Overview of Radiological Reporting and Data System (RADS) Guidelines Currently Applicable in Surgery
Abstract
1. Introduction
2. Materials and Methods
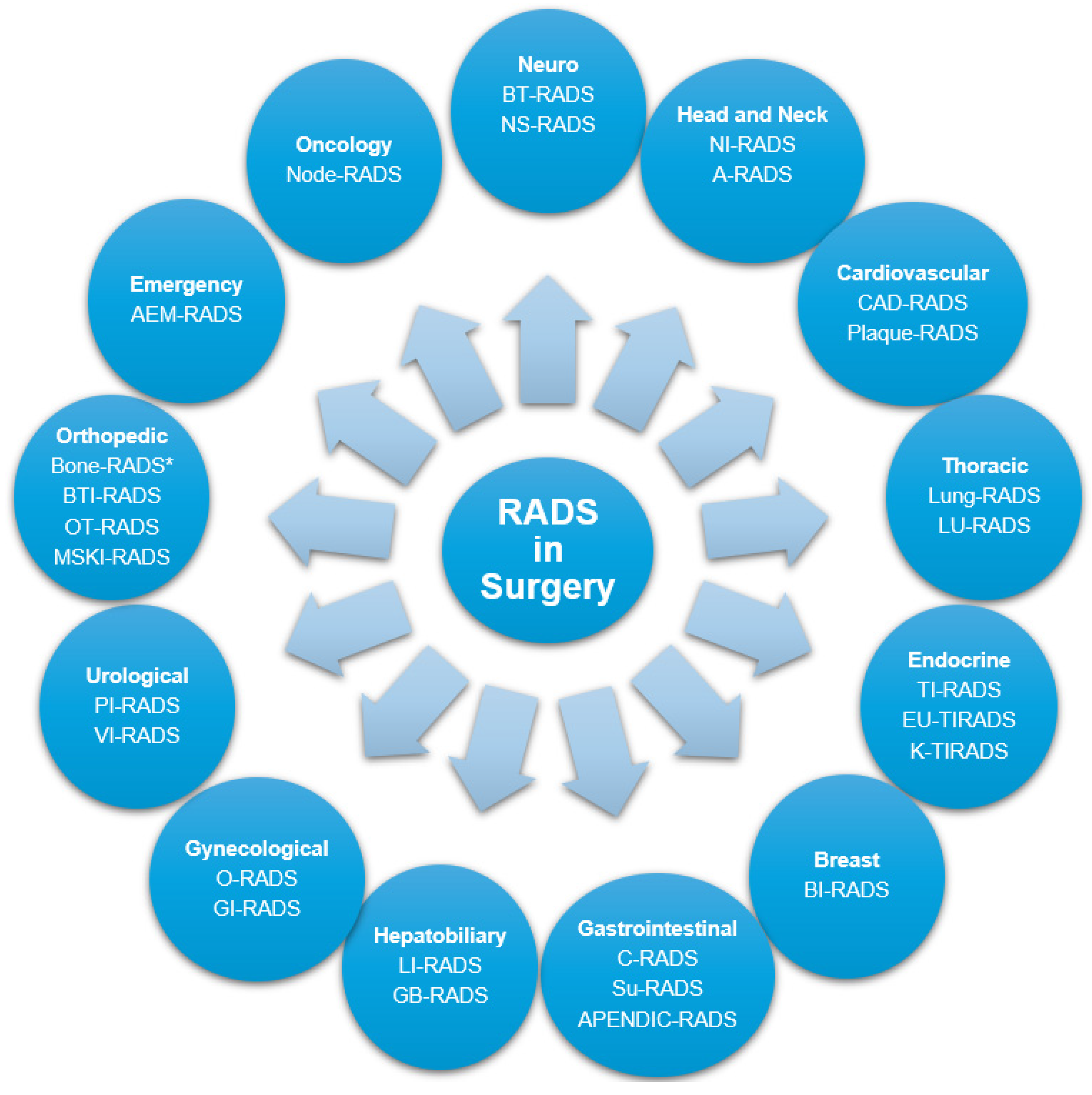
3. Surgery and RADS
3.1. Neurosurgery
3.2. Head and Neck Surgery
3.3. Cardiovascular Surgery
3.4. Thoracic Surgery
3.5. Endocrine Surgery
3.6. Breast Surgery
3.7. Gastrointestinal Surgery
3.8. Hepatobiliary Surgery
3.9. Gynecological Surgery
3.10. Urological Surgery
3.11. Orthopedic Surgery
3.12. Emergency Surgery
3.13. Surgical Oncology
4. Discussion
| RADS | Indication/Type of Surgery | Imaging Techniques | Scores |
|---|---|---|---|
| ACR BI-RADS [5,6] | Breast cancer/Breast surgery | Mammography, US, MRI | 0–6 |
| ACR Bone-RADS [7] | Neoplastic bone lesion/Orthopedic surgery | Radiograph | 0–4 |
| ACR C-RADS [8] | Colon cancer/Gastrointestinal surgery | CT colonography | 0–4 |
| ACR LI-RADS [9,10] | Hepatocellular carcinoma/Hepatobiliary surgery | US, MRI, CT | 1–5, M, TIV |
| ACR Lung-RADS [11] | Lung cancer/Thoracic surgery | CT | 0–4X |
| ACR NI-RADS [12] | Head and neck cancer/Head and neck surgery | CT, MRI | 0–4 |
| ACR O-RADS [13,14] | Ovarian-adnexal mass/Gynecological surgery | US, MRI | 0–5 |
| ACR PI-RADS [15] | Prostate cancer/Urological surgery | MRI | 1–5 |
| ACR TI-RADS [16] | Thyroid cancer/Endocrine surgery | US | 1–5 |
| A-RADS [18] | Cervical adenopathy/Head and neck surgery | US | 1–4 |
| AEM-RADS [19] | Acute abdomen/Emergency surgery | CT | 1–5 |
| APENDIC-RADS [20] | Appendicitis/Gastrointestinal surgery | US | 0–4 |
| Bone-RADS [21,22] | Solitary bone lesion/Orthopedic surgery | CT, MRI | 1–4 |
| BT-RADS [23] | Primary brain tumor/Neurosurgery | MRI | 0–4 |
| BTI-RADS [24] | Solitary bone lesion/Orthopedic surgery | CT, MRI | 1–4 |
| CAD-RADS [17] | Coronary artery disease/Cardiovascular surgery | CT | 0–5 |
| EU-TIRADS [25] | Thyroid cancer/Endocrine surgery | US | 1–5 |
