Stable Isotope Analysis of Ozark Hellbender (Cryptobranchus alleganiensis bishopi) Living and Preserved Museum Tissue Reveals a Shift in Their Generalist Diet Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field-Site Description
2.2. Crayfish Sampling
2.3. Live and Formalin-Preserved Museum Hellbender Sampling
2.4. Fish Sampling
2.5. Laboratory Processing and Stable Isotope Analysis
2.6. Statistical Analyses
2.7. Bayesian Stable Isotope Mixing Model
3. Results
3.1. Crayfish Assemblages
3.2. Among Station Variation in Hellbender Isotopes
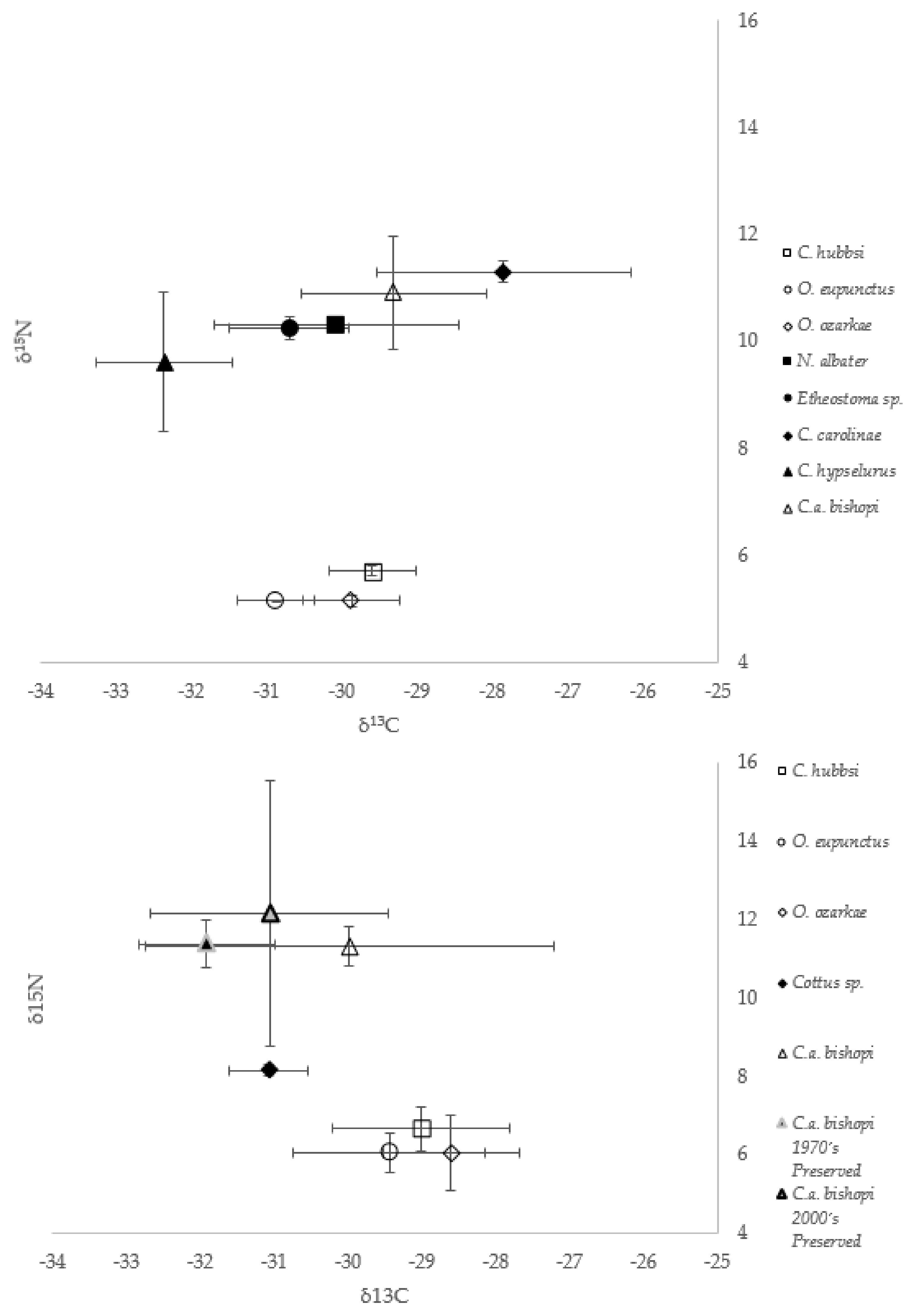
3.3. δ13C and δ15N Values
3.4. Isotopic Mixing Model
4. Discussion
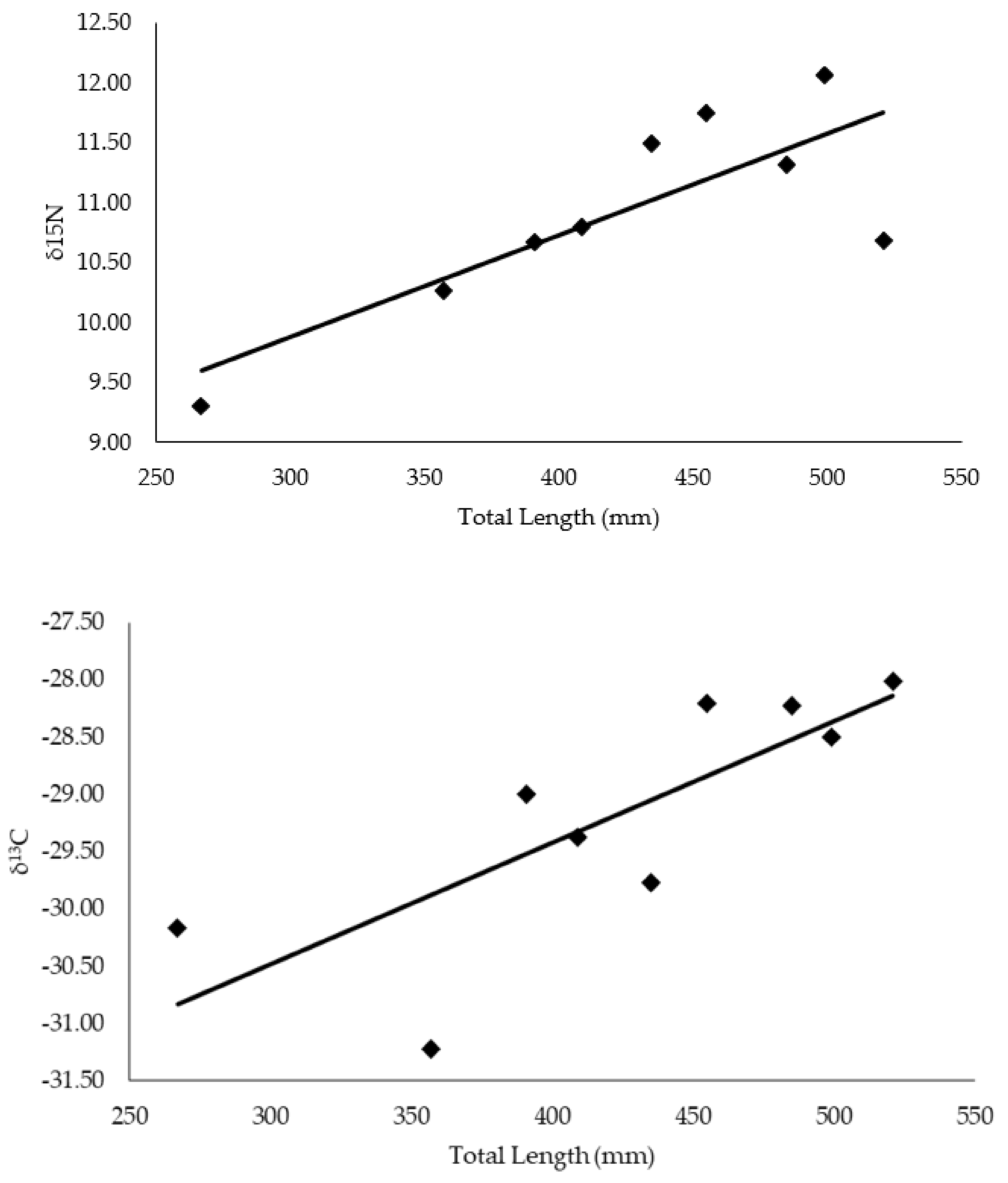
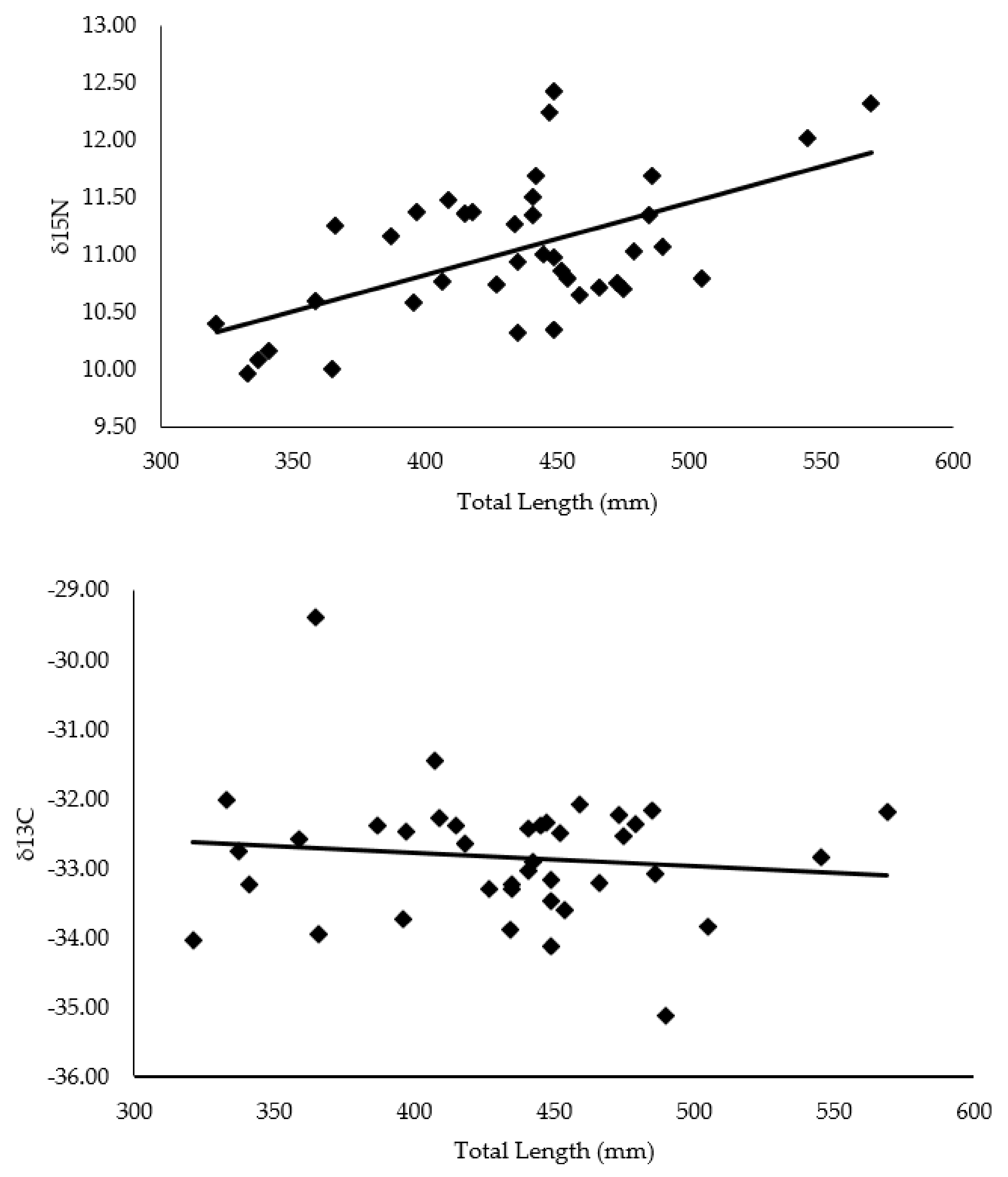
4.1. Hellbender Length Correlation with C and N Isotope Values
4.2. Contemporary Hellbender Diets
4.3. Historical Hellbender Diets
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trauth, S.E.; Wilhide, J.D.; Daniel, P. Status of the Ozark hellbender, Cryptobranchus bishopi (Urodela: Cryptobranchidae), in the Spring River, Fulton County, Arkansas. Proc. Ark. Acad. Sci. 1992, 46, 83–86. [Google Scholar]
