Addressing Peri-Device Leaks in Next-Generation Transcatheter Left Atrial Appendage Occluders: An Open Question
Abstract
:1. Introduction
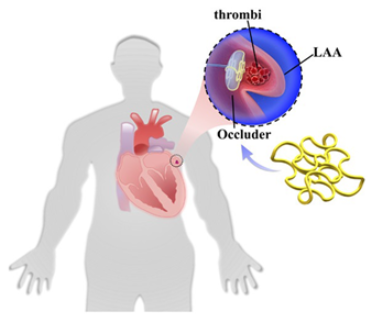
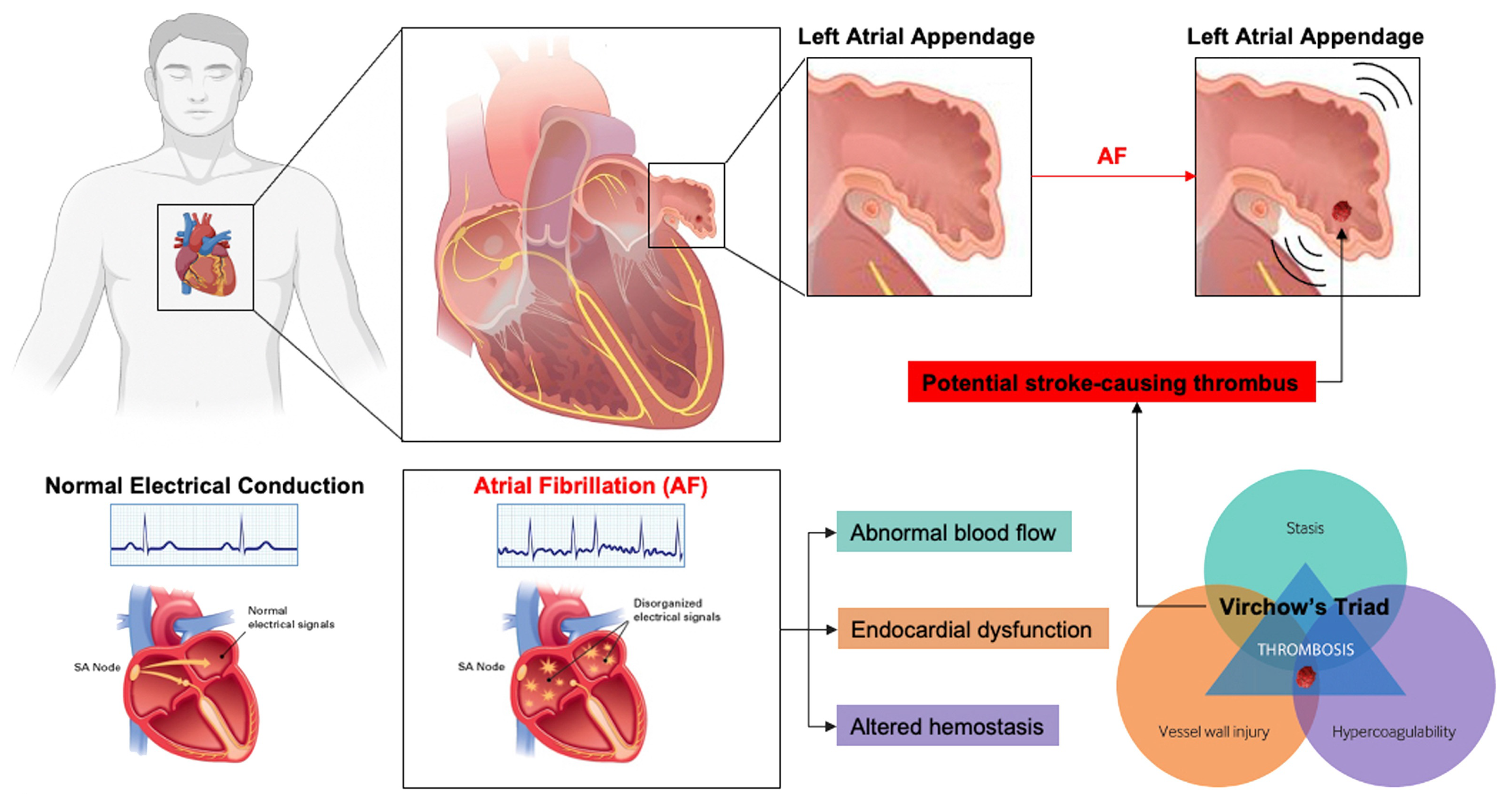
1.1. Left Atrial Appendage and Stroke Prevention in Patients Experiencing Atrial Fibrillation
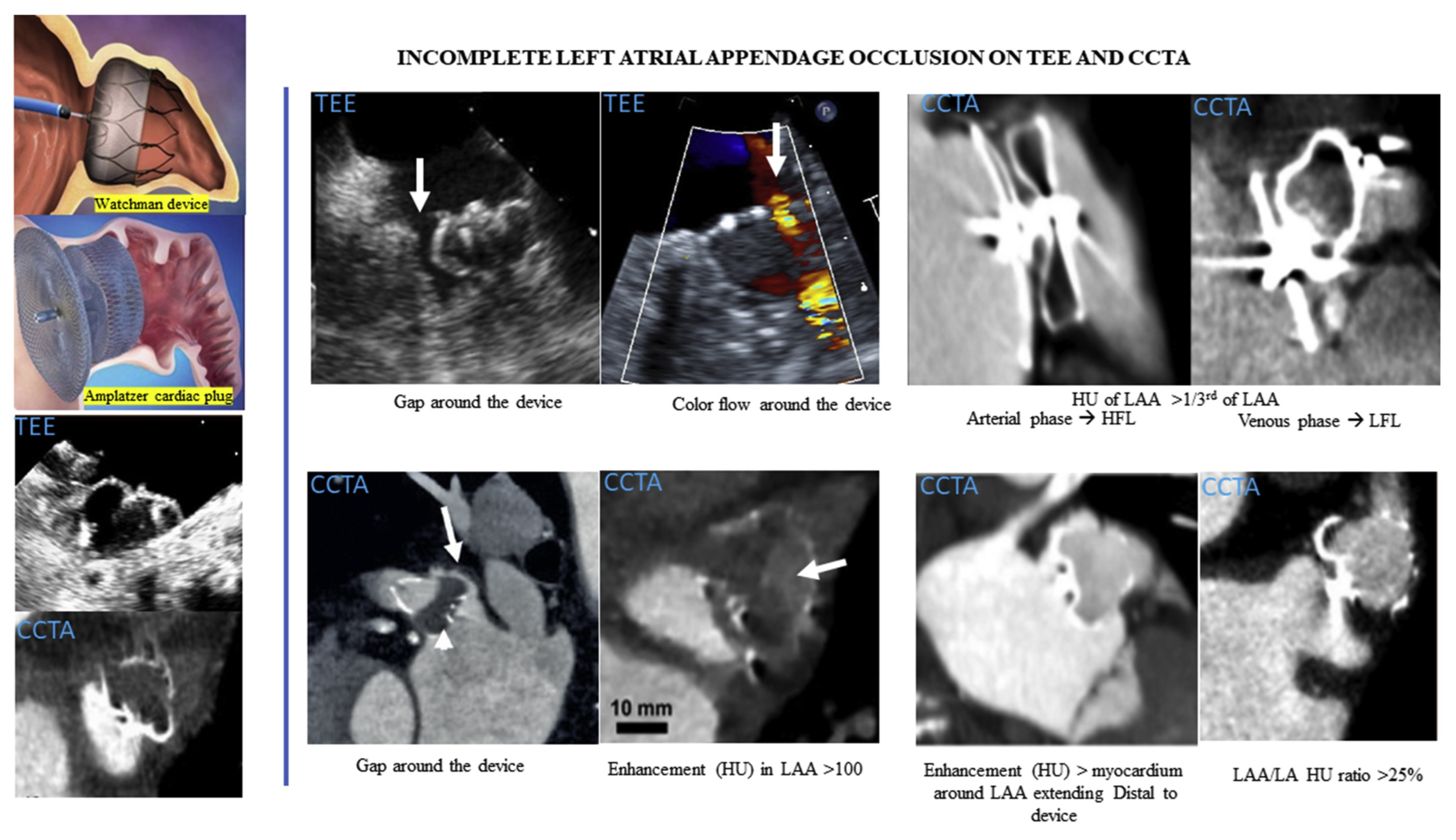
1.2. Definition and Implications of Persistent PDL
1.3. Imaging Modalities for PDL Detection
1.4. PDL Incidence in Patients Experiencing AF
2. Current Solutions for LAA Closure
2.1. WATCHMAN, Amplatzer Amulet, and LARIAT
2.2. CLAAS System
2.3. Occlutech
2.4. LAmbre
2.5. Appligator
2.6. Cormos
2.7. Ultraseal
2.8. Omega
2.9. Laminar
| Device Image | Name, Manufacturer | Key Features | Challenges/Limitations |
|---|---|---|---|
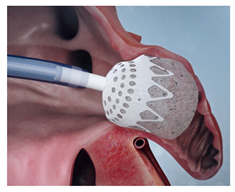 (Photo courtesy Conformal Medical) | CLAAS [81] Conformal Medical | PCUU foam matrix surrounding nitinol endoskeleton; enhanced conformity; atraumatic surface, due to foam folding at distal edge. | Limited to two sizes; may not cover full spectrum of LAA anatomies; long-term efficacy and safety data still being collected. |
 (Photo courtesy Occlutech International) | LAA Oclutech [82] Occlutech International | Self-expanding nitinol wire mesh with conical shape; distally attached loops and additional anchoring elements on flank; delivered via steerable guiding sheath allowing 180-degree rotation. | Distal loops penetrated LAA lobe in some animals; requires oversizing by 3–4 mm; learning curve for proper sizing and deployment. |
 (Photo courtesy LifeTech Scientific) | LAmbre LifeTech Scientific | Double-disc design with umbrella-shaped anchoring disc and cover disc; eight distal hooks and eight U-shaped ends; multiple sizes for LAA up to 40 mm, smaller delivery sheath (10.4–12.3 Fr). | Cases of device misalignment leading to significant PDL and minor PDL not uncommon. |
 (Photo courtesy Append Medical) | Appligator Append Medical | Uses suction mechanism to invert LAA; applies suture loop from inside left atrium; metal-free approach; capitalizes on LAA’s natural tendency to shrink. | Risk of thrombosis due to altered hemodynamics; potential risk of tissue rupture during LAA inversion; optimal patient selection criteria not established. |
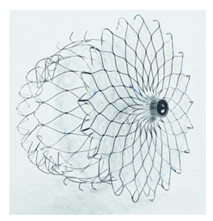 (Photo courtesy Cormos Medical) | Cormos Occluder Cormos Medical | Double membrane system for flexible adaptation; between 15 and 21 J-hooks for anchoring, electropolished surfaces; retrievable at all implantation phases. | Potential learning curve for new deployment technique; long-term durability and efficacy need further evaluation. |
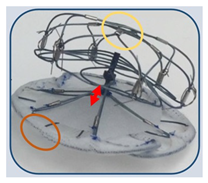 (Photo courtesy Cardia) | Ultraseal [89] Cardia | Flexible design with reduced radial force, allowing adaptation to complex LAA; compact structure with lengths between 10 and 18 mm, available in 10 sizes (16–34 mm). | Limited long-term data available, due to small sample sizes and short follow-up periods; optimal post-procedural antithrombotic regimen not yet established. |
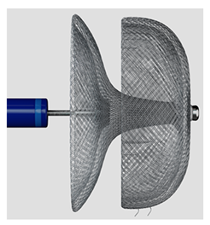 (Photo courtesy Eclipse Medical) | Omega [94] Eclipse Medical | Self-expanding nitinol mesh structure with platinum coating; highly flexible connecting waist to adapt to complex LAA morphologies; available in sizes from 14 to 30 mm, including a unique 14 mm size for small landing zones. | Complex design with multiple components may impact long-term durability; flexible waist could potentially allow more device movement within the LAA; fabric-covered disc relies on variable endothelialization process. |
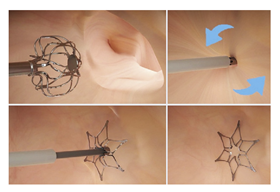 (Photo courtesy Biosense Webster) | Laminar LAAX System [93] Biosense Webster, Inc. | Rotational closure mechanism with integrated ball and lock design; self-expanding nitinol ball structure for LAA tissue engagement; no hooks or barbs required for anchoring, minimal LA-facing surface area; 18-F double-curve steerable guide system; two device sizes (12 mm and 16 mm). | Long-term durability data still being collected; currently in pivotal trial phase; learning curve for novel rotational closure technique; limited real-world experience outside clinical trials; optimal patient selection criteria still being established. |
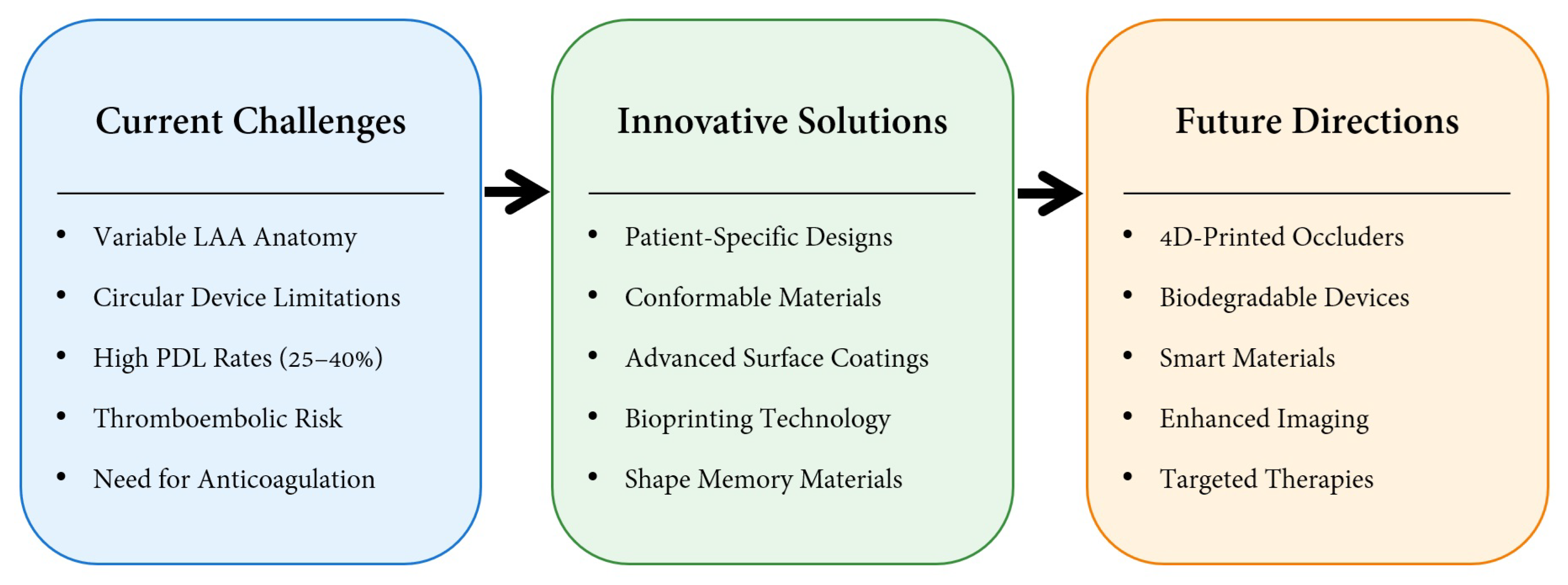
3. Discussion
3.1. Surface Modification
3.2. Upconversion 3D Bioprinting
3.3. Patient-Specific Occluder
3.4. Shape Memory Biodegradable
| Device Image | Device/Method | Key Features | Challenges/Limitations |
|---|---|---|---|
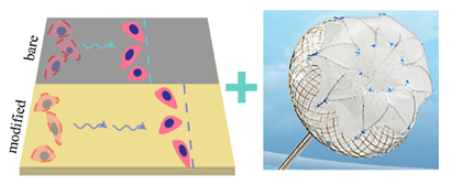 | surface modification [104] | Advanced surface modification, using alternating nanoscale layers of TiN on NiTi alloy, enhancing endothelial cell migration and accelerating endothelialization; successful clinical implantation. | Lack of long-term studies on the effects and potential complications of the nanocoating in humans; limited clinical evidence, with only one reported case. |