| GB-RADS [26] | Gallbladder cancer/Hepatobiliary surgery | US | 0–5 |
| GI-RADS [27] | Ovarian-adnexal mass/Gynecological surgery | US | 1–5 |
| K-TIRADS [28] | Thyroid cancer/Endocrine surgery | US | 1–5 |
| LU-RADS [29] | Lung cancer/Thoracic surgery | CT | 1–6 |
| MSKI-RADS [30] | Extremity infection/Orthopedic surgery | MRI | 0–6 |
| Node-RADS [31] | Lymph node in cancer/Surgical oncology | CT, MRI | 1–5 |
| NS-RADS [32] | Peripheral neuropathy/Neurosurgery | MRI | I 1–5, N 1–4, E 1–3, D 1–2, PI 1–3 |
| OT-RADS [33] | Neoplastic bone lesion/Orthopedic surgery | MRI | 0–6 |
| Plaque-RADS [34] | Cerebrovascular event in carotid artery disease/Cardiovascular surgery | US, CT, MRI | 1–4 |
| Su-RADS [35] | Gastric cancer/Gastrointestinal surgery | US | 0–6 |
| VI-RADS [36] | Bladder cancer/Urological surgery | MRI | 0–5 |
4.1. Potential Strengths, Limitations, and Requirements for RADS Implementation in Surgery
- Enhanced quality assurance: By establishing clear and standardized assessment criteria, RADS contributes to improved diagnostic accuracy and consistency.
- Optimized imaging pathways: RADS facilitates the selection of the most appropriate and efficient imaging examinations for each patient, minimizing unnecessary procedures.
- Precise diagnostic criteria: RADS provides well-defined imaging features and scoring systems, enabling more accurate and consistent interpretation of findings.
- Standardized patient management: RADS outlines clear recommendations for patient follow-up, surgical procedures, and subsequent management strategies.
- Improved interdisciplinary communication: RADS fosters better communication and collaboration among radiologists, as well as with other healthcare professionals involved in patient care, particularly within multidisciplinary teams.
- Enhanced education and training: RADS serves as a valuable tool for educating and training radiologists on best practices in diagnostic imaging and patient management.
