Selective Nerve Root Block in Treatment of Lumbar Radiculopathy: A Narrative Review
Abstract
:1. Introduction
1.1. Low Back Pain and Lumbar Radiculopathy
1.2. Diagnosing Lumbar Radiculopathy
1.3. Management Strategies
2. Selective Nerve Root Block
2.1. Indications for Therapeutic SNRB
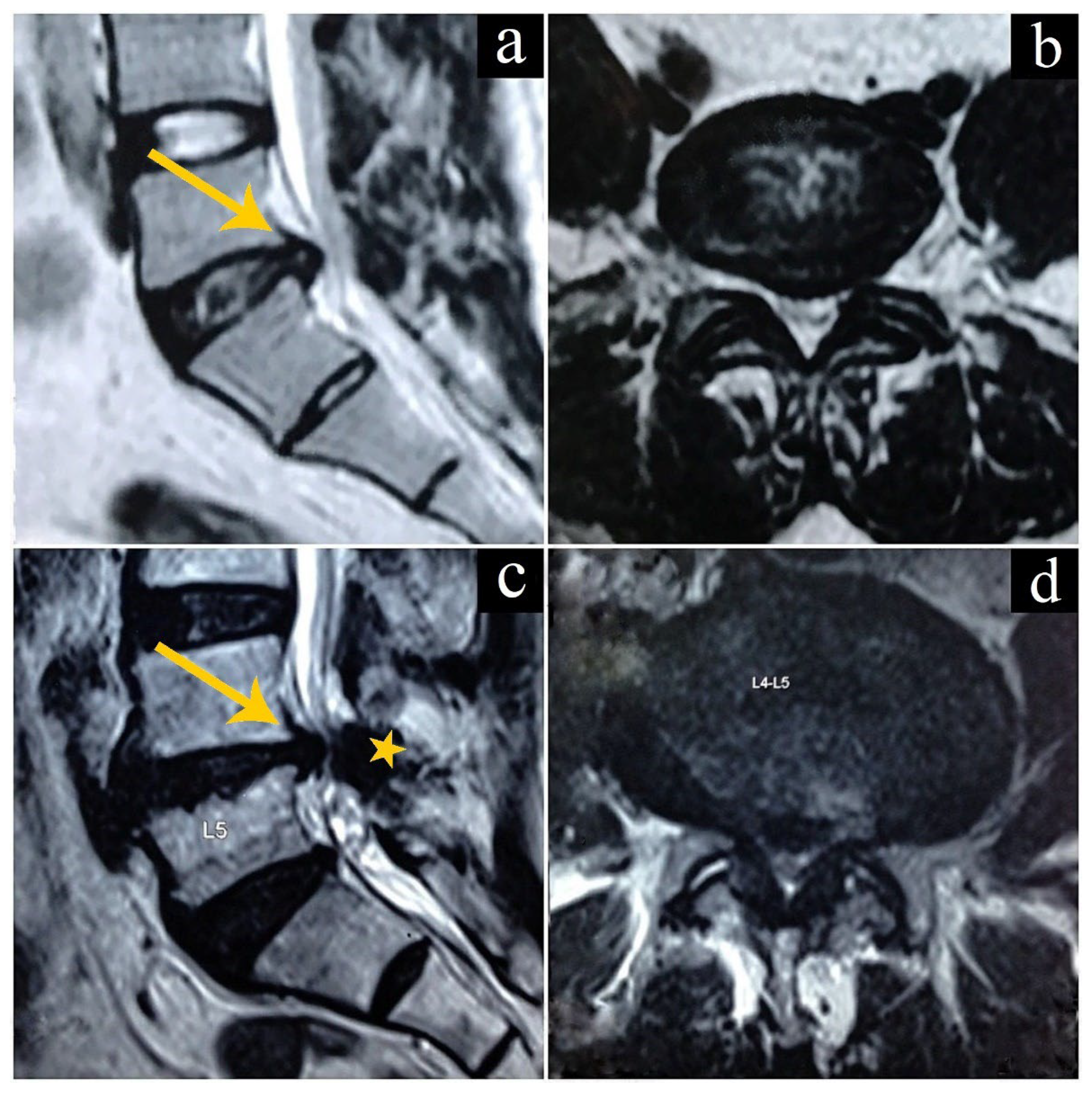
2.2. Intervertebral Disc Herniations
2.3. Spondylosis
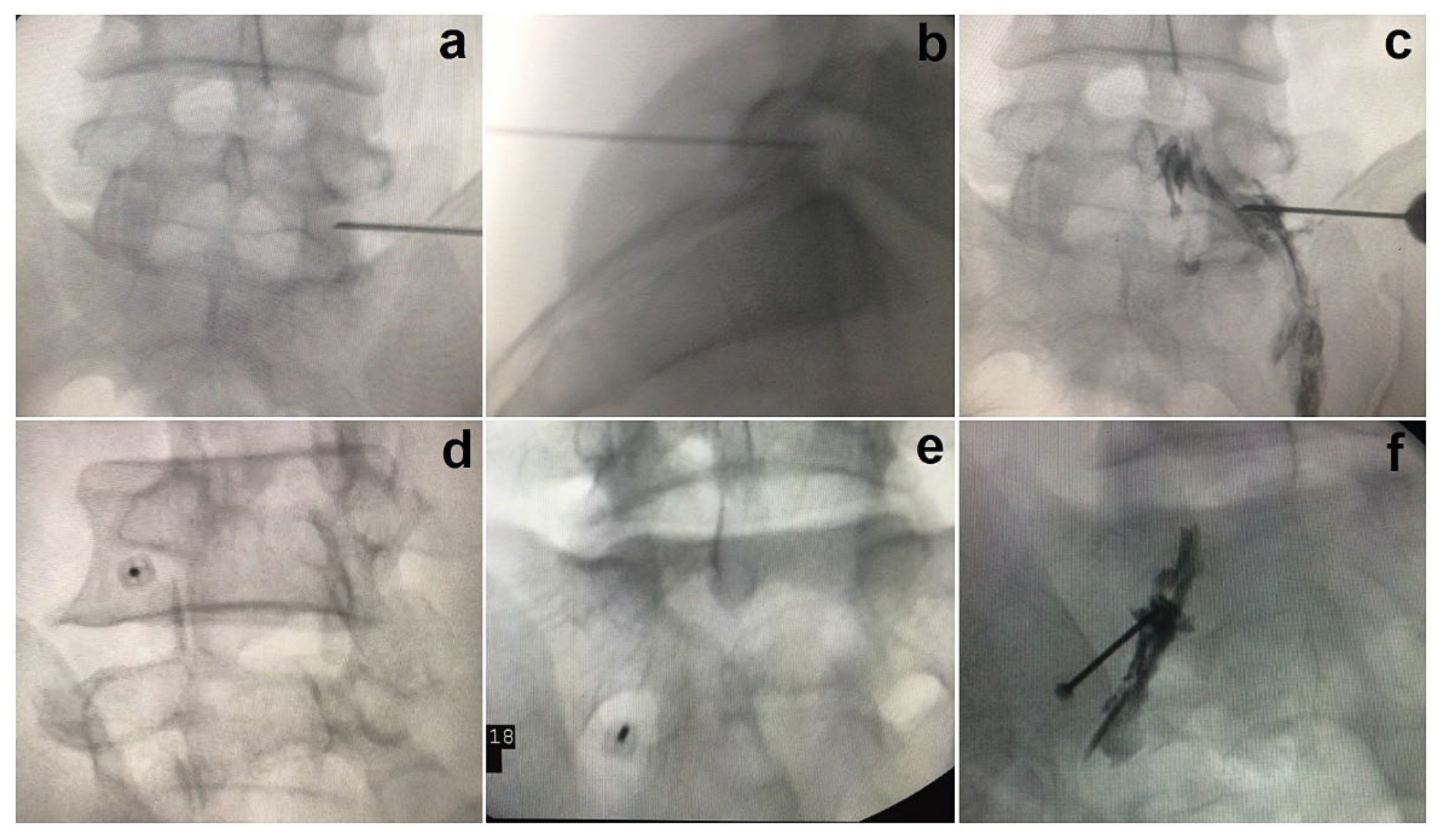
3. Procedure for SNRB
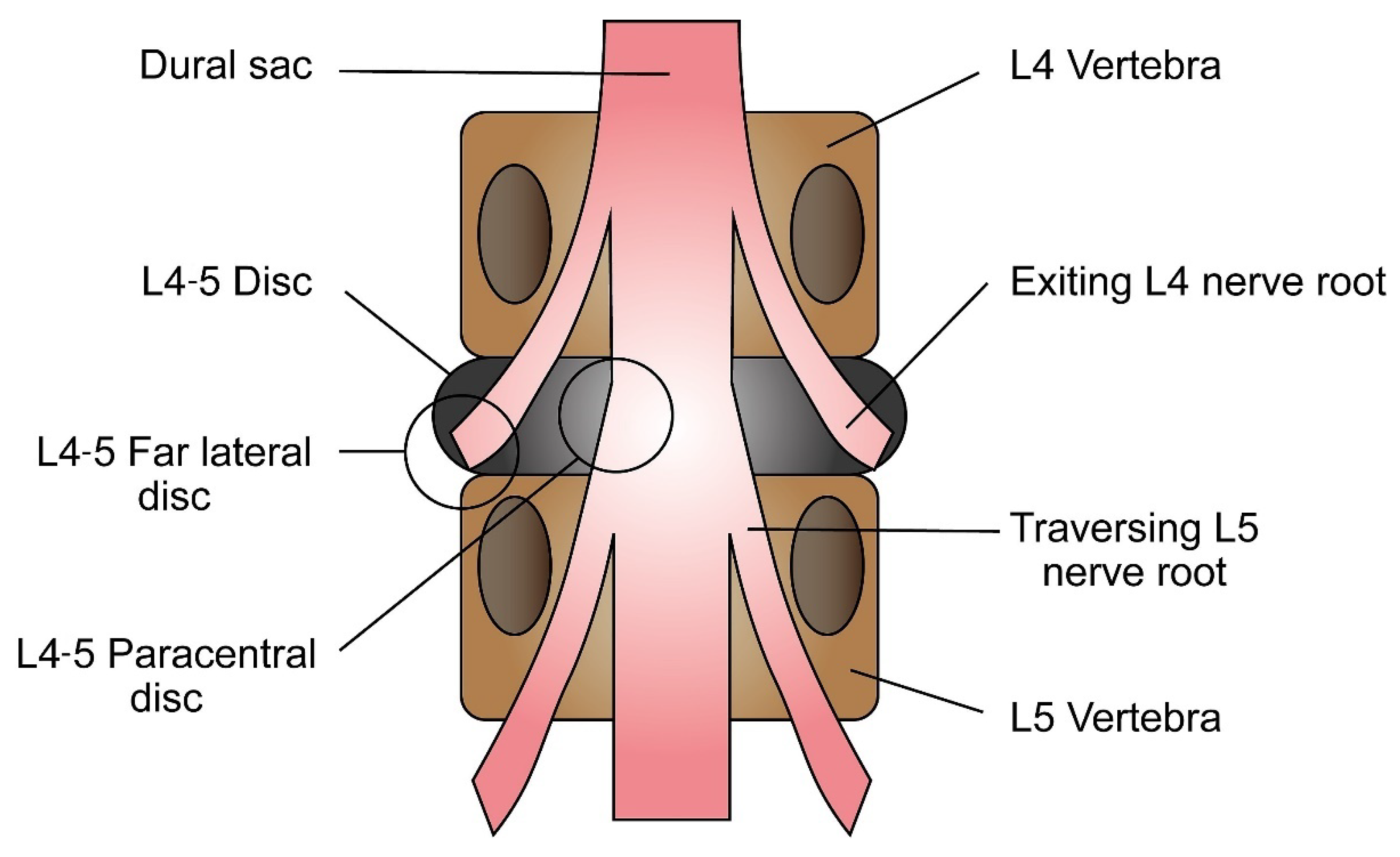
3.1. Identifying the Affected Nerve Root
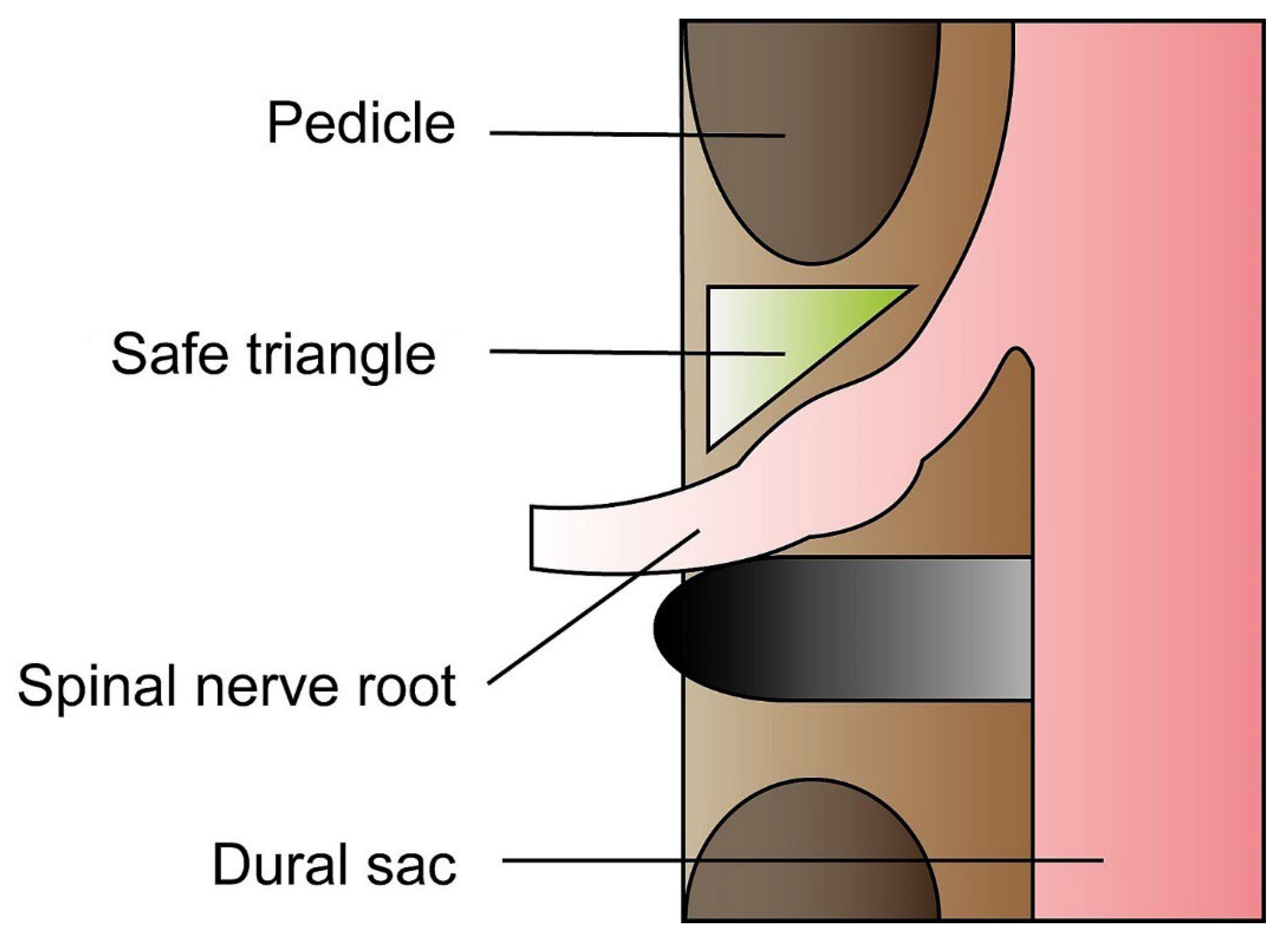
3.2. The Anteroposterior Approach
3.3. The Oblique Scottie Dog Approach
3.4. SNRB Targeting S1
3.5. Ultrasonogram (USG) Guided SNRB
4. The Pharmacological Formulae
5. Outcomes Following SNRB
6. Complications of SNRB
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexander, C.E.; Varacallo, M. Lumbosacral radiculopathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Mattiuzzi, C.; Lippi, G.; Bovo, C. Current epidemiology of low back pain. J. Hosp. Manag. Health Policy 2020, 4, 15. [Google Scholar] [CrossRef]
- Suri, P.; Palmer, M.R.; Tsepilov, Y.A.; Freidin, M.B.; Boer, C.G.; Yau, M.S.; Evans, D.S.; Gelemanovic, A.; Bartz, T.M.; Nethander, M.; et al. Genome-wide meta-analysis of 158,000 individuals of European ancestry identifies three loci associated with chronic back pain. PLoS Genet. 2018, 14, e1007601. [Google Scholar] [CrossRef] [PubMed]
- Suri, P.; Stanaway, I.B.; Zhang, Y.; Freidin, M.B.; Tsepilov, Y.A.; Carrell, D.S.; Williams, F.M.K.; Aulchenko, Y.S.; Hakonarson, H.; Namjou, B.; et al. Genome-wide association studies of low back pain and lumbar spinal disorders using electronic health record data identify a locus associated with lumbar spinal stenosis. Pain 2021, 162, 2263–2272. [Google Scholar] [CrossRef] [PubMed]
- Zafar, F.; Qasim, Y.F.; Farooq, M.U.; Shamael, I.; Khan, I.U.; Khan, D.H. The Frequency of Different Risk Factors for Lower Back Pain in a Tertiary Care Hospital. Cureus 2018, 10, e3183. [Google Scholar] [CrossRef]
- Wong, A.Y.L.; Karppinen, J.; Samartzis, D. Low back pain in older adults: Risk factors, management options and future directions. Scoliosis Spinal Disord. 2017, 12, 14. [Google Scholar] [CrossRef]
- Alhowimel, A.S.; Alodaibi, F.; Alshehri, M.M.; Alqahtani, B.A.; Alotaibi, M.; Alenazi, A.M. Prevalence and Risk Factors Associated with Low Back Pain in the Saudi Adult Community: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18. [Google Scholar] [CrossRef]
- Mukasa, D.; Sung, J. A prediction model of low back pain risk: A population based cohort study in Korea. Korean J. Pain 2020, 33, 153–165. [Google Scholar] [CrossRef]
