Abstract
Introduction: According to the high rate of patients requiring a Re-Do surgery after a primary Sleeve Gastrectomy, due to failure on weight loss, this study proposes a comparison between RYGB and OAGB as a secondary intervention for morbidly obese patients. Methods: A retrospective review of patients who underwent revisional surgery to convert SG to RYGB or OAGB at our institution from November 2011 to November 2019 was performed. Results: A subset of sixty-three patients with previous SG underwent revisional surgery due to failure of the primary intervention. The OAGB group (n = 17) had a mean BMI at the time of the sleeve of 62 kg/m2 and a mean BMI of 50.7 kg/m2, the length of the Omega was 139.35 cm. The RYGB (n = 46) group showed a mean BMI of 47 kg/m2 at the time of the sleeve and a BMI of 34.8 kg/m2 at the time of the revision. The RYGB was performed according to the 70/120 cm standard for all the patients. One patient also had a revision from secondary OAGB to RYGB due to persistent biliary reflux, in this case the biliary branch was settled at 150 cm and the alimentary at 50 cm. Conclusions: The outcomes in the OAGB group showed a 29%WL and a 47%EWL (out of a 17%WL and 28%EWL at the time of the sleeve), on the other side the RYGB group reached a 33%WL and 72%EWL (out of a 25%WL and a 54%EWL at the time of the sleeve). According to our data we assume that RYGB is more effective in terms of weight loss as a revisional surgery after sleeve
1. Introduction
In the time of pandemic, obesity has not stopped its chronic effects affecting a large portion of the world’s population and, even more, it was a major risk factor for those who developed serious complications after a COVID-19 infection [1,2,3]. In order to offer a safe, feasible and simple surgical solution, laparoscopic sleeve gastrectomy (LSG), originally a component of biliopancreatic diversion with duodenal switch (BPD-DS), became the most commonly performed procedure in bariatric surgery [4]. Despite the improvement occurring with LSG in terms of metabolic syndrome, long-term failure rates are up to 64% [5]. According to the American Society for Metabolic and Bariatric Surgery (ASMBS) preoperative surgery is intended to collect corrective, reversal and conversion procedures [6]; the latter is especially increasing all over the world due to insufficient weight loss and weight regain in the long term [7]. It is hard to define how weight regain should be described after LSG, and in the literature a large variety of definitions are offered. It is reasonable to consider an increase of at least 10 kg from nadir weight [8] as a criterion for a re-operation, but also other measures, like BMI or percent excess weight loss (EWL) could guide the choice, giving meaningful data supporting the weight loss failure. A proper distinction between two types of weight loss failure has been proposed by the International Bariatric Club [9]: insufficient weight loss, described as an EWL of <50% at 18 months and progressive weight regain, occurring after reaching a peak of EWL > 50%. Despite criteria and definition, a focus in the literature about proposed causes of weight regain after LSG includes initial sleeve size, sleeve dilation, increased ghrelin levels, inadequate follow-up support and maladaptive lifestyle behaviors [10]. Even with several predictors for weight regain after SG, the lack of a consistent, worldwide shared definition [11] implies that every bariatric center chooses his own strategy to manage those patients. Currently Roux-en-Y gastric bypass (RYGB) is recognized worldwide as the golden standard for revisional procedures, but it is not that easy, feasible and safe as LSG. Revisional RYGB has been described by current studies [12,13,14,15] as a harbinger of early complications such as leakage, bleeding and infection (occurring in 11.8–16.6% of surgeries and up to 8% of these cases need further intervention) and long-term complications like internal herniation (occurring in 3.3–4.9% of surgeries). However, the conversion of LSG to RYGB can be successfully performed laparoscopically with a low rate of conversion and reasonable complication profile, as described by Landreaneau et al. [16], in the same manner for complications from LSG or to enhance weight loss. Rutledge [17], looking for a viable alternative, proposed One Anastomosis Gastric Bypass (OAGB) with the purpose of reaching a shorter operation time, reversibility and technical simplicity. In comparison, as a primary procedure, OAGB is not as effective as RYGB, reporting 60.1% vs. 72.9% excess weight loss five years after surgery, respectively, whereas the complication rate is 3.2% vs. 1.8%, respectively [18,19,20]. Evidently the lower rate of complications and the simplicity of OAGB could drive the choice for revisional procedures, with a large support in the literature. [21,22,23,24,25,26]. The aim of this study was to suggest, according to our experience, which revisional procedure could be more effective for weight regain after LSG, taking into account that all of those revisional procedures were performed by certified and experienced bariatric surgeons in the Adipositas Zentrum Klinikum Stuttgart Bad Cannstatt.