4.2. Artificial Intelligence and RADS in Surgery
4.3. Educational Value and Limitations of This Overview
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- ACR Reporting and Data Systems. Available online: https://www.acr.org/Clinical-Resources/Clinical-Tools-and-Reference/Reporting-and-Data-Systems (accessed on 27 January 2025).
- Bell, D.J. Reporting and Data Systems. Available online: https://radiopaedia.org/articles/reporting-and-data-systems-disambiguation (accessed on 27 January 2025).
- Parillo, M.; Quattrocchi, C.C. Brain Tumor Reporting and Data System (BT-RADS) for the Surveillance of Adult-Type Diffuse Gliomas after Surgery. Surgeries 2024, 5, 764–773. [Google Scholar] [CrossRef]
- Parillo, M.; Quattrocchi, C.C. Node Reporting and Data System 1.0 (Node-RADS) for the Assessment of Oncological Patients’ Lymph Nodes in Clinical Imaging. J. Clin. Med. 2025, 14, 263. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi, C.J.; Sickles, E.A.; Mendelson, E.B.; Morris, E.A. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System; American College of Radiology: Reston, VA, USA, 2013. [Google Scholar]
- Contrast Enhanced Mammography. Available online: https://www.acr.org/-/media/ACR/Files/RADS/BI-RADS/BIRADS_CEM_2022.pdf (accessed on 27 January 2025).
- Caracciolo, J.T.; Ali, S.; Chang, C.Y.; Degnan, A.J.; Flemming, D.J.; Henderson, E.R.; Kransdorf, M.J.; Letson, G.D.; Madewell, J.E.; Murphey, M.D. Bone Tumor Risk Stratification and Management System: A Consensus Guideline from the ACR Bone Reporting and Data System Committee. J. Am. Coll. Radiol. 2023, 20, 1044–1058. [Google Scholar] [CrossRef] [PubMed]
- Yee, J.; Dachman, A.; Kim, D.H.; Kobi, M.; Laghi, A.; McFarland, E.; Moreno, C.; Park, S.H.; Pickhardt, P.J.; Plumb, A.; et al. CT Colonography Reporting and Data System (C-RADS): Version 2023 Update. Radiology 2024, 310, e232007. [Google Scholar] [CrossRef]
- LI-RADS® CT/MRI. Available online: https://Www.Acr.Org/-/Media/ACR/Files/RADS/LI-RADS/LI-RADS-2018-Core.Pdf (accessed on 27 January 2025).
- LI-RADS® CEUS. Available online: https://Www.Acr.Org/-/Media/ACR/Files/RADS/LI-RADS/CEUS-LI-RADS-2017-Core.Pdf (accessed on 27 January 2025).
- Christensen, J.; Prosper, A.E.; Wu, C.C.; Chung, J.; Lee, E.; Elicker, B.; Hunsaker, A.R.; Petranovic, M.; Sandler, K.L.; Stiles, B.; et al. ACR Lung-RADS V2022: Assessment Categories and Management Recommendations. J. Am. Coll. Radiol. 2024, 21, 473–488. [Google Scholar] [CrossRef]
- Aiken, A.H.; Rath, T.J.; Anzai, Y.; Branstetter, B.F.; Hoang, J.K.; Wiggins, R.H.; Juliano, A.F.; Glastonbury, C.; Phillips, C.D.; Brown, R.; et al. ACR Neck Imaging Reporting and Data Systems (NI-RADS): A White Paper of the ACR NI-RADS Committee. J. Am. Coll. Radiol. 2018, 15, 1097–1108. [Google Scholar] [CrossRef]
- Sadowski, E.A.; Thomassin-Naggara, I.; Rockall, A.; Maturen, K.E.; Forstner, R.; Jha, P.; Nougaret, S.; Siegelman, E.S.; Reinhold, C. O-RADS MRI Risk Stratification System: Guide for Assessing Adnexal Lesions from the ACR O-RADS Committee. Radiology 2022, 303, 35–47. [Google Scholar] [CrossRef]
- Strachowski, L.M.; Jha, P.; Phillips, C.H.; Blanchette Porter, M.M.; Froyman, W.; Glanc, P.; Guo, Y.; Patel, M.D.; Reinhold, C.; Suh-Burgmann, E.J.; et al. O-RADS US V2022: An Update from the American College of Radiology’s Ovarian-Adnexal Reporting and Data System US Committee. Radiology 2023, 308, e230685. [Google Scholar] [CrossRef]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef]