- Allegri, M.; Montella, S.; Salici, F.; Valente, A.; Marchesini, M.; Compagnone, C.; Baciarello, M.; Manferdini, M.E.; Fanelli, G. Mechanisms of low back pain: A guide for diagnosis and therapy. F1000Research 2016, 5, F1000 Faculty Rev-1530. [Google Scholar] [CrossRef]
- Zheng, C.J.; Chen, J. Disc degeneration implies low back pain. Theor. Biol. Med. Model 2015, 12, 24. [Google Scholar] [CrossRef]
- Dydyk, A.M.; Khan, M.Z.; Singh, P. Radicular back pain. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Majlesi, J.; Togay, H.; Unalan, H.; Toprak, S. The sensitivity and specificity of the Slump and the Straight Leg Raising tests in patients with lumbar disc herniation. J. Clin. Rheumatol. 2008, 14, 87–91. [Google Scholar] [CrossRef]
- Gonzalez Espinosa de Los Monteros, F.J.; Gonzalez-Medina, G.; Ardila, E.M.G.; Mansilla, J.R.; Exposito, J.P.; Ruiz, P.O. Use of Neurodynamic or Orthopedic Tension Tests for the Diagnosis of Lumbar and Lumbosacral Radiculopathies: Study of the Diagnostic Validity. Int. J. Environ. Res. Public Health 2020, 17, 7046. [Google Scholar] [CrossRef] [PubMed]
- Urban, L.M.; MacNeil, B.J. Diagnostic Accuracy of the Slump Test for Identifying Neuropathic Pain in the Lower Limb. J. Orthop. Sports Phys. Ther. 2015, 45, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.; Scuderi, G.; Scuderi, C.; Grewal, R.; Sandhu, S.J. The Use of Imaging in Management of Patients with Low Back Pain. J. Clin. Imaging Sci. 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Reza Soltani, Z.; Sajadi, S.; Tavana, B. A comparison of magnetic resonance imaging with electrodiagnostic findings in the evaluation of clinical radiculopathy: A cross-sectional study. Eur. Spine J. 2014, 23, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Tamarkin, R.G.; Isaacson, A.C. Electrodiagnostic evaluation of lumbosacral radiculopathy. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Chiu, C.C.; Chuang, T.Y.; Chang, K.H.; Wu, C.H.; Lin, P.W.; Hsu, W.Y. The probability of spontaneous regression of lumbar herniated disc: A systematic review. Clin. Rehabil. 2015, 29, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Gugliotta, M.; da Costa, B.R.; Dabis, E.; Theiler, R.; Juni, P.; Reichenbach, S.; Landolt, H.; Hasler, P. Surgical versus conservative treatment for lumbar disc herniation: A prospective cohort study. BMJ Open 2016, 6, e012938. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Weiner, B.K. Treatment of lumbar disc herniation: Evidence-based practice. Int. J. Gen. Med. 2010, 3, 209–214. [Google Scholar] [CrossRef]