2. Methods
A retrospective analysis of patients undergoing re-operative bariatric surgery after a primary sleeve gastrectomy was performed considering a time frame from January 2010 to December 2019. The patients included in our study had a primary sleeve gastrectomy and afterwards underwent revisional surgery (RYGB or OAGB). The indications for conversion were evaluated for weight loss failure/weight regain and other type of complications—reflux after sleeve, swallowing disturbances and the combinations of others.
Major exclusion criteria were:
- primary surgery not performed in our center
- patients receiving a primary LSG after other bariatric procedure (e.g., adjustable gastric band)
3. General Operative Approach
While there are unique technical aspects to every SG to RYGB conversion, our center has endeavored to standardize the evaluation and operation. Patients undergo upper endoscopy prior to being operated upon. All operations are laparoscopically performed and perioperative prophylactic antibiotics are administered. Optical entry trocar is utilized most often, positioned away from any prior surgical incisions to minimize the risk of inadvertent visceral injury. In most cases, abdominal adhesions are not severe and usually divided with an ultrasonic dissector. Despite the differences between patients who already went through an intervention by RYGP, we regularly perform the same steps. Following radiolysis, identification and preservation of the left gastric pedicle is the first critical step of the operation. The sleeve is typically divided just distal to the left gastric artery resulting in a pouch of 30–50 mL volume. The biliopancreatic limb is typically 70 cm, and the Roux limb is typically 150 cm, ante-gastric/ante-colic. The