- Tessler, F.N.; Middleton, W.D.; Grant, E.G.; Hoang, J.K.; Berland, L.L.; Teefey, S.A.; Cronan, J.J.; Beland, M.D.; Desser, T.S.; Frates, M.C.; et al. ACR Thyroid Imaging, Reporting and Data System (TI-RADS): White Paper of the ACR TI-RADS Committee. J. Am. Coll. Radiol. 2017, 14, 587–595. [Google Scholar] [CrossRef]
- Cury, R.C.; Leipsic, J.; Abbara, S.; Achenbach, S.; Berman, D.; Bittencourt, M.; Budoff, M.; Chinnaiyan, K.; Choi, A.D.; Ghoshhajra, B.; et al. CAD-RADSTM 2.0—2022 Coronary Artery Disease-Reporting and Data System: An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR), and the North America Society of Cardiovascular Imaging (NASCI). JACC Cardiovasc. Imaging 2022, 15, 1974–2001. [Google Scholar] [CrossRef] [PubMed]
- Alamdaran, S.A.; Randian, A.; Rasoulian, B.; Jafarian, A.H.; Aminzadeh, B.; Niroumand, S. Correlation of Sonographic Classification of Neck Adenopathy (A-RADS) and Malignancy. Iran. J. Otorhinolaryngol. 2023, 35, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, A.G.; Başaran, H.; İdilman, İ.S.; Onur, M.R.; Akpınar, E. Introducing AEM-RADS: A Novel Reporting and Data System for Abdominal Emergencies. Abdom. Radiol. 2024, 49, 4175–4184. [Google Scholar] [CrossRef]
- De Queiroz, M.R.G.; Jabour, V.A.; de Souza Junior, J.L.; Paixão, M.R.; da Silva, P.S.D.; Kang, D.W.W.; Barboza, G.C.Y.G.; Bourroul, G.M.; de Lamare, J.M.H.; de Macedo Pontes, I.C.; et al. APENDIC-RADS: An Ultrasound Reporting System for the Diagnosis of Acute Appendicitis. Einstein 2024, 22, eAO1164. [Google Scholar] [CrossRef]
- Chang, C.Y.; Garner, H.W.; Ahlawat, S.; Amini, B.; Bucknor, M.D.; Flug, J.A.; Khodarahmi, I.; Mulligan, M.E.; Peterson, J.J.; Riley, G.M.; et al. Society of Skeletal Radiology—White Paper. Guidelines for the Diagnostic Management of Incidental Solitary Bone Lesions on CT and MRI in Adults: Bone Reporting and Data System (Bone-RADS). Skelet. Radiol. 2022, 51, 1743–1764. [Google Scholar] [CrossRef]
- Haseli, S.; Park, C.; Azhideh, A.; Karande, G.; Chalian, M. Performance and Reliability Comparison: Original vs. Revised Bone Reporting and Data System (Bone-RADS). Skelet. Radiol. 2025; epub ahead of print. [Google Scholar] [CrossRef]
- Weinberg, B.D.; Gore, A.; Shu, H.-K.G.; Olson, J.J.; Duszak, R.; Voloschin, A.D.; Hoch, M.J. Management-Based Structured Reporting of Posttreatment Glioma Response with the Brain Tumor Reporting and Data System. J. Am. Coll. Radiol. 2018, 15, 767–771. [Google Scholar] [CrossRef]
- Ribeiro, G.J.; Gillet, R.; Hossu, G.; Trinh, J.-M.; Euxibie, E.; Sirveaux, F.; Blum, A.; Teixeira, P.A.G. Solitary Bone Tumor Imaging Reporting and Data System (BTI-RADS): Initial Assessment of a Systematic Imaging Evaluation and Comprehensive Reporting Method. Eur. Radiol. 2021, 31, 7637–7652. [Google Scholar] [CrossRef]
- Russ, G.; Bonnema, S.J.; Erdogan, M.F.; Durante, C.; Ngu, R.; Leenhardt, L. European Thyroid Association Guidelines for Ultrasound Malignancy Risk Stratification of Thyroid Nodules in Adults: The EU-TIRADS. Eur. Thyroid. J. 2017, 6, 225–237. [Google Scholar] [CrossRef]
- Gupta, P.; Dutta, U.; Rana, P.; Singhal, M.; Gulati, A.; Kalra, N.; Soundararajan, R.; Kalage, D.; Chhabra, M.; Sharma, V.; et al. Gallbladder Reporting and Data System (GB-RADS) for Risk Stratification of Gallbladder Wall Thickening on Ultrasonography: An International Expert Consensus. Abdom. Radiol. 2022, 47, 554–565. [Google Scholar] [CrossRef]
- Amor, F.; Vaccaro, H.; Alcázar, J.L.; León, M.; Craig, J.M.; Martinez, J. Gynecologic Imaging Reporting and Data System: A New Proposal for Classifying Adnexal Masses on the Basis of Sonographic Findings. J. Ultrasound Med. 2009, 28, 285–291. [Google Scholar] [CrossRef]