- Hahne, A.J.; Ford, J.J.; McMeeken, J.M. Conservative management of lumbar disc herniation with associated radiculopathy: A systematic review. Spine 2010, 35, E488–E504. [Google Scholar] [CrossRef]
- Hakan, T.; Gurcan, S. Spontaneous Regression of Herniated Lumbar Disc with New Disc Protrusion in the Adjacent Level. Case Rep. Orthop. 2016, 2016, 1538072. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Wen, J.; Lu, J.; Sun, Y.; Sang, D. The effectiveness of therapeutic strategies for patients with radiculopathy: A network meta-analysis. Mol. Pain 2018, 14, 1744806918768972. [Google Scholar] [CrossRef]
- Sabnis, A.B.; Diwan, A.D. The timing of surgery in lumbar disc prolapse: A systematic review. Indian J. Orthop. 2014, 48, 127–135. [Google Scholar] [CrossRef]
- Gregory, D.S.; Seto, C.K.; Wortley, G.C.; Shugart, C.M. Acute lumbar disk pain: Navigating evaluation and treatment choices. Am. Fam. Physician 2008, 78, 835–842. [Google Scholar] [PubMed]
- Yoon, W.W.; Koch, J. Herniated discs: When is surgery necessary? EFORT Open Rev. 2021, 6, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Lorio, M.; Kim, C.; Araghi, A.; Inzana, J.; Yue, J.J. International Society for the Advancement of Spine Surgery Policy 2019-Surgical Treatment of Lumbar Disc Herniation with Radiculopathy. Int. J. Spine Surg. 2020, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Abram, S.E. Treatment of lumbosacral radiculopathy with epidural steroids. Anesthesiology 1999, 91, 1937–1941. [Google Scholar] [CrossRef]
- Hakim, B.R.; Munakomi, S. Interlaminar epidural injection. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Viswanathan, V.K.; Kanna, R.M.; Farhadi, H.F. Role of transforaminal epidural injections or selective nerve root blocks in the management of lumbar radicular syndrome—A narrative, evidence-based review. J. Clin. Orthop. Trauma 2020, 11, 802–809. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, S.; Chahal, G.; Verma, R. Selective nerve root blocks vs. caudal epidural injection for single level prolapsed lumbar intervertebral disc—A prospective randomized study. J. Clin. Orthop. Trauma 2017, 8, 142–147. [Google Scholar] [CrossRef]
- Murakibhavi, V.G.; Khemka, A.G. Caudal epidural steroid injection: A randomized controlled trial. Evid. Based Spine Care J. 2011, 2, 19–26. [Google Scholar] [CrossRef]
- Arun-Kumar, K.; Jayaprasad, S.; Senthil, K.; Lohith, H.; Jayaprakash, K.V. The Outcomes of Selective Nerve Root Block for Disc Induced Lumbar Radiculopathy. Malays. Orthop. J. 2015, 9, 17–22. [Google Scholar] [CrossRef]