- Wheeler, B.A.; Prosen, E.; Mathis, A.; Wilkinson, R.F. Population declines of a long-lived salamander: A 20+ year study of hellbenders, Cryptobranchus alleganiensis. Biol. Conserv. 2003, 109, 151–156. [Google Scholar] [CrossRef]
- Hiler, W.R.; Wheeler, B.A.; Trauth, S.E. The decline of the Ozark Hellbender (Cryptobranchus alleganiensis bishopi) in the Spring River, Arkansas, USA. Herpetol. Conserv. Biol. Herpetol. Conserv. Biol. 2003, 8, 114–121. [Google Scholar]
- National Archives and Records Administration. Endangered and Threatened Wildlife and Plants: Endangered Status for the Ozark Hellbender Salamander, Department of the Interior, Fish and Wildlife Service; Federal Register: Washington, DC, USA, 2011; Volume 76, pp. 61956–61978. [Google Scholar]
- Nickerson, M.; Briggler, J. Harvesting as a factor in population decline of a long-lived salamander; the Ozark hellbender, Cryptobranchus alleganiensis bishopi Grobman. Appl. Herpetol. Brill 2007, 4, 207–216. [Google Scholar]
- Solis, M.E.; Liu, C.C.; Nam, P.; Niyogi, D.K.; Bandeff, J.M.; Huang, Y.-W. Occurrence of organic chemicals in two rivers inhabited by Ozark hellbenders (Cryptobranchus alleganiensis bishopi). Arch. Environ. Contam. Toxicol. 2007, 53, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-C.; Xu, Y.; Briggler, J.T.; McKee, M.; Nam, P.; Huang, Y. Heavy metals, hematology, plasma chemistry, and parasites in adult hellbenders (Cryptobranchus alleganiensis). Environ. Toxicol. Chem. 2010, 29, 1132–1137. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, C.A.; Ott, C.M.; Castro, S.L.; Garcia, V.M.; Molina, T.C.; Briggler, J.T.; Pitt, A.L.; Tavano, J.J.; Byram, J.K.; Barrila, J. Evaluation of microorganisms cultured from injured and repressed tissue regeneration sites in endangered giant aquatic Ozark hellbender salamanders. PLoS ONE 2011, 6, e28906. [Google Scholar] [CrossRef] [Green Version]
- Unger, S. A comparison of sperm health in declining and stable populations of Hellbenders (Cryptobranchus alleganiensis alleganiensis and Ca bishopi). Am. Midl. Nat. 2013, 170, 382–392. [Google Scholar] [CrossRef]