jejunojejunostomy is performed with a linear stapled technique, the mesenteric defects are closed. Regarding the One Anastomosis Gastric Bypass, after the identification of the lesser curvature of the stomach, a partial transection of the lesser sac is performed with a stapler. Using a 36 Fr bougie, the vertical transection in order to obtain the gastric pouch is achieved excluding the fundus. Thereafter, the small bowel is measured starting from the ligament of Treitz up to 150–180 cm, then the gastrojejunostomy is performed with a linear stapled technique. A blue methylene test is regularly performed for both procedures.
4. Calculations and Statistics
The purpose of this evaluation is only descriptive, since the choice of one operation instead of another is partly down to patient choice. Continuous variables are described in terms of the mean and standard deviation, or the median and interquartile range. Categorical variable data (Table 1) were used to calculate the change in BMI, percent total weight loss (%TWL) and percent excess weight loss (%EWL).

Table 1.
Short overview of the two populations.
The short overview of the two populations shows a major difference in numbers between groups and outcomes (Figure 1). This is firstly because of the historical reliability of RYGB and the younger age of OAGB, but most importantly the occurrence of gastroesophageal reflux after sleeve, which drives the use of RYGB (30). As shown in Table 2, Table 3 and Table 4, GERD is one of the most recurrent comorbidities in the RYGB group.
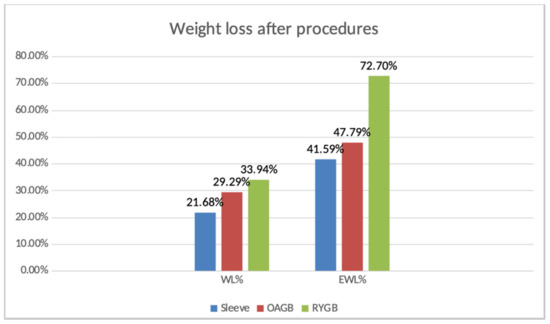
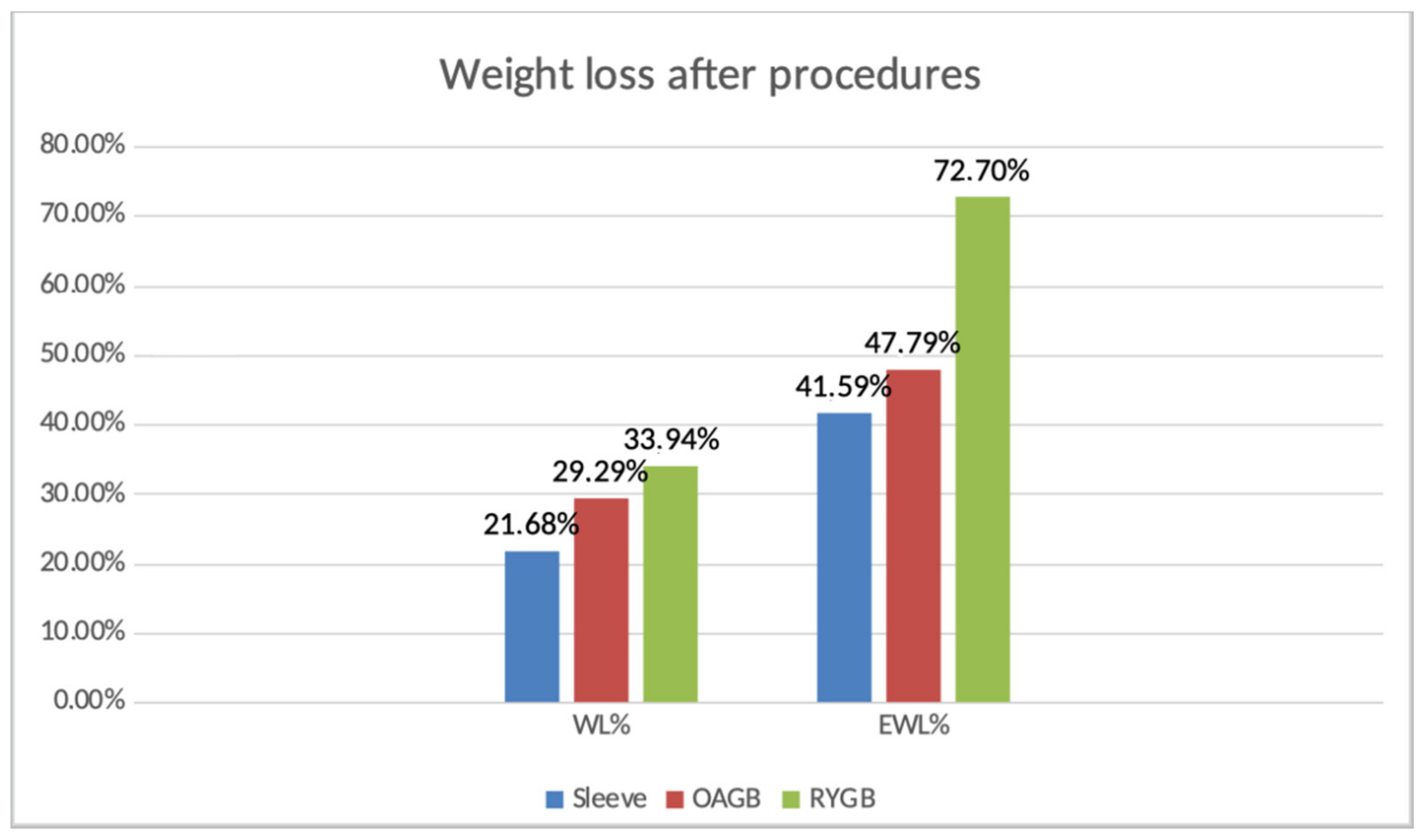
Figure 1.
Weight loss after procedures.

Table 2.
Percentage of comorbidities in the two examined groups.

Table 3.
