-17. https://doi.org/10.3390/surgeries3010003
Chicago/Turabian StyleKhanijaun, Sukhwant, Rajkumar Cheluvappa, Selwyn Selvendran, and Tony Pang. 2022. "Confluent Small Bowel Lipomatosis: A Rare Cause of Recurrent Abdominal Pain" Surgeries 3, no. 1: 11-17. https://doi.org/10.3390/surgeries3010003
APA StyleKhanijaun, S., Cheluvappa, R., Selvendran, S., & Pang, T. (2022). Confluent Small Bowel Lipomatosis: A Rare Cause of Recurrent Abdominal Pain. Surgeries, 3(1), 11-17. https://doi.org/10.3390/surgeries3010003