- Dhakal, G.R.; Hamal, P.K.; Dhungana, S.; Kawaguchi, Y. Clinical Efficacy of Selective Nerve Root Block in Lumbar Radiculopathy due to Disc Prolapse. J. Nepal Health Res. Counc. 2019, 17, 242–246. [Google Scholar] [CrossRef]
- Yeom, J.S.; Lee, J.W.; Park, K.W.; Chang, B.S.; Lee, C.K.; Buchowski, J.M.; Riew, K.D. Value of diagnostic lumbar selective nerve root block: A prospective controlled study. AJNR Am. J. Neuroradiol. 2008, 29, 1017–1023. [Google Scholar] [CrossRef]
- Huston, C.W.; Slipman, C.W. Diagnostic selective nerve root blocks: Indications and usefulness. Phys. Med. Rehabil. Clin. N. Am. 2002, 13, 545–565. [Google Scholar] [CrossRef]
- Kanaan, T.; Abusaleh, R.; Abuasbeh, J.; Al Jammal, M.; Al-Haded, S.; Al-Rafaiah, S.; Kanaan, A.; Alnaimat, F.; Khreesha, L.; Al Hadidi, F.; et al. The Efficacy of Therapeutic Selective Nerve Block in Treating Lumbar Radiculopathy and Avoiding Surgery. J. Pain Res. 2020, 13, 2971–2978. [Google Scholar] [CrossRef] [PubMed]
- Narozny, M.; Zanetti, M.; Boos, N. Therapeutic efficacy of selective nerve root blocks in the treatment of lumbar radicular leg pain. Swiss Med. Wkly. 2001, 131, 75–80. [Google Scholar]
- Beynon, R.; Elwenspoek, M.M.C.; Sheppard, A.; Higgins, J.N.; Kolias, A.G.; Laing, R.J.; Whiting, P.; Hollingworth, W. The utility of diagnostic selective nerve root blocks in the management of patients with lumbar radiculopathy: A systematic review. BMJ Open 2019, 9, e025790. [Google Scholar] [CrossRef] [PubMed]
- Slipman, C.W.; Issac, Z. The role of diagnostic selective nerve root blocks in the management of spinal pain. Pain Physician 2001, 4, 214–226. [Google Scholar] [CrossRef] [PubMed]
- Bartynski, W.S.; Kang, M.D.; Rothfus, W.E. Adjacent double-nerve root contributions in unilateral lumbar radiculopathy. AJNR Am. J. Neuroradiol. 2010, 31, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Sharrak, S.; Al Khalili, Y. StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Fardon, D.F.; Williams, A.L.; Dohring, E.J.; Murtagh, F.R.; Gabriel Rothman, S.L.; Sze, G.K. Lumbar disc nomenclature: Version 2.0: Recommendations of the combined task forces of the North American Spine Society, the American Society of Spine Radiology and the American Society of Neuroradiology. Spine J. 2014, 14, 2525–2545. [Google Scholar] [CrossRef]
- Fardon, D.F.; Milette, P.C. Nomenclature and classification of lumbar disc pathology. Recommendations of the Combined task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine 2001, 26, E93–E113. [Google Scholar] [CrossRef]