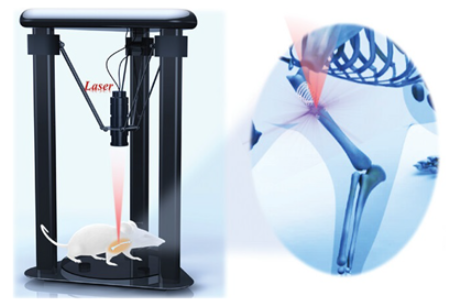 | upconversion 3D bioprinting [106] | Patient-specific 3D-printed implants; enhanced tissue integration; in vivo non-invasive fabrication. | Long-term concerns; precision challenges for bioprinting in cardiac environment; complex regulatory pathway. |
 | patient-specific occluder | Customized geometry for each patient’s LAA; thin-walled, inflatable balloons made from soft, biocompatible materials; incorporates biomimetic surface patterns for enhanced adhesion; superior anchoring force and reduced leak rates in vitro. | Complex manufacturing process; challenges in ensuring consistent quality across custom devices; long-term durability of soft materials needs evaluation; potentially higher cost, due to customization. |
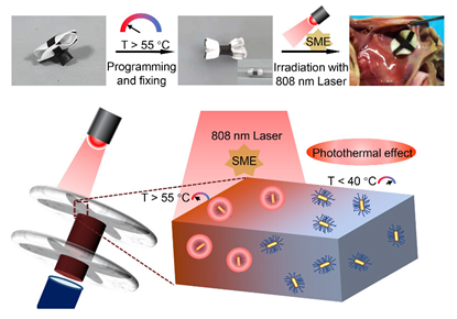 | shape memory biodegradable [110] | NIR light-triggered shape memory effect for controlled deployment; fully biodegradable composition, using PLCL-GNR/PEG composite; maintained mechanical strength during initial tissue regeneration phase. | Long-term effects of gold nanoparticles require further study; NIR light stimulation may complicate clinical deployment procedures; primarily tested in vitro/ex vivo; needs further in vivo validation. |
 | bio-inspired absorbable [111] | Bio-inspired design with wavy microstructures mimicking collagen fibrils, exhibiting “J-shaped” stress–strain behavior; 4D printing enabling patient-specific geometries and shape transformation. | Need for optimization of shape memory transition temperature; preliminary nature of in vitro feasibility testing. |
3.5. Bio-Inspired Absorbable
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AF | atrial fibrillation |
| CT | computed tomography |
| FDA | Food and Drug Administration |
| ICE | intracardiac echocardiography |
| LAA | left atrial appendage |
| NCDR | National Cardiovascular Data Registry |
| OAC | oral anticoagulant |
| PDL | peri-device leak |
| PS | patient-specific |
| RCT | randomized controlled trials |
| TEE | trans-esophageal echocardiogram |
References
- Bergau, L.; Bengel, P.; Sciacca, V.; Fink, T.; Sohns, C.; Sommer, P. Atrial fibrillation and heart failure. J. Clin. Med. 2022, 11, 2510. [Google Scholar] [CrossRef]
- Escudero-Martinez, I.; Morales-Caba, L.; Segura, T. Atrial fibrillation and stroke: A review and new insights. Trends Cardiovasc. Med. 2023, 33, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Ganjehei, L.; Massumi, A.; Razavi, M.; Rasekh, A. Stroke prevention in nonvalvular atrial fibrillation. Tex. Heart Inst. J. 2011, 38, 350. [Google Scholar]
- Katritsis, D.G.; Gersh, B.J.; Camm, A.J. Anticoagulation in atrial fibrillation–current concepts. Arrhythmia Electrophysiol. Rev. 2015, 4, 100. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar]
- Komen, J.J.; Pottegård, A.; Mantel-Teeuwisse, A.K.; Forslund, T.; Hjemdahl, P.; Wettermark, B.; Hallas, J.; Olesen, M.; Bennie, M.; Mueller, T.; et al. Oral anticoagulants in patients with atrial fibrillation at low stroke risk: A multicentre observational study. Eur. Heart J. 2022, 43, 3528–3538. [Google Scholar] [CrossRef] [PubMed]
- Agasthi, P.; Pujari, S.H. Peri-and Post-procedural Anticoagulation with Left Atrial Appendage Occlusion Devices. Heart Int. 2023, 17, 54. [Google Scholar] [PubMed]
- John, R.M.; Sharma, D. Contraindication to Anticoagulation in Nonvalvular Atrial Fibrillation: Are We Still to Fear the Clot and Not the Bleed? JACC Clin. Electrophysiol. 2019, 5, 1393–1395. [Google Scholar] [CrossRef] [PubMed]
- Lucà, F.; Oliva, F.; Abrignani, M.G.; Di Fusco, S.A.; Parrini, I.; Canale, M.L.; Giubilato, S.; Cornara, S.; Nesti, M.; Rao, C.M.; et al. Management of patients treated with direct oral anticoagulants in clinical practice and challenging scenarios. J. Clin. Med. 2023, 12, 5955. [Google Scholar] [CrossRef] [PubMed]
- Ballestri, S.; Romagnoli, E.; Arioli, D.; Coluccio, V.; Marrazzo, A.; Athanasiou, A.; Di Girolamo, M.; Cappi, C.; Marietta, M.; Capitelli, M. Risk and management of bleeding complications with direct oral anticoagulants in patients with atrial fibrillation and venous thromboembolism: A narrative review. Adv. Ther. 2023, 40, 41–66. [Google Scholar] [CrossRef]
- Kong, B.; Liu, Y.; Huang, H.; Jiang, H.; Huang, C. Left atrial appendage closure for thromboembolism prevention in patients with atrial fibrillation: Advances and perspectives. J. Thorac. Dis. 2015, 7, 199. [Google Scholar] [PubMed]
- Sulague, R.M.; Whitham, T.; Danganan, L.M.L.; Effiom, V.; Candelario, K.; Latif, N.; Hameed, I. The Left Atrial Appendage and Atrial Fibrillation—A Contemporary Review. J. Clin. Med. 2023, 12, 6909. [Google Scholar] [CrossRef] [PubMed]
- Sarzani, R.; Allevi, M.; Di Pentima, C.; Schiavi, P.; Spannella, F.; Giulietti, F. Role of cardiac natriuretic peptides in heart structure and function. Int. J. Mol. Sci. 2022, 23, 14415. [Google Scholar] [CrossRef]
- Kanderian, A.S.; Gillinov, A.M.; Pettersson, G.B.; Blackstone, E.; Klein, A.L. Success of surgical left atrial appendage closure: Assessment by transesophageal echocardiography. J. Am. Coll. Cardiol. 2008, 52, 924–929. [Google Scholar] [CrossRef]