- Shin, J.H.; Baek, J.H.; Chung, J.; Ha, E.J.; Kim, J.-H.; Lee, Y.H.; Lim, H.K.; Moon, W.-J.; Na, D.G.; Park, J.S.; et al. Ultrasonography Diagnosis and Imaging-Based Management of Thyroid Nodules: Revised Korean Society of Thyroid Radiology Consensus Statement and Recommendations. Korean J. Radiol. 2016, 17, 370–395. [Google Scholar] [CrossRef] [PubMed]
- Manos, D.; Seely, J.M.; Taylor, J.; Borgaonkar, J.; Roberts, H.C.; Mayo, J.R. The Lung Reporting and Data System (LU-RADS): A Proposal for Computed Tomography Screening. Can. Assoc. Radiol. J. 2014, 65, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, A.; Alaia, E.F.; Ashikyan, O.; Wong, P.K.; Eajazi, A.; Taneja, A.K.; Colucci, P.; Bajaj, G.; Vossen, J.A.; Pezeshk, P.; et al. MSKI-RADS: An MRI-Based Musculoskeletal Infection Reporting and Data System for the Diagnosis of Extremity Infections. Radiology 2024, 312, e232914. [Google Scholar] [CrossRef] [PubMed]
- Elsholtz, F.H.J.; Asbach, P.; Haas, M.; Becker, M.; Beets-Tan, R.G.H.; Thoeny, H.C.; Padhani, A.R.; Hamm, B. Introducing the Node Reporting and Data System 1.0 (Node-RADS): A Concept for Standardized Assessment of Lymph Nodes in Cancer. Eur. Radiol. 2021, 31, 6116–6124. [Google Scholar] [CrossRef]
- Chhabra, A.; Deshmukh, S.D.; Lutz, A.M.; Fritz, J.; Andreisek, G.; Sneag, D.B.; Subhawong, T.; Singer, A.D.; Wong, P.K.; Thakur, U.; et al. Neuropathy Score Reporting and Data System: A Reporting Guideline for MRI of Peripheral Neuropathy with a Multicenter Validation Study. AJR Am. J. Roentgenol. 2022, 219, 279–291. [Google Scholar] [CrossRef]
- Chhabra, A.; Gupta, A.; Thakur, U.; Pezeshk, P.; Dettori, N.; Callan, A.; Xi, Y.; Weatherall, P. Osseous Tumor Reporting and Data System-Multireader Validation Study. J. Comput. Assist. Tomogr. 2021, 45, 571–585. [Google Scholar] [CrossRef]
- Saba, L.; Cau, R.; Murgia, A.; Nicolaides, A.N.; Wintermark, M.; Castillo, M.; Staub, D.; Kakkos, S.K.; Yang, Q.; Paraskevas, K.I.; et al. Carotid Plaque-RADS: A Novel Stroke Risk Classification System. JACC Cardiovasc. Imaging 2024, 17, 62–75. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, W.; Guo, J.; Zhao, Y.; Sun, S.; Li, Y.; Liu, Z. Preliminary Opinion on Assessment Categories of Stomach Ultrasound Report and Data System (Su-RADS). Gastric Cancer 2018, 21, 879–888. [Google Scholar] [CrossRef]
- Panebianco, V.; Narumi, Y.; Altun, E.; Bochner, B.H.; Efstathiou, J.A.; Hafeez, S.; Huddart, R.; Kennish, S.; Lerner, S.; Montironi, R.; et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting And Data System). Eur. Urol. 2018, 74, 294–306. [Google Scholar] [CrossRef]
- Parillo, M.; Vaccarino, F.; Di Gennaro, G.; Kumar, S.; Van Goethem, J.; Beomonte Zobel, B.; Quattrocchi, C.C.; Parizel, P.M.; Mallio, C.A. Overview of the Current Knowledge and Conventional MRI Characteristics of Peri- and Para-Vascular Spaces. Brain Sci. 2024, 14, 138. [Google Scholar] [CrossRef]
- Parillo, M.; Vertulli, D.; Mallio, C.A.; Quattrocchi, C.C. Imaging Findings in Carcinomatous Encephalitis Secondary to Malignant Melanoma. Egypt. J. Neurol. Psychiatry Neurosurg. 2023, 59, 76. [Google Scholar] [CrossRef]
- Parillo, M.; Quattrocchi, C.C.; Pilato, F.; Di Lazzaro, V.; Beomonte Zobel, B. Whole-Body Computed Tomography as First-Line Imaging Procedure to Exclude Cancer in Patients with Neurological Suspicion of Paraneoplastic Syndromes: Shall Clinical Practice Adhere to Recommendations? Radiography 2023, 29, 8–13. [Google Scholar] [CrossRef] [PubMed]
- NIRADS® MRI. Available online: https://edge.sitecorecloud.io/americancoldf5f-acrorgf92a-productioncb02-3650/media/ACR/Files/RADS/NI-RADS/NIRADS-MRI-Management-Table.pdf (accessed on 27 January 2025).