Comparison of Delta BMI, %WL and %EWL before and after One Anastomosis Gastric Bypass.

Table 4.
Comparison of Delta BMI, %WL and %EWL before and after Roux-en-Y Gastric Bypass.
Another important difference favoring RYGB is the chance to use it as a “rescue” procedure. In fact, this group also includes patients who developed postoperative complications like stapler leaks and bleeding or long-term problems such as swallowing failure.
The OAGB group includes patients operated on (for revisional surgery) in a short time span of 3 years.
The larger number of cases in the RYGB group is due to a longer time span (5 years) and the inclusion of cases where the combination of failure of weight loss and general complaints after sleeve drove the patients to a new operation.
5. Results
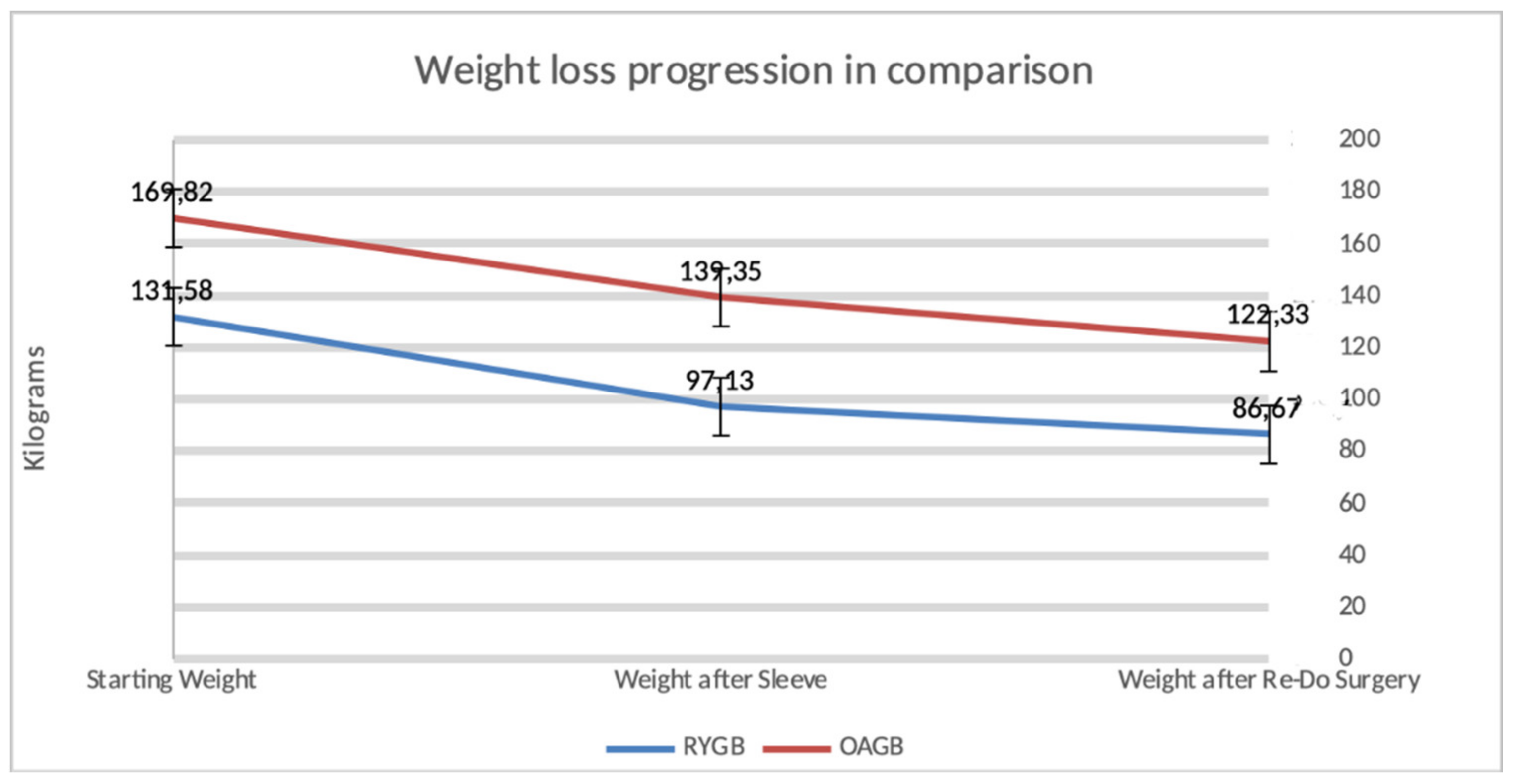
There were 63 patients who underwent conversion from sleeve to another bariatric solution. The OAGB group involved 17 patients, 76.4% of them were female with a mean age of 37.35 years. The BMI before sleeve was 62.05 kg/m2 and after a time span of 3 years and 10 months on average, the patients underwent One Anastomosis Gastric Bypass, reaching, after one year, a BMI of 50.72 kg/m2. The RYGB group consisted of 46 patients, with 93.47% being female. The mean age was 40.6 years. The average BMI before sleeve reached a mean of 47.23 kg/m2, which dropped to 34.88 kg/m2 in one year after RYGB.
Regarding a comparison between the two groups, the EWL was increased in RYGB group, 72% versus 47.7% for the OAGB group.
Figure 2 describes the weight loss progression after a secondary bariatric surgery.
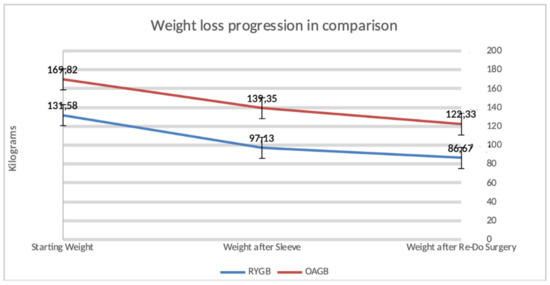
Figure 2.
Progression of weight loss occurring in our study population after a secondary bariatric procedure.