- Mysliwiec, L.W.; Cholewicki, J.; Winkelpleck, M.D.; Eis, G.P. MSU classification for herniated lumbar discs on MRI: Toward developing objective criteria for surgical selection. Eur. Spine J. 2010, 19, 1087–1093. [Google Scholar] [CrossRef]
- Pfirrmann, C.W.; Dora, C.; Schmid, M.R.; Zanetti, M.; Hodler, J.; Boos, N. MR image-based grading of lumbar nerve root compromise due to disk herniation: Reliability study with surgical correlation. Radiology 2004, 230, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Middleton, K.; Fish, D.E. Lumbar spondylosis: Clinical presentation and treatment approaches. Curr. Rev. Musculoskelet. Med. 2009, 2, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Rustenburg, C.M.E.; Emanuel, K.S.; Peeters, M.; Lems, W.F.; Vergroesen, P.A.; Smit, T.H. Osteoarthritis and intervertebral disc degeneration: Quite different, quite similar. JOR Spine 2018, 1, e1033. [Google Scholar] [CrossRef] [PubMed]
- Rider, S.M.; Mizuno, S.; Kang, J.D. Molecular Mechanisms of Intervertebral Disc Degeneration. Spine Surg. Relat. Res. 2019, 3, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mihara, A.; Nishida, N.; Jiang, F.; Ohgi, J.; Imajo, Y.; Suzuki, H.; Funaba, M.; Yamagata, H.; Chen, X.; Sakai, T. Tensile Test of Human Lumbar Ligamentum Flavum: Age-Related Changes of Stiffness. Appl. Sci. 2021, 11, 3337. [Google Scholar] [CrossRef]
- Sairyo, K.; Biyani, A.; Goel, V.; Leaman, D.; Booth, R., Jr.; Thomas, J.; Gehling, D.; Vishnubhotla, L.; Long, R.; Ebraheim, N. Pathomechanism of ligamentum flavum hypertrophy: A multidisciplinary investigation based on clinical, biomechanical, histologic, and biologic assessments. Spine 2005, 30, 2649–2656. [Google Scholar] [CrossRef]
- Kolte, V.S.; Khambatta, S.; Ambiye, M.V. Thickness of the ligamentum flavum: Correlation with age and its asymmetry-an magnetic resonance imaging study. Asian Spine J. 2015, 9, 245–253. [Google Scholar] [CrossRef]
- Inoue, N.; Orias, A.A.E.; Segami, K. Biomechanics of the Lumbar Facet Joint. Spine Surg. Relat. Res. 2020, 4, 1–7. [Google Scholar] [CrossRef]
- Gilchrist, R.V.; Slipman, C.W.; Bhagia, S.M. Anatomy of the intervertebral foramen. Pain Physician 2002, 5, 372–378. [Google Scholar] [CrossRef]
- Jaumard, N.V.; Welch, W.C.; Winkelstein, B.A. Spinal facet joint biomechanics and mechanotransduction in normal, injury and degenerative conditions. J. Biomech. Eng. 2011, 133, 071010. [Google Scholar] [CrossRef]
