1. Introduction
Breast cancer is one of the most diagnosed cancers in the world, and with improved survival and a high incidence rate, it is the most prevalent cancer among women in the USA with 2.6 million women reporting a history of the disease [
1,
2]. In 2018, there were nearly 2.1 million new breast cancer cases, accounting for more than 10% of all cancer diagnoses across both sexes [
3]. Fortunately, with improvements in management, the 5-year breast cancer survival rate now approaches 90% [
4]. Surgery is a critical component in breast cancer management and involves either a mastectomy (surgical removal of the entire breast) or a lumpectomy (removing only cancerous tissue and a rim of normal tissue). However, since patients with breast cancer are living longer, long-term complications of management and surgery are becoming increasingly apparent [
5].
Persistent pain is a devastating yet common complication after breast cancer surgery. The International Association for the Study of Pain (IASP) defines persistent pain after surgery as pain localized to the surgical field or area, or innervation territory, that persists beyond the healing process (i.e., at least 3 months after surgery) [
6]. A meta-analysis of 146 observational studies (137,675 patients) found that the median prevalence of any type of persistent pain after breast cancer surgery was 37% (IQR 22–48%) 3 to >120 months after surgery [
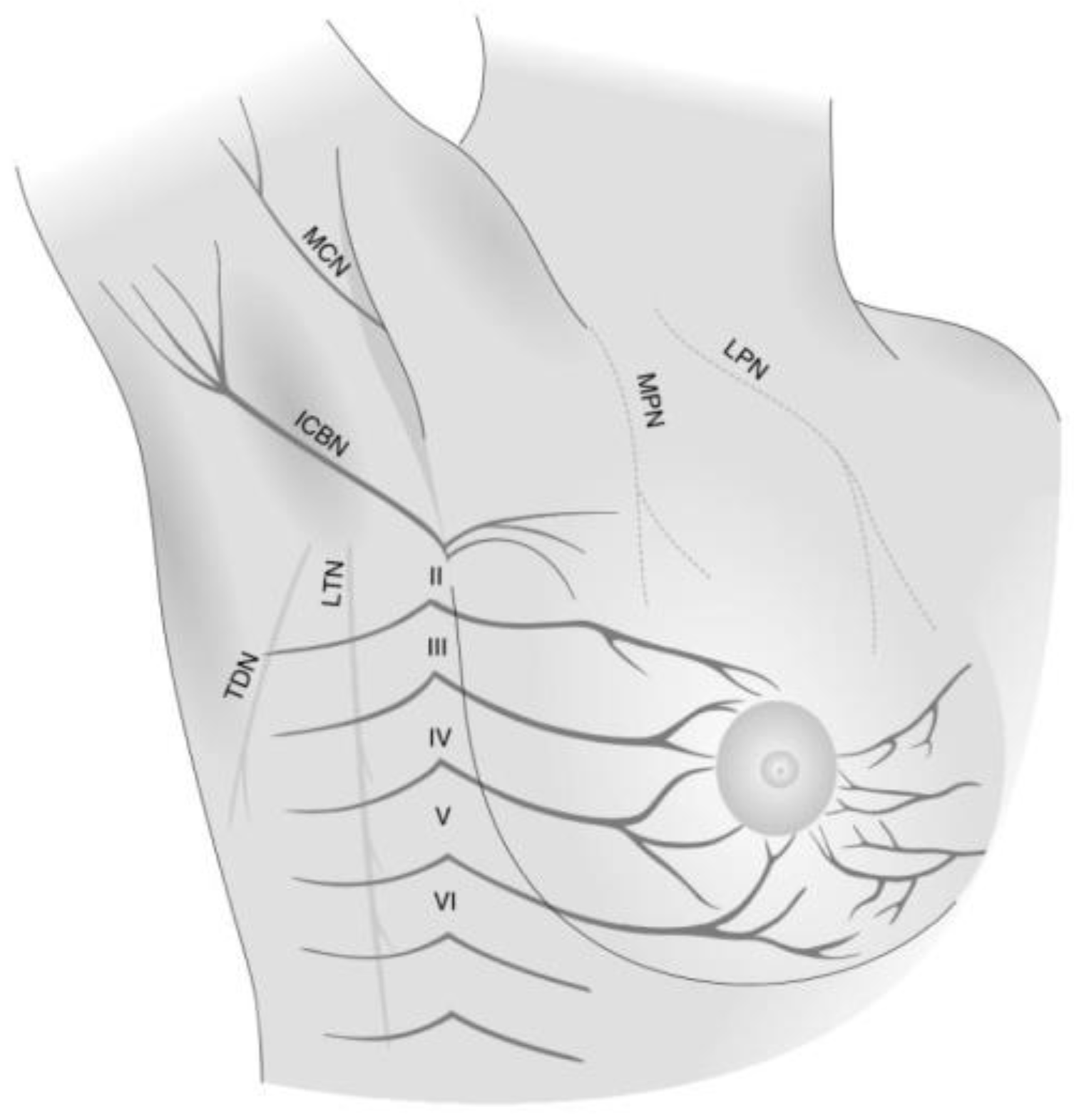
7]. One reason for this high prevalence after breast cancer surgery is due to the rich neuronal innervation of the breast tissue and chest wall (
Figure 1) [
8]. Longitudinal data also suggest that this pain disorder is chronic and that over 50% of those diagnosed will continue to suffer from it 7–12 years after surgery [
9]. The pain intensity experienced by those afflicted is not trivial and the median intensity of pain is estimated to be 3.9 cm (95% CI 3.6 to 4.2) on a 0 to 10 cm visual analog scale (VAS). Further, 20% of patients (95% CI 17–23%; 78 studies, 29,939 patients) suffer from moderate-to-severe pain [
7].