- NIRADS® PET/CT. Available online: https://edge.sitecorecloud.io/americancoldf5f-acrorgf92a-productioncb02-3650/media/ACR/Files/RADS/NI-RADS/NIRADS-PET-CT-Management-Table.pdf (accessed on 27 January 2025).
- Pesce, K.; Orruma, M.B.; Hadad, C.; Bermúdez Cano, Y.; Secco, R.; Cernadas, A. BI-RADS Terminology for Mammography Reports: What Residents Need to Know. Radiographics 2019, 39, 319–320. [Google Scholar] [CrossRef] [PubMed]
- LI-RADS® Ultrasound Surveillance. Available online: https://Www.Acr.Org/-/Media/ACR/Files/RADS/LI-RADS/LI-RADS-US-Surveillance-V2024-Core.Pdf (accessed on 27 January 2025).
- Ribeiro, G.J.; Gillet, R.; Blum, A.; Teixeira, P.A.G. Imaging Report and Data System (RADS) for Bone Tumors: Where Do We Stand and Future Directions. Skelet. Radiol. 2023, 52, 151–156. [Google Scholar] [CrossRef]
- Ganau, M.; Foroni, R.I.; Gerosa, M.; Zivelonghi, E.; Longhi, M.; Nicolato, A. Radiosurgical Options in Neuro-Oncology: A Review on Current Tenets and Future Opportunities. Part I: Therapeutic Strategies. Tumori 2014, 100, 459–465. [Google Scholar] [CrossRef]
- Ganau, M.; Foroni, R.I.; Gerosa, M.; Ricciardi, G.K.; Longhi, M.; Nicolato, A. Radiosurgical Options in Neuro-Oncology: A Review on Current Tenets and Future Opportunities. Part II: Adjuvant Radiobiological Tools. Tumori 2015, 101, 57–63. [Google Scholar] [CrossRef]
- Marcon, M.; Fuchsjäger, M.H.; Clauser, P.; Mann, R.M. ESR Essentials: Screening for Breast Cancer—General Recommendations by EUSOBI. Eur. Radiol. 2024, 34, 6348–6357. [Google Scholar] [CrossRef]
- Isautier, J.M.J.; Houssami, N.; Hadlow, C.; Marinovich, M.L.; Hope, S.; Zackrisson, S.; Brennan, M.E.; Nickel, B. Clinical Guidelines for the Management of Mammographic Density: A Systematic Review of Breast Screening Guidelines Worldwide. JNCI Cancer Spectr. 2024, 8, pkae103. [Google Scholar] [CrossRef]
- EAU Prostate Cancer Guidelines. Available online: https://uroweb.org/guidelines/prostate-cancer/chapter/diagnostic-evaluation (accessed on 20 February 2025).