- Alkhouli, M.; Busu, T.; Shah, K.; Osman, M.; Alqahtani, F.; Raybuck, B. Incidence and clinical impact of device-related thrombus following percutaneous left atrial appendage occlusion: A meta-analysis. JACC Clin. Electrophysiol. 2018, 4, 1629–1637. [Google Scholar] [CrossRef]
- Busu, T.; Khan, S.U.; Alhajji, M.; Alqahtani, F.; Holmes, D.R.; Alkhouli, M. Observed versus expected ischemic and bleeding events following left atrial appendage occlusion. Am. J. Cardiol. 2020, 125, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.J.; Du, C.; Wang, Y.; Agarwal, V.; Varosy, P.D.; Masoudi, F.A.; Holmes, D.R.; Reddy, V.Y.; Price, M.J.; Curtis, J.P.; et al. Patient-level analysis of Watchman left atrial appendage occlusion in practice versus clinical trials. Cardiovasc. Interv. 2022, 15, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Dukkipati, S.R.; Holmes Jr, D.R.; Doshi, S.K.; Kar, S.; Singh, S.M.; Gibson, D.; Price, M.J.; Natale, A.; Mansour, M.; Sievert, H.; et al. Impact of peridevice leak on 5-year outcomes after left atrial appendage closure. J. Am. Coll. Cardiol. 2022, 80, 469–483. [Google Scholar] [CrossRef]
- Holmes, D.R.; Reddy, V.Y.; Gordon, N.T.; Delurgio, D.; Doshi, S.K.; Desai, A.J.; Stone, J.E.; Kar, S. Long-term safety and efficacy in continued access left atrial appendage closure registries. J. Am. Coll. Cardiol. 2019, 74, 2878–2889. [Google Scholar] [CrossRef]
- Simard, T.; Jung, R.G.; Lehenbauer, K.; Piayda, K.; Pracoń, R.; Jackson, G.G.; Flores-Umanzor, E.; Faroux, L.; Korsholm, K.; Chun, J.K.; et al. Predictors of device-related thrombus following percutaneous left atrial appendage occlusion. J. Am. Coll. Cardiol. 2021, 78, 297–313. [Google Scholar] [CrossRef]
- Alkhouli, M.; De Backer, O.; Ellis, C.R.; Nielsen-Kudsk, J.E.; Sievert, H.; Natale, A.; Lakkireddy, D.; Holmes, D.R. Peridevice leak after left atrial appendage occlusion: Incidence, mechanisms, clinical impact, and management. Cardiovasc. Interv. 2023, 16, 627–642. [Google Scholar]
- Jang, S.J.; Wong, S.C.; Mosadegh, B. Leaks after left atrial appendage closure: Ignored or neglected? Cardiology 2021, 146, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Mosadegh, B.; Jang, S.J. Expanding beyond conventional indications for left atrial appendage closure. AsiaIntervention 2023, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, M.R.; Eid, M.M.; Abuelazm, M.; Al-Abdouh, A.; Najim, M.; Hassan, A.R.; El-Sakka, A.A.; Renjithal, S.L.M.; Malik, M.A.; Mohamed, S.; et al. Meta-Analysis of the Outcomes of Peri-Device Leak After Left Atrial Appendage Closure. Am. J. Cardiol. 2023, 204, 325–332. [Google Scholar] [CrossRef] [PubMed]
- He, Y.G.; Yang, S.H.; Xu, L.; Wang, Y.; Qin, X.T.; Chen, P.P.; Zhao, Y.J. The Effect of Peri-Device Leaks on Ischaemic Stroke/Transient Ischaemic Attack/Systemic Embolism after Left Atrial Appendage Closure: A Systematic Review and Meta-Analysis. Cardiology 2023, 148, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Raphael, C.E.; Friedman, P.A.; Saw, J.; Pislaru, S.V.; Munger, T.M.; Holmes Jr, D.R. Residual leaks following percutaneous left atrial appendage occlusion: Assessment and management implications. EuroIntervention 2017, 13, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Korsholm, K.; Jensen, J.M.; Nørgaard, B.L.; Samaras, A.; Saw, J.; Berti, S.; Tzikas, A.; Nielsen-Kudsk, J.E. Peridevice leak following Amplatzer left atrial appendage occlusion: Cardiac computed tomography classification and clinical outcomes. Cardiovasc. Interv. 2021, 14, 83–93. [Google Scholar]
- Viles-Gonzalez, J.F.; Kar, S.; Douglas, P.; Dukkipati, S.; Feldman, T.; Horton, R.; Holmes, D.; Reddy, V.Y. The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: A PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) substudy. J. Am. Coll. Cardiol. 2012, 59, 923–929. [Google Scholar] [PubMed]
- Samaras, A.; Papazoglou, A.S.; Balomenakis, C.; Bekiaridou, A.; Moysidis, D.V.; Patsiou, V.; Orfanidis, A.; Giannakoulas, G.; Kassimis, G.; Fragakis, N.; et al. Residual leaks following percutaneous left atrial appendage occlusion and outcomes: A meta-analysis. Eur. Heart J. 2024, 45, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M.; Du, C.; Killu, A.; Simard, T.; Noseworthy, P.A.; Friedman, P.A.; Curtis, J.P.; Freeman, J.V.; Holmes, D.R. Clinical impact of residual leaks following left atrial appendage occlusion: Insights from the NCDR LAAO registry. Clin. Electrophysiol. 2022, 8, 766–778. [Google Scholar] [CrossRef]
- Święczkowski, M.; Dąbrowski, E.J.; Muszyński, P.; Pogorzelski, P.; Jemielita, P.; Dudzik, J.M.; Januszko, T.; Duzinkiewicz, M.; Południewski, M.; Kuźma, Ł.; et al. A Comprehensive Review of Percutaneous and Surgical Left Atrial Appendage Occlusion. J. Cardiovasc. Dev. Dis. 2024, 11, 234. [Google Scholar] [PubMed]
- Whitlock, R.P.; Belley-Cote, E.P.; Paparella, D.; Healey, J.S.; Brady, K.; Sharma, M.; Reents, W.; Budera, P.; Baddour, A.J.; Fila, P.; et al. Left atrial appendage occlusion during cardiac surgery to prevent stroke. N. Engl. J. Med. 2021, 384, 2081–2091. [Google Scholar] [CrossRef]