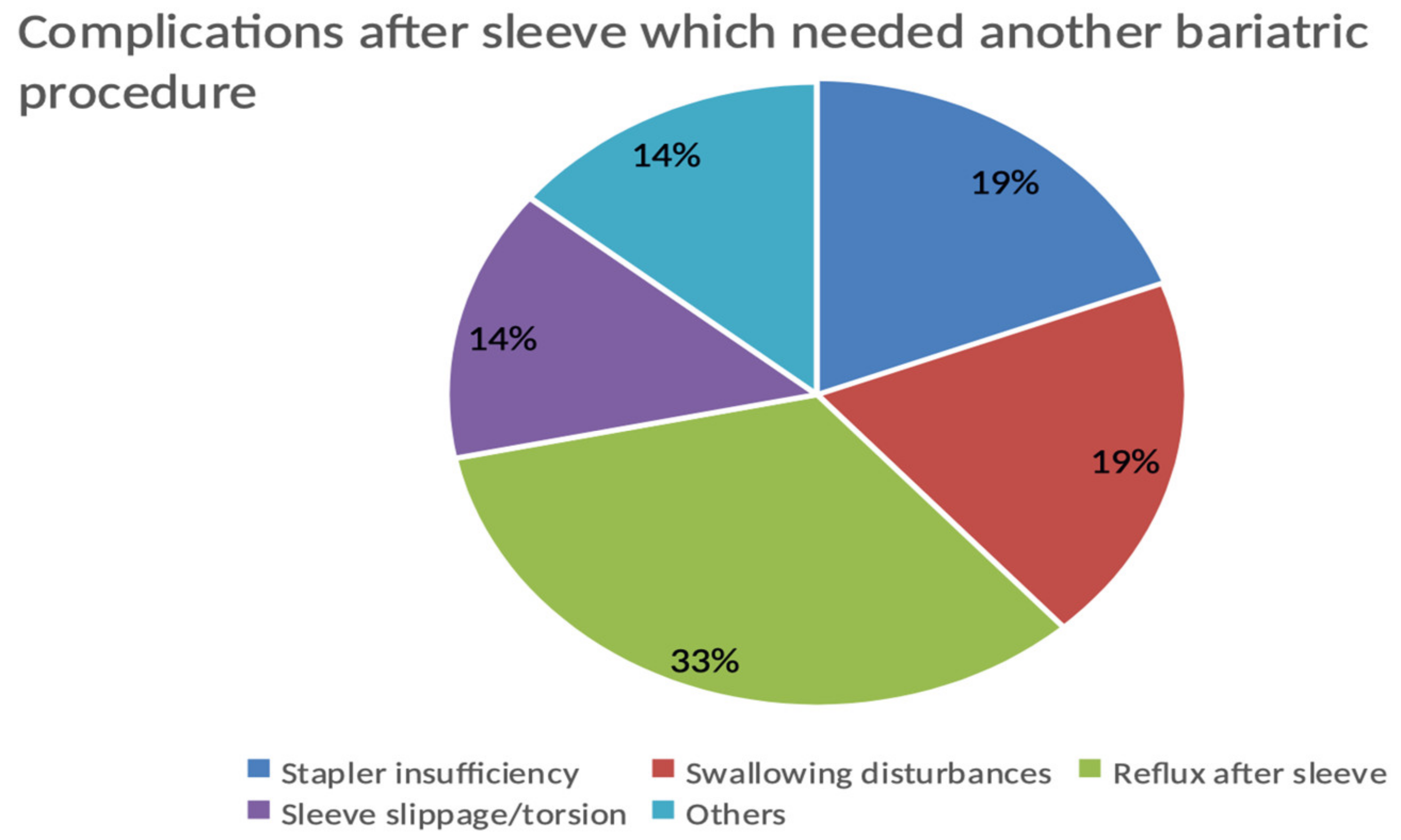
The complications after sleeve driving towards revisional operation in the whole population count, 21 patients out of 63 (33.33%), are shown in Figure 3.
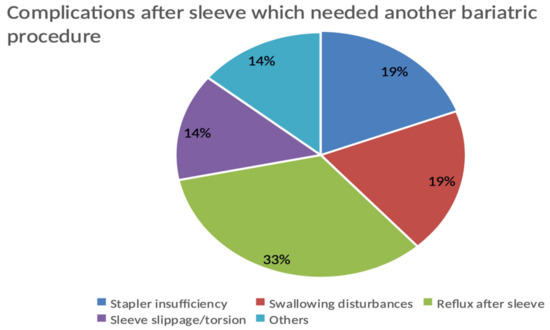
Figure 3.
Complications after sleeve driving towards revisional operation in the whole population.
6. Discussion
Despite the small number of patients and the descriptive statistics, this evaluation offers different topics of discussion and opens reflections about revisional surgery after sleeve gastrectomy. First it is necessary to state that the feasibility of one operation instead of another depends on a large variety of fac tors, including patient choice and surgeon skill. The modern trend for OAGB [18] suggests that this type of operation offers good outcomes and a better chance for the surgeon to spare time and minimize the risks. Furthermore, arising randomized studies [27] show results from OAGB, in terms of weight loss and reduced comorbidities, are even better than those for RYGB and sleeve. Our data went against the main trend because a large proportion of our patients in the RYGB group asked for this revisional operation in order to solve long-term complications like gastroesophageal reflux; so this population also included patients with a good projection for weight loss after sleeve gastrectomy. Regarding reflux, it has to be reported that in the OAGB group one of our patients needed a further revisional operation due to biliary reflux, which made a switch to RYGB necessary. It should be a good time to switch the focus not only of the literature trends and the astonishing results coming from OAGB surgery, but also on approaching the complexity of the patient, the symptoms developed after sleeve gastrectomy, and most of all, to try to avoid other long-term complications. Since dumping syndrome is a probable complication to be handled for both procedures, even if it is a little more frequent in the RYGB group [28], educating the patients and regular follow-ups must been taken into account [29], in order to avoid recurrent dumping episodes.