- Bureau, N.J.; Kaplan, P.A.; Dussault, R.G. Lumbar facet joint synovial cyst: Percutaneous treatment with steroid injections and distention--clinical and imaging follow-up in 12 patients. Radiology 2001, 221, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Kanna, R.M.; Shetty, A.P.; Rajasekaran, S. Predictors of Successful Outcomes of Selective Nerve Root Blocks for Acute Lumbar Disc Herniation. Glob. Spine J. 2019, 9, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.M.; Andrade, N.S.; Neuman, B.J. Lumbar Disc Herniation. Curr. Rev. Musculoskelet. Med. 2017, 10, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Kaliya-Perumal, A.K.; Yeh, Y.C.; Luo, C.A.; Joey-Tan, K.Y. Assessment of Anteroposterior Subpedicular Approach and Oblique Scotty Dog Subpedicular Approach for Selective Nerve Root Block. Clin. Orthop. Surg. 2017, 9, 71–76. [Google Scholar] [CrossRef]
- Mansfeld, E.E. Sacral Transforaminal Epidural Injection (Selective Nerve Root Block), Interventional Pain ed.; Stogicza, A.R., Mansano, A.M., Trescot, A.M., Staats, P.S., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Emami, S.A.; Sanatkar, M.; Espahbodi, E.; Pestehei, S.K. Ultrasound and nerve stimulator guidance lumbar transforaminal epidural block for the treatment of patients with lumbosacral radicular pain. Sci. Rep. 2022, 12, 5954. [Google Scholar] [CrossRef]
- Soni, P.; Punj, J. Ultrasound-Guided Lumbar Transforaminal Epidural Injection: A Narrative Review. Asian Spine J. 2021, 15, 261–270. [Google Scholar] [CrossRef]
- Korbe, S.; Udoji, E.N.; Ness, T.J.; Udoji, M.A. Ultrasound-guided interventional procedures for chronic pain management. Pain Manag. 2015, 5, 465–482. [Google Scholar] [CrossRef] [PubMed]
- Sahu, D.K.; Sharma, A.; Kothari, K.; Wani, P.; Patel, C.; Parampill, R. Ultrasound-guided fluoroscopic-verified lumbar transforaminal epidural injection: A clinical evaluation of technique. Indian J. Pain 2016, 30, 158–161. [Google Scholar] [CrossRef]
- Chatterjee, N.; Roy, C.; Das, S.; Al Ajmi, W.; Al Sharji, N.S.; Al Mandhari, A. Comparative Efficacy of Methylprednisolone Acetate and Dexamethasone Disodium Phosphate in Lumbosacral Transforaminal Epidural Steroid Injections. Turk. J. Anaesthesiol. Reanim. 2019, 47, 414–419. [Google Scholar] [CrossRef]
- Guyot, J.P. Lumbar Selective Nerve Root Block: Comparative Study Using Two Pharmacological Formulae. Global Spine J. 2018, 8, 374–377. [Google Scholar] [CrossRef]
- Jonayed, S.A.; Kamruzzaman, M.; Saha, M.K.; Alam, S.; Akter, S. The Role of Selective Nerve Root Block in the Treatment of Lumbar Radicular Leg Pain. Mymensingh Med. J. 2016, 25, 141–147. [Google Scholar] [PubMed]