- Caliskan, E.; Cox, J.L.; Holmes Jr, D.R.; Meier, B.; Lakkireddy, D.R.; Falk, V.; Salzberg, S.P.; Emmert, M.Y. Interventional and surgical occlusion of the left atrial appendage. Nat. Rev. Cardiol. 2017, 14, 727–743. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Holmes, D.; Doshi, S.K.; Neuzil, P.; Kar, S. Safety of percutaneous left atrial appendage closure: Results from the Watchman Left Atrial Appendage System for Embolic Protection in Patients with AF (PROTECT AF) clinical trial and the Continued Access Registry. Circulation 2011, 123, 417–424. [Google Scholar] [CrossRef]
- Ansari, U.; Brachmann, J.; Lewalter, T.; Zeymer, U.; Sievert, H.; Ledwoch, J.; Geist, V.; Hochadel, M.; Schneider, S.; Senges, J.; et al. LAA occlusion is effective and safe in very high-risk atrial fibrillation patients with prior stroke: Results from the multicentre German LAARGE registry. Clin. Res. Cardiol. 2024, 113, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Behnes, M.; Akin, I.; Sartorius, B.; Fastner, C.; El-Battrawy, I.; Borggrefe, M.; Haubenreisser, H.; Meyer, M.; Schoenberg, S.O.; Henzler, T. -LAA Occluder View for post-implantation Evaluation (LOVE)-standardized imaging proposal evaluating implanted left atrial appendage occlusion devices by cardiac computed tomography. BMC Med. Imaging 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R.; Reddy, V.Y.; Turi, Z.G.; Doshi, S.K.; Sievert, H.; Buchbinder, M.; Mullin, C.M.; Sick, P. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomised non-inferiority trial. Lancet 2009, 374, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. Left atrial appendage closure versus direct oral anticoagulants in high-risk patients with atrial fibrillation. J. Am. Coll. Cardiol. 2020, 75, 3122–3135. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Jiang, C.; Lin, P.; Yu, Y.; Li, J.; Zhao, F.; Hu, H.; Sun, S. Management of complications associated with percutaneous left atrial appendage closure with or without ablation: Experience from 512 cases over a 4-year period. Front. Cardiovasc. Med. 2024, 11, 1388024. [Google Scholar] [CrossRef]
- Clarke, J.R.D.; Higgins, A.Y.; Wang, Y.; Faridi, K.F.; Curtis, J.A.; Freeman, J.V.; Friedman, D.J. Impact of preprocedure imaging for left atrial appendage occlusion: Insights from the NCDR LAAO registry. Cardiovasc. Interv. 2023, 16, 1317–1328. [Google Scholar]
- Kleinecke, C.; Park, J.W.; Gödde, M.; Zintl, K.; Schnupp, S.; Brachmann, J. Twelve-month follow-up of left atrial appendage occlusion with Amplatzer Amulet. Cardiol. J. 2017, 24, 131–138. [Google Scholar] [CrossRef]
- Meier, B.; Blaauw, Y.; Khattab, A.A.; Lewalter, T.; Sievert, H.; Tondo, C.; Glikson, M.; ESC Scientific Document Group Lip Gregory YH (UK) Lopez-Minguez Jose (Spain) Roffi Marco (Switzerland) Israel Carsten (Germany) Dudek Dariusz (Poland) Savelieva Irene (on behalf of EP-Europace, U. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion. Europace 2014, 16, 1397–1416. [Google Scholar] [CrossRef]
- Brundel, B.J.; Ai, X.; Hills, M.T.; Kuipers, M.F.; Lip, G.Y.; de Groot, N. Atrial fibrillation. Nat. Rev. Dis. Prim. 2022, 8, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Mendez, K.; Kennedy, D.G.; Wang, D.D.; O’Neill, B.; Roche, E.T. Left atrial appendage occlusion: Current stroke prevention strategies and a shift toward data-driven, patient-specific approaches. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100405. [Google Scholar] [CrossRef]
- Glikson, M.; Wolff, R.; Hindricks, G.; Mandrola, J.; Camm, A.J.; Lip, G.Y.; Fauchier, L.; Betts, T.R.; Lewalter, T.; Saw, J.; et al. EHRA/EAPCI expert consensus statement on catheter-based left atrial appendage occlusion–an update. EP Eur. 2020, 22, 184. [Google Scholar]
- Helal, B.; Khan, J.; AlJayar, D.; Khan, M.S.; Alabdaljabar, M.S.; Asad, Z.U.A.; DeSimone, C.V.; Deshmukh, A. Risk factors, clinical implications, and management of peridevice leak following left atrial appendage closure: A systematic review. J. Interv. Card. Electrophysiol. 2024, 67, 865–885. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Doshi, S.K.; Kar, S.; Gibson, D.N.; Price, M.J.; Huber, K.; Horton, R.P.; Buchbinder, M.; Neuzil, P.; Gordon, N.T.; et al. 5-year outcomes after left atrial appendage closure: From the PREVAIL and PROTECT AF trials. J. Am. Coll. Cardiol. 2017, 70, 2964–2975. [Google Scholar] [CrossRef]
- Korsholm, K.; Berti, S.; Iriart, X.; Saw, J.; Wang, D.D.; Cochet, H.; Chow, D.; Clemente, A.; De Backer, O.; Møller Jensen, J.; et al. Expert recommendations on cardiac computed tomography for planning transcatheter left atrial appendage occlusion. Cardiovasc. Interv. 2020, 13, 277–292. [Google Scholar] [CrossRef]
- Sedaghat, A.; Nickenig, G.; Schrickel, J.W.; Ince, H.; Schmidt, B.; Protopopov, A.V.; Betts, T.R.; Gori, T.; Sievert, H.; Mazzone, P.; et al. Incidence, predictors and outcomes of device-related thrombus after left atrial appendage closure with the WATCHMAN device—insights from the EWOLUTION Real World Registry. Catheter. Cardiovasc. Interv. 2021, 97, E1019–E1024. [Google Scholar] [CrossRef] [PubMed]