With the increasing popularity of sleeve gastrectomy, we can anticipate a growing number of patients who require re-operation, predominantly to enhance weight loss. Laparoscopic conversion of SG to RYGB or OAGB is a safe and technically feasible operation, and our experience shows better outcomes after an RYGB. Long-term follow-ups [30] are showing high rates of GERD after sleeve gastrectomy and the recommended solution for Barrett’s esophagus [31], as described by Prager’s group, is the Roux-en-Y Gastric Bypass. Nonetheless, the controversial bile reflux after OAGB [32], seems to be crucial for a Re-Do surgery, since the patients are asking for another intervention not only in order to solve weight regain or weight loss failure but also for other problems, as shown in our population and described in the literature [33]. There is evidence about OAGB efficacy as revisional surgery, mostly for weight regain [34], thereby a multidimensional evaluation should not focus only on weight loss but also other aspects. Results regarding the quality of life (QoL) after primary bariatric surgery have been already described [35], here, there was no difference in long-term QoL between sleeve and RYGB, but OAGB appeared better than sleeve [36].
7. Conclusions
We could assume that RYGB appears to be a better solution for revisional surgery taking into account good reliability in terms of reducing side effects like GERD, and a good outcome in weight loss. A solid statistical evaluation could be obtained with a larger number of patients, but the overall description of our population could stimulate discussions about the actual trend of using OAGB more than RYGB for revisional surgery.
Author Contributions
Conceptualization, P.A. and T.M.; Methodology P.A.; Software, P.A.; Validation, E.T., C.K. and J.K.; Formal analysis, Investigation, Resources, Data curation, P.A. and T.M.; writing, P.A.; Visualization, E.T.; Supervision and Project Administration, T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to a retrospective and observative analysis.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used in this study are available on the DGAV-StuDoQ Deutschen Gesellschaft für Allgemein-und Viszeralchirurgie (DGAV).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hussain, A.; Mahawar, K.; El-Hasani, S. The Impact of COVID-19 Pandemic on Obesity and Bariatric Surgery. Obes. Surg. 2020, 30, 3222–3223. [Google Scholar] [CrossRef] [PubMed]
- Chiappetta, S.; Schaack, H.M.; Wölnerhannsen, B.; Stier, C.; Squillante, S.; Weiner, R.A. The Impact of Obesity and Metabolic Surgery on Chronic Inflammation. Obes. Surg. 2018, 28, 3028–3040. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, G.P.D.; de Melo, H.A.B.; Faria, S.S.; Santos, I.D.O.; Kobinger, G.P.; Magalhães, K.G. Hypercoagulopathy and Adipose Tissue Exacerbated Inflammation May Explain Higher Mortality in COVID-19 Patients With Obesity. Front. Endocrinol. 2020, 11, 530. [Google Scholar] [CrossRef] [PubMed]
- Switzer, N.J.; Karmali, S.; Gill, R.S.; Sherman, V. Revisional Bariatric Surgery. Surg. Clin. 2016, 96, 827–842. [Google Scholar] [CrossRef]
- Mann, J.P.; Jakes, A.D.; Hayden, J.D.; Barth, J.H. Systematic Review of Definitions of Failure in Revisional Bariatric Surgery. Obes. Surg. 2015, 25, 571–574. [Google Scholar] [CrossRef]
- Brethauer, S.A.; Kothari, S.; Sudan, R.; Williams, B.; English, W.J.; Brengman, M.; Kurian, M.; Hutter, M.; Stegemann, L.; Kallies, K.; et al. Systematic review on reoperative bariatric surgery. Surg. Obes. Relat. Dis. 2014, 10, 952–972. [Google Scholar] [CrossRef]
- Van Rutte, P.W.J.; Smulders, J.F.; de Zoete, J.P.; Nienhuijs, S.W. Outcome of sleeve gastrectomy as a primary bariatric procedure. Br. J. Surg. 2014, 101, 661–668. [Google Scholar] [CrossRef]