Persistent pain also has negative consequences on patients’ sleep, function, social interactions, mood and psychological well-being, and quality of life [
9,
10,
11,
12,
13,
14]. Furthermore, economic analyses suggest that persistent pain after breast cancer surgery places an immense financial burden on the healthcare system, totaling about
$1 billion USD annually to the US healthcare system alone [
15].
Despite the common occurrence and significant impact of persistent pain after breast cancer surgery, limited data have been published on the characteristics and qualities of this pain disorder [
16]. Information on the typical clinical presentation such as pain location, pain intensity levels, neuropathic qualities, features of the surgical scar, and analgesic consumption are important as they can aid clinicians in better screening, diagnosing, and managing this chronic pain disorder [
17].
Additionally, further data are needed on specific risk factors that may be associated with developing persistent pain after breast cancer surgery. In a study by Gärtner et al. in 2009, which analyzed questionnaires completed by 3754 women 2–3 years after their breast cancer surgery, a 47% prevalence of persistent pain was observed with younger age, adjuvant radiotherapy, and axillary lymph node dissection (ALND) as predictors [
18]. A systematic review of 30 studies (19,813 patients) provided further moderate-to-high quality evidence that younger age, radiotherapy, ALND, greater acute postoperative pain, and presence of preoperative pain are associated with persistent pain after breast cancer surgery [
19]. However, despite the comprehensive nature of this review, many of the included studies did not explore associations between specific aspects of routine perioperative care, such as surgical or anesthetic-related interventions.
The purpose of this investigation was to identify novel risk factors seldom explored, and corroborate or refute those with previous contradictory findings.
5. Discussion
In this study of 100 patients undergoing breast cancer surgery, our data suggest that persistent pain is an exceedingly common complication, is mild-moderate in intensity, primarily exists in the axilla and chest, has neuropathic pain features, is associated with interference across all domains of daily living, worse quality of life, and increases the need for non-opioid analgesics. Our univariate analysis suggests that employment status, pain catastrophizing, preoperative anxiety, use of intraoperative IV dexamethasone, higher postoperative pain scores, and adjuvant chemotherapy may be associated with persistent pain at 3 months after surgery. Findings of our adjusted analyses identified employment status, worse postoperative pain on movement, and adjuvant chemotherapy as independent predictors of persistent pain.
There are several strengths to this analysis. Given that our analysis utilized data from a previously completed randomized controlled trial, data were prospectively collected and avoided the typical issues with case-control studies such as recall bias and missing data. Furthermore, our study collected specific anesthetic and surgical-related factors that have not been previously explored as potential risk factors for persistent pain after surgery. Lastly, our study obtained a notable follow-up rate of 100% at 3 months, allowing greater outcome data for our analyses and avoiding bias caused by loss to follow-up.
Our study also contributes novel insights to our understanding of persistent pain after breast cancer surgery. Our data suggest that patients who are employed at the time of surgery may be more likely to develop persistent pain. This is an interesting and novel finding and only a few perioperative studies have sought to evaluate this relationship [
32,
33,
34]. A retrospective survey of 408 patients who underwent breast cancer surgery also found that employment status at the time of surgery was significantly associated with the development of persistent post-surgical pain [
32]. Further, a study of 226 patients who underwent open inguinal hernia repair also reported that those who had full-time employment at the time of surgery were more likely to suffer from chronic pain [
33]. The mechanism responsible for this relationship is not readily apparent. Outside the perioperative period, work-related stress and high psychological demands have been identified as risk factors for chronic pain [
35]. For those who are employed at the time of surgery, similar stressors may exist such as stress related to taking time off work, uncertainty surrounding their return-to-work date, the possibility of job loss, returning to work too soon, and financial strains of missing work. The added work-related stress may be an important contribution to the development of chronic post-surgical pain, and future studies will be needed to further evaluate this association.