- Hildick-Smith, D.; Landmesser, U.; Camm, A.J.; Diener, H.C.; Paul, V.; Schmidt, B.; Settergren, M.; Teiger, E.; Nielsen-Kudsk, J.E.; Tondo, C. Left atrial appendage occlusion with the Amplatzer™ Amulet™ device: Full results of the prospective global observational study. Eur. Heart J. 2020, 41, 2894–2901. [Google Scholar] [CrossRef] [PubMed]
- Nielsen-Kudsk, J.E.; Berti, S.; Caprioglio, F.; Ronco, F.; Arzamendi, D.; Betts, T.; Tondo, C.; Christen, T.; Allocco, D.J. Intracardiac echocardiography to guide Watchman FLX implantation: The ICE LAA study. Cardiovasc. Interv. 2023, 16, 643–651. [Google Scholar]
- Pastormerlo, L.E.; De Caterina, A.R.; Esposito, A.; Korsholm, K.; Berti, S. State-of-the-Art of Transcatheter Left Atrial Appendage Occlusion. J. Clin. Med. 2024, 13, 939. [Google Scholar] [CrossRef]
- Lakkireddy, D.; Thaler, D.; Ellis, C.R.; Swarup, V.; Sondergaard, L.; Carroll, J.; Gold, M.R.; Hermiller, J.; Diener, H.C.; Schmidt, B.; et al. Amplatzer Amulet left atrial appendage occluder versus Watchman device for stroke prophylaxis (Amulet IDE): A randomized, controlled trial. Circulation 2021, 144, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. 4-year outcomes after left atrial appendage closure versus nonwarfarin oral anticoagulation for atrial fibrillation. J. Am. Coll. Cardiol. 2022, 79, 1–14. [Google Scholar] [CrossRef]
- Rajiah, P.; Alkhouli, M.; Thaden, J.; Foley, T.; Williamson, E.; Ranganath, P. Pre-and postprocedural CT of transcatheter left atrial appendage closure devices. Radiographics 2021, 41, 680–698. [Google Scholar] [CrossRef]
- Qamar, S.R.; Jalal, S.; Nicolaou, S.; Tsang, M.; Gilhofer, T.; Saw, J. Comparison of cardiac computed tomography angiography and transoesophageal echocardiography for device surveillance after left atrial appendage closure. EuroIntervention 2019, 15, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Banga, S.; Osman, M.; Sengupta, P.P.; Benjamin, M.M.; Shrestha, S.; Challa, A.; Zeb, I.; Kadiyala, M.; Mills, J.; Balla, S.; et al. CT assessment of the left atrial appendage post-transcatheter occlusion–a systematic review and meta analysis. J. Cardiovasc. Comput. Tomogr. 2021, 15, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Bonanni, M.; Trimarchi, G.; Benedetti, G.; D’Agostino, A.; Iuliano, G.; Manzo, R.; Capasso, R.; Cerone, E.; Paradossi, U.; Berti, S.; et al. Standardized 3D Transoesophageal Echocardiography Manoeuvre for Enhanced Tenting Height Evaluation During Transcatheter Mitral Valve Edge-to-Edge Repair. J. Clin. Med. 2024, 13, 6525. [Google Scholar] [CrossRef]
- Della Rocca, D.G.; Magnocavallo, M.; Gianni, C.; Mohanty, S.; Al-Ahmad, A.; Bassiouny, M.; Denora, M.; La Fazia, V.M.; Lavalle, C.; Gallinghouse, G.J.; et al. Three-dimensional intracardiac echocardiography for left atrial appendage sizing and percutaneous occlusion guidance. Europace 2024, 26, euae010. [Google Scholar] [CrossRef] [PubMed]
- Serpa, F.; Rivera, A.; Fernandes, J.M.; Braga, M.A.P.; Araújo, B.; Ferreira Felix, I.; Ferro, E.G.; Zimetbaum, P.J.; d’Avila, A.; Kramer, D.B. Intracardiac vs transesophageal echocardiography for left atrial appendage occlusion: An updated systematic review and meta-analysis. Heart Rhythm 2024, 24, 1547–5271. [Google Scholar] [CrossRef]
- Wazni, O.M.; Saliba, W.I.; Nair, D.G.; Marijon, E.; Schmidt, B.; Hounshell, T.; Ebelt, H.; Skurk, C.; Oza, S.; Patel, C.; et al. Left atrial appendage closure after ablation for atrial fibrillation. N. Engl. J. Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Saw, J.; Holmes, D.R.; Cavalcante, J.L.; Freeman, J.V.; Goldsweig, A.M.; Kavinsky, C.J.; Moussa, I.D.; Munger, T.M.; Price, M.J.; Reisman, M.; et al. SCAI/HRS expert consensus statement on transcatheter left atrial appendage closure. Cardiovasc. Interv. 2023, 16, 1384–1400. [Google Scholar]
- Boersma, L.V.; Schmidt, B.; Betts, T.R.; Sievert, H.; Tamburino, C.; Teiger, E.; Pokushalov, E.; Kische, S.; Schmitz, T.; Stein, K.M.; et al. Implant success and safety of left atrial appendage closure with the WATCHMAN device: Peri-procedural outcomes from the EWOLUTION registry. Eur. Heart J. 2016, 37, 2465–2474. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Doshi, S.K.; Sadhu, A.; Horton, R.; Osorio, J.; Ellis, C.; Stone Jr, J.; Shah, M.; Dukkipati, S.R.; Adler, S.; et al. Primary outcome evaluation of a next-generation left atrial appendage closure device: Results from the PINNACLE FLX trial. Circulation 2021, 143, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Price, M.J.; Ellis, C.R.; Nielsen-Kudsk, J.E.; Thaler, D.; Gupta, N.; Koulogiannis, K.; Anderson, J.A.; Gage, R.; Lakkireddy, D. Peridevice leak after transcatheter left atrial appendage occlusion: An analysis of the Amulet IDE trial. Cardiovasc. Interv. 2022, 15, 2127–2138. [Google Scholar]
- Korsholm, K.; Jensen, J.M.; Nørgaard, B.L.; Nielsen-Kudsk, J.E. Temporal changes and clinical significance of peridevice leak following left atrial appendage occlusion with Amplatzer devices. Catheter. Cardiovasc. Interv. 2022, 99, 2071–2079. [Google Scholar] [CrossRef]