- Langer, F.B.; Bohdjalian, A.; Shakeri-Leidenmuhler, S.; Schoppmann, S.F.; Zacherl, J.; Prager, G. Conversion from sleeve gastrectomy to Rouxen-Y gastric bypass—Indications and outcome. Obes. Surg. 2010, 20, 835–840. [Google Scholar] [CrossRef]
- Nedelcu, M.; Khwaja, H.A.; Rogula, T.G. Weight regain after bariatric surgery—How should it be defined? Surg. Obes. Relat. Dis. 2016, 12, 1129–1130. [Google Scholar] [CrossRef]
- Lauti, M.; Kularatna, M.; Hill, A.G.; MacCormick, A.D. Weight Regain Following Sleeve Gastrectomy—A Systematic Review. Obes. Surg. 2016, 26, 1326–1334. [Google Scholar] [CrossRef]
- Yu, Y.; Klem, M.L.; Kalarchian, M.A.; Ji, M.; Burke, L.E. Predictors of weight regain after sleeve gastrectomy: An integrative review. Surg. Obes. Relat. Dis. 2019, 15, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, H.; Cabrera, A.; Cabrera, K.; Zerrweck, C.; Mosti, M.; Sierra, M.; Dominguez, G.; Herrera, M.F. Laparoscopic Roux-en-Y Gastric Bypass as a Revision Procedure after Restrictive Bariatric Surgery. Obes. Surg. 2008, 18, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Suter, M.; Ralea, S.; Millo, P.; Allé, J.L. Laparoscopic Roux-en-Y Gastric Bypass After Failed Vertical Banded Gastroplasty: A Multicenter Experience with 203 Patients. Obes. Surg. 2012, 22, 1554–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, P.E.; Hindle, A.; Brennan, L.; Skinner, S.; Burton, P.; Smith, A.; Crosthwaite, G.; Brown, W. Long-Term Outcomes After Bariatric Surgery: A Systematic Review and Meta-analysis of Weight Loss at 10 or More Years for All Bariatric Procedures and a Single-Centre Review of 20-Year Outcomes After Adjustable Gastric Banding. Obes. Surg. 2019, 29, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Apers, J.A.; Wens, C.; Van Vlodrop, V.; Michiels, M.; Ceulemans, R.; Van Daele, G.; Jacobs, I. Perioperative outcomes of revisional laparoscopic gastric bypass after failed adjustable gastric banding and after vertical banded gastroplasty: Experience with 107 cases and subgroup analysis. Surg. Endosc. 2013, 27, 558–564. [Google Scholar] [CrossRef]
- Landreneau, J.P.; Strong, A.T.; Rodriguez, J.H.; Aleassa, E.M.; Aminian, A.; Brethauer, S.; Schauer, P.R.; Kroh, M.D. Conversion of Sleeve Gastrectomy to Roux-en-Y Gastric Bypass. Obes. Surg. 2018, 28, 3843–3850. [Google Scholar] [CrossRef]
- Rutledge, R. The Mini-Gastric Bypass: Experience with the First 1274 Cases. Obes. Surg. 2001, 11, 276–280. [Google Scholar] [CrossRef]
- Musella, M.; Apers, J.; Rheinwalt, K.P.; Ribeiro, R.; Manno, E.; Greco, F.; Čierny, M.; Milone, M.; Di Stefano, C.; Guler, S.; et al. Efficacy of Bariatric Surgery in Type 2 Diabetes Mellitus Remission: The Role of Mini Gastric Bypass/One Anastomosis Gastric Bypass and Sleeve Gastrectomy at 1 Year of Follow-up. A European survey. Obes. Surg. 2015, 26, 933–940. [Google Scholar] [CrossRef]
- Lee, W.-J.; Chong, K.; Lin, Y.-H.; Wei, J.H.; Chen, S.C. Laparoscopic Sleeve Gastrectomy Versus Single Anastomosis (Mini-) Gastric Bypass for the Treatment of Type 2 Diabetes Mellitus: 5-Year Results of a Randomized Trial and Study of Incretin Effect. Obes. Surg. 2014, 24, 1552–1562. [Google Scholar] [CrossRef]
- Quan, Y.; Huang, A.; Ye, M.; Xu, M.; Zhuang, B.; Zhang, P.; Yu, B.; Min, Z. Efficacy of Laparoscopic Mini Gastric Bypass for Obesity and Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2015, 2015, 152852. [Google Scholar] [CrossRef]