- McCormick, Z.; Kennedy, D.J.; Garvan, C.; Rivers, E.; Temme, K.; Margolis, S.; Zander, E.; Rohr, A.; Smith, M.C.; Plastaras, C. Comparison of Pain Score Reduction Using Triamcinolone vs. Betamethasone in Transforaminal Epidural Steroid Injections for Lumbosacral Radicular Pain. Am. J. Phys. Med. Rehabil. 2015, 94, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- MacMahon, P.J.; Huang, A.J.; Palmer, W.E. Spine Injectables: What Is the Safest Cocktail? AJR Am. J. Roentgenol. 2016, 207, 526–533. [Google Scholar] [CrossRef]
- Bensler, S.; Sutter, R.; Pfirrmann, C.W.A.; Peterson, C.K. Particulate versus non-particulate corticosteroids for transforaminal nerve root blocks: Comparison of outcomes in 494 patients with lumbar radiculopathy. Eur. Radiol. 2018, 28, 946–952. [Google Scholar] [CrossRef]
- MacMahon, P.J.; Eustace, S.J.; Kavanagh, E.C. Injectable corticosteroid and local anesthetic preparations: A review for radiologists. Radiology 2009, 252, 647–661. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.H.; Lee, I.S.; Choi, J.A.; Choi, J.Y.; Hong, S.H.; Kang, H.S. Therapeutic effect and outcome predictors of sciatica treated using transforaminal epidural steroid injection. AJR Am. J. Roentgenol. 2006, 187, 1427–1431. [Google Scholar] [CrossRef]
- Benny, B.; Azari, P. The efficacy of lumbosacral transforaminal epidural steroid injections: A comprehensive literature review. J. Back Musculoskelet. Rehabil. 2011, 24, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.T.; Willick, S.E.; Rho, M.E.; Rittenberg, J.D. Efficacy of lumbosacral transforaminal epidural steroid injections: A systematic review. PM R 2009, 1, 657–668. [Google Scholar] [CrossRef]
- Bhatia, A.; Flamer, D.; Shah, P.S.; Cohen, S.P. Transforaminal Epidural Steroid Injections for Treating Lumbosacral Radicular Pain from Herniated Intervertebral Discs: A Systematic Review and Meta-Analysis. Anesth. Analg. 2016, 122, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Manchikanti, L.; Malla, Y.; Wargo, B.W.; Cash, K.A.; Pampati, V.; Fellows, B. A prospective evaluation of complications of 10,000 fluoroscopically directed epidural injections. Pain Physician 2012, 15, 131–140. [Google Scholar] [CrossRef]
- Karaman, H.; Kavak, G.O.; Tufek, A.; Yldrm, Z.B. The complications of transforaminal lumbar epidural steroid injections. Spine 2011, 36, E819–E824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGrath, J.M.; Schaefer, M.P.; Malkamaki, D.M. Incidence and characteristics of complications from epidural steroid injections. Pain Med. 2011, 12, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Ng, A.T. Complications Associated with Lumbar Transforaminal Epidural Steroid Injections. Curr. Pain Headache Rep. 2020, 24, 67. [Google Scholar] [CrossRef]