- Afzal, M.R.; Gabriels, J.K.; Jackson, G.G.; Chen, L.; Buck, B.; Campbell, S.; Sabin, D.F.; Goldner, B.; Ismail, H.; Liu, C.F.; et al. Temporal changes and clinical implications of delayed peridevice leak following left atrial appendage closure. Clin. Electrophysiol. 2022, 8, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Piayda, K.; Sievert, K.; Della Rocca, D.; Adeola, O.; Alkhouli, M.; Yoo, D.; Benito-Gonzalez, T.; Cruz-Gonzalez, I.; Galea, R.; Skurk, C.; et al. Safety and feasibility of peri-device leakage closure after LAAO: An international, multicentre collaborative study: Interventional leakage closure after LAAO. EuroIntervention 2021, 17, e1033. [Google Scholar] [CrossRef] [PubMed]
- Musikantow, D.R.; Shivamurthy, P.; Croft, L.B.; Kawamura, I.; Turagam, M.K.; Whang, W.; Dukkipati, S.R.; Goldman, M.E.; Reddy, V.Y. Transcatheter embolic coils to treat peridevice leaks after left atrial appendage closure. Heart Rhythm 2021, 18, 717–722. [Google Scholar] [CrossRef]
- Killu, A.M.; Gbolabo Adeola, O.; Della Rocca, D.G.; Ellis, C.; Sugrue, A.M.; Simard, T.; Friedman, P.A.; Kawsara, A.; Horton, R.P.; Natale, A.; et al. Leak closure following left atrial appendage exclusion procedures: A multicenter registry. Catheter. Cardiovasc. Interv. 2022, 99, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Della Rocca, D.G.; Murtaza, G.; Di Biase, L.; Akella, K.; Krishnan, S.C.; Magnocavallo, M.; Mohanty, S.; Gianni, C.; Trivedi, C.; Lavalle, C.; et al. Radiofrequency energy applications targeting significant residual leaks after watchman implantation: A prospective, multicenter experience. Clin. Electrophysiol. 2021, 7, 1573–1584. [Google Scholar] [CrossRef]
- Alkhouli, M.; Chaker, Z.; Clemetson, E.; Alqahtani, F.; Al Hajji, M.; Lobban, J.; Sengupta, P.P.; Raybuck, B. Incidence, characteristics and management of persistent peri-device flow after percutaneous left atrial appendage occlusion. Struct. Heart 2019, 3, 491–498. [Google Scholar] [CrossRef]
- Korsholm, K.; Samaras, A.; Andersen, A.; Jensen, J.M.; Nielsen-Kudsk, J.E. The Watchman FLX device: First European experience and feasibility of intracardiac echocardiography to guide implantation. Clin. Electrophysiol. 2020, 6, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Safavi-Naeini, P.; Razavi, M.; Saeed, M.; Rasekh, A.; Massumi, A. A review of the LARIAT suture delivery device for left atrial appendage closure. J. Tehran Univ. Heart Cent. 2015, 10, 69. [Google Scholar]
- Srivastava, M.C.; See, V.Y.; Dawood, M.Y.; Price, M.J. A review of the LARIAT device: Insights from the cumulative clinical experience. Springerplus 2015, 4, 1–13. [Google Scholar] [CrossRef]
- Chow, D.H.; Wong, Y.H.; Park, J.W.; Lam, Y.Y.; De Potter, T.; Rodés-Cabau, J.; Asmarats, L.; Sandri, M.; Sideris, E.; McCaw, T.; et al. An overview of current and emerging devices for percutaneous left atrial appendage closure. Trends Cardiovasc. Med. 2019, 29, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Lakkireddy, D.; Ellis, C.R.; Thaler, D.; Swarup, V.; Gambhir, A.; Hermiller, J.; Nielsen-Kudsk, J.E.; Worthley, S.; Nair, D.; Schmidt, B.; et al. 5-Year Results From the AMPLATZER Amulet Left Atrial Appendage Occluder Randomized Controlled Trial. J. Am. Coll. Cardiol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Korsholm, K.; Kramer, A.; Andersen, A.; Saw, J.; Nørgaard, B.L.; Jensen, J.M.; Nielsen-Kudsk, J.E. Left atrial appendage sealing performance of the Amplatzer Amulet and Watchman FLX device. J. Interv. Card. Electrophysiol. 2023, 66, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Sommer, R.J.; Lamport, R.; Melanson, D.; Devellian, C.; Levine, A.; Cain, C.M.; Kaplan, A.V.; Gray, W.A. Preclinical Assessment of a Novel Conformable Foam-Based Left Atrial Appendage Closure Device. Biomed Res. Int. 2021, 2021, 4556400. [Google Scholar] [CrossRef] [PubMed]
- Turagam, M.K.; Neuzil, P.; Hala, P.; Mraz, T.; Dukkipati, S.R.; Reddy, V.Y. Intracardiac echocardiography-guided left atrial appendage closure with a novel foam-based conformable device: Safety and 1-year outcomes. Clin. Electrophysiol. 2022, 8, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.V.; Devellian, C.; Kim, J.H.; Rotschafer, J.; Levine, A.; Sommer, R.J.; Gray, W.A. Foam-based Left Atrial Appendage Closure (CLAAS) Device: Evaluation in a Chronic Canine Model. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 100555. [Google Scholar] [CrossRef] [PubMed]
- Reinthaler, M.; Grosshauser, J.; Schmidt, T.; Unger, J.; Morgan, R.; Zimmermann, F.; Hartung, J.; Seppelt, C.; Meteva, D.; Haider, W.; et al. Preclinical assessment of a modified Occlutech left atrial appendage closure device in a porcine model. Sci. Rep. 2021, 11, 2988. [Google Scholar] [CrossRef] [PubMed]
- Bellmann, B.; Schnupp, S.; Kuehnlein, P.; Javernik, C.; Kleinecke, C.; Rillig, A.; Landmesser, U.; Brachmann, J.; PARK, J.W. Left atrial appendage closure with the new Occlutech® device: First in man experience and neurological outcome. J. Cardiovasc. Electrophysiol. 2017, 28, 315–320. [Google Scholar] [CrossRef]
- Chen, S.; Chun, K.J.; Bordignon, S.; Weise, F.K.; Nagase, T.; Perrotta, L.; Bologna, F.; Schmidt, B. Left atrial appendage occlusion using LAmbre Amulet and Watchman in atrial fibrillation. J. Cardiol. 2019, 73, 299–306. [Google Scholar] [CrossRef] [PubMed]