Additionally, our data suggest that acute pain scores (i.e., in the 3 days following surgery), specifically on movement, are positively associated with developing persistent pain. This finding is also consistent with prior data in patients undergoing breast cancer [
19] and non-breast cancer-related surgeries [
36]. Movement-evoked pain after surgery has been shown to be a strong predictor of chronic post-surgical pain, and even greater than acute pain scores at rest [
36]. The mechanism for the development of persistent postsurgical pain is complex and involves various molecular and cellular changes in the peripheral and central nervous systems. Inflammation [
37] and nerve injury result in long-term synaptic plasticity that amplifies pain signaling, also known as pain sensitization [
38]. Animal studies have assisted in identifying the neurobiological mechanism of pain sensitization after surgery, including the role of specific receptors, mediators, and neurotransmitters involved [
39]. On a molecular level, when peripheral nerves are damaged, there are changes within sensory nerves that involve substance P, calcitonin gene-related peptide, and particularly, the upregulation of sodium channels [
40], specifically Nav1.3 which is an embryonic voltage-gated sodium channel that has fast-activating and fast-inactivating current [
41]. These changes result in spontaneous ectopic discharges (resting pain) and greater sensitivity to discharge (i.e., decreased activation threshold and increased amplitude of pain response [
42]). Inflammation and nerve injury induces transcriptional changes in the dorsal horn, strengthening the offending neural pathway, and resulting in greater neuroplastic changes in the central and peripheral nervous system, resulting in persistent pain [
43]. Nonetheless, while acute pain intensities may be associated with persistent pain [
44], prior trials aimed at improving postoperative pain intensities have not consistently demonstrated a reduction in the development of chronic pain [
45]. This may be in part related to statistical power, and thus larger trials in this area are needed.
Another predictor of persistent pain identified in our univariable and multivariable models is adjuvant chemotherapy. This finding is highly controversial, with contradicting reports in the literature [
9,
19,
46,
47,
48,
49]. Nevertheless, adjuvant chemotherapy as a significant risk factor for persistent pain should be the focus of future large prospective studies, as perhaps a better balance between its advantage of improved disease-free duration and risk of persistent pain may be achieved. While dexamethasone was associated with persistent pain in the univariate analysis, significance was lost in the adjusted analysis. Several studies document a beneficial effect of perioperative dexamethasone use and acute analgesic outcomes (pain intensities and opioid consumption), but few studies have demonstrated an effect on persistent pain [
50]. A secondary analysis of 310 patients who underwent breast cancer surgery also reported a non-significant difference in the incidence of chronic pain between those who received and those who did not receive dexamethasone [
51]. Further, an analysis of 1043 cardiac surgical patients also found no association between steroid use around the time of surgery and the development of persistent pain [
52].
Our data provide additional insights into the characteristics of persistent pain after surgery. Our analysis suggests that there are unique characteristics of the surgical scar in those with persistent pain. Specifically, patients with pain report changes in the color and contour of their scars compared to those without pain. Furthermore, consistent with prior studies, our data suggest that persistent pain after breast cancer surgery is largely a neuropathic pain disorder. The vast majority of patients with persistent pain reported a neuropathic feature such as burning, shooting, numbness, and tingling pain, and possibly allodynia and hyperalgesia [
14,
53]. The high prevalence of neuropathic features suggests that perioperative peripheral nerve injury may be a key component in the pathogenesis of this pain disorder. It also suggests that a neuropathic pain approach could be considered in the management of persistent pain after breast cancer surgery through the use of pain medications [
54,
55], targeted nerve blocks [
56,
57], or even neuromodulation [
58,
59]. A recently published review of studies assessed various treatment modalities for chronic pain after breast cancer surgery, including physical and psychological approaches as well as pharmacological (e.g., anti-depressants, anti-convulsants, topical analgesic creams) and interventional strategies (e.g., PEC I or II blocks, serratus plane blocks, neuromodulation). However, high-quality evidence of beneficial interventions is lacking, stressing the need to identify novel treatments and, more importantly, find ways to prevent this type of pain from occurring [
16,
60,
61].
There are several limitations to our investigation. First and foremost, this study included a relatively small sample size of 100 patients, which restricted both the number of variables that could be included in a multivariable model, as well as limiting our statistical power to identify possible relationships. Our small sample size also increased our chances of spurious findings. Second, while there are benefits of using secondary data, the primary trial was not designed for this exploratory analysis and thus many perioperative factors that may have been worthwhile collecting were not recorded. Third, given that patients were only assessed 3 months after surgery, we do not know if our findings are also predictive of pain at a much longer follow-up period after surgery (i.e., 1 year). However, previously published data suggest that this pain may persist for years to follow [
18]. Furthermore, while the prospective nature of this study, together with a 100% response rate to the study questionnaires, makes it less prone to several sources of bias, a selection bias may have occurred since patients in this cohort were only those who agreed to participate in the randomized controlled trial at the participating centers. Prospective data collection also does not eliminate information bias and measurement errors, although our use of validated questionnaires probably mitigated this risk. Lastly, we conducted many statistical tests both in our univariate and multivariate analysis, which raises the possibility of spurious findings by chance.
While ongoing efforts are aimed at prolonging life for patients with cancer, it is also important that resources be directed at improving the quality of life for these survivors. Persistent pain after breast cancer surgery is a detrimental yet common complication, which should be addressed preemptively. While this exploratory analysis provides both confirmatory and novel findings, larger studies are needed to verify these predictive factors, and whether interventions modifying these factors could indeed adjust the risk of developing persistent pain.