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Zawaideh, J.P.; Sala, E.; Pantelidou, M.; Shaida, N.; Koo, B.; Caglic, I.; Warren, A.Y.; Carmisciano, L.; Saeb-Parsy, K.; Gnanapragasam, V.J.; et al. Comparison of Likert and PI-RADS Version 2 MRI Scoring Systems for the Detection of Clinically Significant Prostate Cancer. Br. J. Radiol. 2020, 93, 20200298. [Google Scholar] [CrossRef]
- Munari, A.M.; Monti, C.B.; Viglio, C.; Folco, G.; Rizzetto, F.; Zirpoli, S. MRI of Pediatric Ovarian Masses: Validation of the O-RADS Framework. Eur. Radiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Richman, D.M.; Benson, C.B.; Doubilet, P.M.; Wassner, A.J.; Asch, E.; Cherella, C.E.; Smith, J.R.; Frates, M.C. Assessment of American College of Radiology Thyroid Imaging Reporting and Data System (TI-RADS) for Pediatric Thyroid Nodules. Radiology 2020, 294, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Balaji, R. The RADS-Panacea or Pain? Indian J. Radiol. Imaging 2022, 32, 149–150. [Google Scholar] [CrossRef]
- Saleh, G.A.; Batouty, N.M.; Gamal, A.; Elnakib, A.; Hamdy, O.; Sharafeldeen, A.; Mahmoud, A.; Ghazal, M.; Yousaf, J.; Alhalabi, M.; et al. Impact of Imaging Biomarkers and AI on Breast Cancer Management: A Brief Review. Cancers 2023, 15, 5216. [Google Scholar] [CrossRef]
- Shao, Y.; Cheng, Y.; Shah, R.U.; Weir, C.R.; Bray, B.E.; Zeng-Treitler, Q. Shedding Light on the Black Box: Explaining Deep Neural Network Prediction of Clinical Outcomes. J. Med. Syst. 2021, 45, 5. [Google Scholar] [CrossRef]
- Laino, M.E.; Viganò, L.; Ammirabile, A.; Lofino, L.; Generali, E.; Francone, M.; Lleo, A.; Saba, L.; Savevski, V. The Added Value of Artificial Intelligence to LI-RADS Categorization: A Systematic Review. Eur. J. Radiol. 2022, 150, 110251. [Google Scholar] [CrossRef]
- Tsuboyama, T.; Yanagawa, M.; Fujioka, T.; Fujita, S.; Ueda, D.; Ito, R.; Yamada, A.; Fushimi, Y.; Tatsugami, F.; Nakaura, T.; et al. Recent Trends in AI Applications for Pelvic MRI: A Comprehensive Review. Radiol. Med. 2024, 129, 1275–1287. [Google Scholar] [CrossRef]
- Alqahtani, S. Systematic Review of AI-Assisted MRI in Prostate Cancer Diagnosis: Enhancing Accuracy Through Second Opinion Tools. Diagnostics 2024, 14, 2576. [Google Scholar] [CrossRef]
- Verpalen, V.A.; Coerkamp, C.F.; Henriques, J.P.S.; Isgum, I.; Planken, R.N. Automated Classification of Coronary LEsions fRom Coronary Computed Tomography Angiography Scans with an Updated Deep Learning Model: ALERT Study. Eur. Radiol. 2025, 35, 1543–1551. [Google Scholar] [CrossRef]
- Bhayana, R. Chatbots and Large Language Models in Radiology: A Practical Primer for Clinical and Research Applications. Radiology 2024, 310, e232756. [Google Scholar] [CrossRef]
- Parillo, M.; Vaccarino, F.; Beomonte Zobel, B.; Mallio, C.A. ChatGPT and Radiology Report: Potential Applications and Limitations. Radiol. Med. 2024, 129, 1849–1863. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parillo, M.; Quattrocchi, C.C. Overview of Radiological Reporting and Data System (RADS) Guidelines Currently Applicable in Surgery. Surgeries 2025, 6, 23. https://doi.org/10.3390/surgeries6010023
Parillo M, Quattrocchi CC. Overview of Radiological Reporting and Data System (RADS) Guidelines Currently Applicable in Surgery. Surgeries. 2025; 6(1):23. https://doi.org/10.3390/surgeries6010023
Chicago/Turabian StyleParillo, Marco, and Carlo Cosimo Quattrocchi. 2025. "Overview of Radiological Reporting and Data System (RADS) Guidelines Currently Applicable in Surgery" Surgeries 6, no. 1: 23. https://doi.org/10.3390/surgeries6010023
APA StyleParillo, M., & Quattrocchi, C. C. (2025). Overview of Radiological Reporting and Data System (RADS) Guidelines Currently Applicable in Surgery. Surgeries, 6(1), 23. https://doi.org/10.3390/surgeries6010023