- So, C.y.; Li, S.; Fu, G.h.; Chen, W.; Kam, K.; Lee, A.; Chu, H.m.; Xu, Y.w.; Yan, B.; Lam, Y.y. Procedural and short-term outcomes of occluding large left atrial appendages with the LAmbre device: LAmbre for occluding large left atrial appendage. EuroIntervention 2021, 17, 90. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Wang, J.; Liao, Q. A planted LAmbre device gets dislocated during the procedure: A case report with literature review. Authorea Prepr. 2022. [Google Scholar] [CrossRef]
- Asmarats, L.; Masson, J.B.; Pagnotta, P.A.; Cook, S.; Foresti, M.; Ibrahim, R.; Sukiennik, A.; Sabiniewicz, R.; Maffeo, D.; Carballo, J.; et al. Percutaneous left atrial appendage closure with the ultraseal device: Insights from the initial multicenter experience. JACC Cardiovasc. Interv. 2018, 11, 1932–1941. [Google Scholar] [CrossRef] [PubMed]
- Cruz-González, I.; PL, S.F.; Pérez, M.I.B.; Fernández, B.L.S. First-in-man percutaneous closure of left atrial appendage using the Ultraseal II device. Eurointervention J. Eur. Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2019, 15, e816–e817. [Google Scholar] [CrossRef] [PubMed]
- Pivato, C.A.; Liccardo, G.; Sanz-Sanchez, J.; Pelloni, E.; Pujdak, K.; Xuareb, R.G.; Cruz-Gonzalez, I.; Pisano, F.; Scotti, A.; Tarantini, G.; et al. Left atrial appendage closure with the II generation Ultraseal device: An international registry. The LIGATE study. Catheter. Cardiovasc. Interv. 2022, 100, 620–627. [Google Scholar] [CrossRef]
- Ahlgrimm, B.; Pottgiesser, T.; Stachon, P.; Zehender, M.; Bugger, H.; Helbing, T.; Siepe, M.; Grundmann, S.; Bode, C.; Diehl, P. The Cardia Ultraseal left atrial appendage occluder: A case series with significant device-related complications. JACC Cardiovasc. Interv. 2019, 12, 1987–1989. [Google Scholar] [CrossRef]
- De Backer, O.; Hafiz, H.; Fabre, A.; Lertsapcharoen, P.; Srimahachota, S.; Foley, D.; Sondergaard, L. State-of-the-art preclinical testing of the OMEGATM left atrial appendage occluder. Catheter. Cardiovasc. Interv. 2021, 97, E1011–E1018. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, B.; Srimahachota, S.; De Backer, O.; Boonyartavej, S.; Lertsuwunseri, V.; Tumkosit, M.; Søndergaard, L. First-in-human results of the Omega left atrial appendage occluder for patients with non-valvular atrial fibrillation: First-in-human results of the Omega LAA occluder. EuroIntervention 2021, 17, e376. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.X.; Kar, S.; Smith, T.W.; Spangler, T.; Bolling, S.F.; Rogers, J.H. Transcatheter left atrial appendage exclusion: Preclinical and early clinical results with the laminar device. Cardiovasc. Interv. 2023, 16, 1347–1357. [Google Scholar]
- Albors, C.; Mill, J.; Olivares, A.L.; Iriart, X.; Cochet, H.; Camara, O. Impact of occluder device configurations in in-silico left atrial hemodynamics for the analysis of device-related thrombus. PLoS Comput. Biol. 2024, 20, e1011546. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.R.; Doshi, S.K.; Kar, S.; Price, M.J.; Sanchez, J.M.; Sievert, H.; Valderrabano, M.; Reddy, V.Y. Left atrial appendage closure as an alternative to warfarin for stroke prevention in atrial fibrillation: A patient-level meta-analysis. J. Am. Coll. Cardiol. 2015, 65, 2614–2623. [Google Scholar] [CrossRef] [PubMed]
- Alkhouli, M. Over Flexing of the WATCHMAN FLX Muscles in the Real World. Circulation Cardiovasc. Interv. 2024, 17, e014465. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, L.; Bordignon, S.; Dugo, D.; Fürnkranz, A.; Konstantinou, A.; Ricciardi, G.; Pieragnoli, P.; Schmidt, B.; Chun, K.J. Complications from left atrial appendage exclusion devices. J. Atr. Fibrillation 2014, 7, 1034. [Google Scholar]
- Noelck, N.; Papak, J.; Freeman, M.; Paynter, R.; Low, A.; Motu’apuaka, M.; Kondo, K.; Kansagara, D. Effectiveness of left atrial appendage exclusion procedures to reduce the risk of stroke: A systematic review of the evidence. Circulation Cardiovasc. Qual. Outcomes 2016, 9, 395–405. [Google Scholar] [CrossRef]
- Izquierdo-Garcia, D.; Désogère, P.; Philip, A.L.; Mekkaoui, C.; Weiner, R.B.; Catalano, O.A.; Iris Chen, Y.C.; DeFaria Yeh, D.; Mansour, M.; Catana, C.; et al. Detection and characterization of thrombosis in humans using fibrin-targeted positron emission tomography and magnetic resonance. Cardiovasc. Imaging 2022, 15, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Tzolos, E.; Bing, R.; Andrews, J.; MacAskill, M.G.; Tavares, A.A.; Macnaught, G.; Clark, T.; Mills, N.L.; Fujisawa, T.; Nash, J.; et al. Noninvasive in vivo coronary artery thrombus imaging. Cardiovasc. Imaging 2023, 16, 820–832. [Google Scholar] [CrossRef]
- Luis, S.A.; Roper, D.; Incani, A.; Poon, K.; Haqqani, H.; Walters, D.L. Non-Pharmacological Therapy for Atrial Fibrillation: Managing the Left Atrial Appendage. Cardiol. Res. Pract. 2012, 2012, 304626. [Google Scholar] [CrossRef]
- Wunderlich, N.C.; Beigel, R.; Swaans, M.J.; Ho, S.Y.; Siegel, R.J. Percutaneous interventions for left atrial appendage exclusion: Options, assessment, and imaging using 2D and 3D echocardiography. JACC Cardiovasc. Imaging 2015, 8, 472–488. [Google Scholar] [CrossRef]