- Moszkowicz, D.; Rau, C.; Guenzi, M.; Zinzindohoué, F.; Berger, A.; Chevallier, J.M. Laparoscopic omega-loop gastric bypass for the conversion of failed sleeve gastrectomy: Early experience. J. Visc. Surg. 2013, 150, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Bruzzi, M.; Voron, T.; Zinzindohoue, F.; Berger, A.; Douard, R.; Chevallier, J.M. Revisional single-anastomosis gastric bypass for a failed restrictive procedure: 5-year results. Surg. Obes. Relat. Dis. 2016, 12, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Apers, J.; Wijkmans, R.; Totte, E.; Emous, M. Implementation of mini gastric bypass in the Netherlands: Early and midterm results from a high-volume unit. Surg. Endosc. 2018, 32, 3949–3955. [Google Scholar] [CrossRef] [PubMed]
- Lönroth, H. Laparoscopic Gastric Bypass. Obes. Surg. 1998, 8, 563–564. [Google Scholar] [CrossRef]
- Lee, W.-J.; Yu, P.-J.; Wang, W.; Chen, T.C.; Wei, P.-L.; Huang, M.-T. Laparoscopic Roux-en-Y versus mini-gastric bypass for the treatment of morbid obesity: A prospective randomized controlled clinical trial. Ann. Surg. 2005, 242, 20–28. [Google Scholar] [CrossRef]
- Lee, W.-J.; Wang, W.; Lee, Y.-C.; Huang, M.T.; Ser, K.H.; Chen, J.C. Laparoscopic Mini-gastric Bypass: Experience with Tailored Bypass Limb According to Body Weight. Obes. Surg. 2008, 18, 294–299. [Google Scholar] [CrossRef]
- Ruiz-Tovar, J.; Carbajo, M.A.; Jimenez, J.M.; Castro, M.J.; Gonzalez, G.; Ortiz-De-Solorzano, J.; Zubiaga, L. Long-term follow-up after sleeve gastrectomy versus Roux-en-Y gastric bypass versus one-anastomosis gastric bypass: A prospective randomized comparative study of weight loss and remission of comorbidities. Surg. Endosc. 2018, 33, 401–410, Retraction in Surg. Endosc. 2021, 35, 1492. [Google Scholar] [CrossRef]
- Rheinwalt, K.P.; Plamper, A.; Rückbeil, M.V.; Kroh, A.; Neumann, U.P.; Ulmer, T.F. One Anastomosis Gastric Bypass–Mini-Gastric Bypass (OAGB-MGB) Versus Roux-en-Y Gastric Bypass (RYGB)—A Mid-Term Cohort Study with 612 Patients. Obes. Surg. 2020, 30, 1230–1240. [Google Scholar] [CrossRef]
- Trotta, M.; Ferrari, C.; D’Alessandro, G.; Sarra, G.; Piscitelli, G.; Marinari, G.M. Enhanced recovery after bariatric surgery (ERABS) in a high-volume bariatric center. Surg. Obes. Relat. Dis. 2019, 15, 1785–1792. [Google Scholar] [CrossRef]
- Stenard, F. Laparoscopic sleeve gastrectomy and gastroesophageal reflux. World J. Gastroenterol. 2015, 21, 10348–10357. [Google Scholar] [CrossRef]
- Felsenreich, D.M.; Langer, F.B.; Bichler, C.; Eilenberg, M.; Jedamzik, J.; Kristo, I.; Vock, N.; Gensthaler, L.; Rabl, C.; Todoroff, A.; et al. Roux-en-Y Gastric Bypass as a Treatment for Barrett’s Esophagus after Sleeve Gastrectomy. Obes. Surg. 2020, 30, 1273–1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csendes, A. Bile Reflux After One Anastomosis Gastric Bypass. Obes. Surg. 2020, 30, 2802–2803. [Google Scholar] [CrossRef] [PubMed]
- Hasenberg, T.; Niedergethmann, M. Redo-Eingriffe und Komplikationsmanagement in der bariatrischen Chirurgie. Der Chir. 2014, 85, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Kermansaravi, M.; Shahmiri, S.S.; DavarpanahJazi, A.H.; Valizadeh, R.; Berardi, G.; Vitiello, A.; Musella, M.; Carbajo, M. One Anastomosis/Mini-Gastric Bypass (OAGB/MGB) as Revisional Surgery Following Primary Restrictive Bariatric Procedures: A Systematic Review and Meta-Analysis. Obes. Surg. 2021, 31, 370–383. [Google Scholar] [CrossRef] [PubMed]
- Grönroos, S.; Helmiö, M.; Juuti, A.; Tiusanen, R.; Hurme, S.; Löyttyniemi, E.; Ovaska, J.; Leivonen, M.; Peromaa-Haavisto, P.; Mäklin, S.; et al. Effect of Laparoscopic Sleeve Gastrectomy vs Roux-en-Y Gastric Bypass on Weight Loss and Quality of Life at 7 Years in Patients With Morbid Obesity. JAMA Surg. 2021, 156, 137. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Tantia, O.; Goyal, G.; Chaudhuri, T.; Khanna, S.; Poddar, A.; Majumdar, K.; Gupta, S. LSG vs MGB-OAGB: 5-Year Follow-up Data and Comparative Outcome of the Two Procedures over Long Term—Results of a Randomised Control Trial. Obes. Surg. 2021, 31, 1223–1232. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).