- Lyders, E.M.; Morris, P.P. A case of spinal cord infarction following lumbar transforaminal epidural steroid injection: MR imaging and angiographic findings. AJNR Am. J. Neuroradiol. 2009, 30, 1691–1693. [Google Scholar] [CrossRef] [PubMed]
- Kabbara, A.; Rosenberg, S.K.; Untal, C. Methicillin-resistant Staphylococcus aureus epidural abscess after transforaminal epidural steroid injection. Pain Physician 2004, 7, 269–272. [Google Scholar] [CrossRef]
- Houten, J.K.; Errico, T.J. Paraplegia after lumbosacral nerve root block: Report of three cases. Spine J. 2002, 2, 70–75. [Google Scholar] [CrossRef]
- Huntoon, M.A.; Martin, D.P. Paralysis after transforaminal epidural injection and previous spinal surgery. Reg. Anesth. Pain Med. 2004, 29, 494–495. [Google Scholar] [CrossRef]
- Kennedy, D.J.; Dreyfuss, P.; Aprill, C.N.; Bogduk, N. Paraplegia following image-guided transforaminal lumbar spine epidural steroid injection: Two case reports. Pain Med. 2009, 10, 1389–1394. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, D.H.; Kim, S.H.; Cho, Y.H. Spinal epidural hematoma occurring at a distance from the transforaminal epidural injection site: A case report. Medicine 2019, 98, e16654. [Google Scholar] [CrossRef]
- Gungor, S.; Aiyer, R. Epidural hematoma development contralateral to dura after lumbar transforaminal epidural steroid injection. Pain Manag. 2017, 7, 367–375. [Google Scholar] [CrossRef]
- Goodman, B.S.; Bayazitoglu, M.; Mallempati, S.; Noble, B.R.; Geffen, J.F. Dural puncture and subdural injection: A complication of lumbar transforaminal epidural injections. Pain Physician 2007, 10, 697–705. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.C.R.; Chiu, S.T.; Oh, J.Y.-L.; Kaliya-Perumal, A.-K. Selective Nerve Root Block in Treatment of Lumbar Radiculopathy: A Narrative Review. Surgeries 2022, 3, 259-270. https://doi.org/10.3390/surgeries3030028
Yang JCR, Chiu ST, Oh JY-L, Kaliya-Perumal A-K. Selective Nerve Root Block in Treatment of Lumbar Radiculopathy: A Narrative Review. Surgeries. 2022; 3(3):259-270. https://doi.org/10.3390/surgeries3030028
Chicago/Turabian StyleYang, Jacqueline Chu Ruo, Shi Ting Chiu, Jacob Yoong-Leong Oh, and Arun-Kumar Kaliya-Perumal. 2022. "Selective Nerve Root Block in Treatment of Lumbar Radiculopathy: A Narrative Review" Surgeries 3, no. 3: 259-270. https://doi.org/10.3390/surgeries3030028
APA StyleYang, J. C. R., Chiu, S. T., Oh, J. Y.-L., & Kaliya-Perumal, A.-K. (2022). Selective Nerve Root Block in Treatment of Lumbar Radiculopathy: A Narrative Review. Surgeries, 3(3), 259-270. https://doi.org/10.3390/surgeries3030028