- Bshennaty, A.; Vogl, B.J.; Bavo, A.M.; Sularz, A.; Kramer, A.D.; Jia, Y.; De Beule, M.; Nielsen-Kudsk, J.E.; De Backer, O.; Alkhouli, M.; et al. Understanding the role of the left atrial appendage on the flow in the atrium. Catheter. Cardiovasc. Interv. 2024, 104, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, W.; Xie, Y.; Li, A.; Wang, X.; Chen, X.; Liu, Q.; Wang, Q.; Zhang, G.; Liu, Q.; et al. Surface modification to enhance cell migration on biomaterials and its combination with 3D structural design of occluders to improve interventional treatment of heart diseases. Biomaterials 2021, 279, 121208. [Google Scholar] [CrossRef]
- Tarabanis, C.; Klapholz, J.; Zahid, S.; Jankelson, L. A systematic review of the use of 3D printing in left atrial appendage occlusion procedures. J. Cardiovasc. Electrophysiol. 2022, 33, 2367–2374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Teng, Z.; Zhou, M.; Yu, X.; Wen, H.; Niu, J.; Liu, Z.; Zhang, Z.; Liu, Y.; Qiu, J.; et al. Upconversion 3D Bioprinting for Noninvasive In Vivo Molding. Adv. Mater. 2024, 36, 2310617. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.S.; Alaie, S.; Sidoti, H.; Auge, J.; Baskaran, L.; Avilés-Fernández, K.; Hollenberg, S.D.; Shepherd, R.F.; Min, J.K.; Dunham, S.N.; et al. Patient-specific design of a soft occluder for the left atrial appendage. Nat. Biomed. Eng. 2018, 2, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.S.; Aubin, C.A.; Wallin, T.J.; Gharaie, S.; Xu, P.A.; Wang, K.; Dunham, S.N.; Mosadegh, B.; Shepherd, R.F. Stereolithography for personalized left atrial appendage occluders. Adv. Mater. Technol. 2018, 3, 1800233. [Google Scholar] [CrossRef]
- Alaie, S.; Robinson, S.S.; Amiri Moghadam, A.A.; Augé, J.; Caprio, A.; Kolli, K.; Al’Aref, S.J.; Min, J.K.; Mosadegh, B.; Dunham, S. Advanced Manufacturing of Patient-Specific Occluders for the Left Atrial Appendage with Minimally Invasive Delivery. Adv. Eng. Mater. 2020, 22, 1901074. [Google Scholar] [CrossRef]
- Xiang, Z.; Zhang, J.; Zhou, C.; Zhang, B.; Chen, N.; Li, M.; Fu, D.; Wang, Y. Near-Infrared Remotely Controllable Shape Memory Biodegradable Occluder Based on Poly (l-lactide-co-ε-caprolactone)/Gold Nanorod Composite. ACS Appl. Mater. Interfaces 2023, 15, 42341–42353. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liu, L.; Liu, Y.; Leng, J. 4D printing of bioinspired absorbable left atrial appendage occluders: A proof-of-concept study. ACS Appl. Mater. Interfaces 2021, 13, 12668–12678. [Google Scholar] [CrossRef] [PubMed]
| Study | Device | Number of Patients | Assessment Timing | PDL Definition | PDL Rate (%) |
|---|---|---|---|---|---|
| OPTION Trial | WATCHMAN FLX | 803 | 3 months | Any PDL | 19.0% |
| 12 months | 20.3% | ||||
| Amulet IDE | Amplatzer Amulet | 801 | 45 days | Any PDL | 37.0% |
| PDL ≥ 3 mm | 11.2% | ||||
| PROTECT AF | WATCHMAN | 485 | 45 days | Any PDL | 40.9% |
| PDL ≥ 3 mm | 13.3% | ||||
| WATCHMAN 2.5 Registry | WATCHMAN 2.5 | 792 | 45 days | Any PDL | 53.9% |
| PDL ≥ 3 mm | 25.9% | ||||
| PINNACLE FLX | WATCHMAN FLX | 400 | 45 days | Any PDL | 17.4% |
| 12 months | 10.5% | ||||
| SURPASS | WATCHMAN FLX | 16,048 | 45 days | Any PDL | 18.0% |
| Device Image | Device Name | Manufacturer | Key Features | Challenges/Limitations |
|---|---|---|---|---|
 (Photo courtesy Boston Scientific) | WATCHMAN FLX and FLX Pro [78] | Boston Scientific | Thromboresistant coating; radiopaque markers; larger size option (FLX Pro). | PDL; risk of device-related thrombus. |
 (Photo courtesy Abbott) | Amplatzer Amulet [78] | Abbott | Lobe (7.5–10 mm); Disk (22–41 mm); enhanced LAA sealing. | PDL, device size, and fit. |
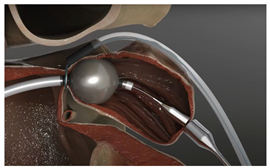 (Photo courtesy SentreHEART) | LARIAT suture delivery | SentreHEART | Minimally invasive; suture loop around LAA; guided by magnetic catheter. | Two access points; large LAA size; specific orientations; scar tissue; risk of incomplete sealing. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roshanfar, M.; Jang, S.-J.; Sinusas, A.; Wong, S.-C.; Mosadegh, B. Addressing Peri-Device Leaks in Next-Generation Transcatheter Left Atrial Appendage Occluders: An Open Question. Surgeries 2025, 6, 15. https://doi.org/10.3390/surgeries6010015
Roshanfar M, Jang S-J, Sinusas A, Wong S-C, Mosadegh B. Addressing Peri-Device Leaks in Next-Generation Transcatheter Left Atrial Appendage Occluders: An Open Question. Surgeries. 2025; 6(1):15. https://doi.org/10.3390/surgeries6010015
Chicago/Turabian StyleRoshanfar, Majid, Sun-Joo Jang, Albert Sinusas, Shing-Chiu Wong, and Bobak Mosadegh. 2025. "Addressing Peri-Device Leaks in Next-Generation Transcatheter Left Atrial Appendage Occluders: An Open Question" Surgeries 6, no. 1: 15. https://doi.org/10.3390/surgeries6010015
APA StyleRoshanfar, M., Jang, S.-J., Sinusas, A., Wong, S.-C., & Mosadegh, B. (2025). Addressing Peri-Device Leaks in Next-Generation Transcatheter Left Atrial Appendage Occluders: An Open Question. Surgeries, 6(1), 15. https://doi.org/10.3390/surgeries6010015