- Bodinof, C.M.; Briggler, J.T.; Duncan, M.C.; Beringer, J.; Millspaugh, J.J. Historic occurrence of the amphibian chytrid fungus Batrachochytrium dendrobatidis in hellbender Cryptobranchus alleganiensis populations from Missouri. Dis. Aquat. Org. 2011, 96, 1–7. [Google Scholar] [CrossRef]
- Gall, B.G.; Mathis, A. Innate predator recognition and the problem of introduced trout. Ethology 2010, 116, 47–58. [Google Scholar] [CrossRef]
- Cundall, D.; Lorenz-Elwood, J.; Groves, J.D. Asymmetric suction feeding in primitive salamanders. Experientia 1987, 43, 1229–1231. [Google Scholar] [CrossRef]
- Hecht, K.A.; Nickerson, M.A.; Colclough, P.B. Hellbenders (Cryptobranchus alleganiensis) may exhibit an ontogenetic dietary shift. Southeast. Nat. 2017, 16, 157–162. [Google Scholar] [CrossRef]
- Unger, S.D.; Williams, L.A.; Diaz, L.; Jachowski, C.B. DNA barcoding to assess diet of larval eastern hellbenders in North Carolina. Food Webs 2020, 22, e00134. [Google Scholar] [CrossRef]
- Smith, B.G. The life history and habits of Cryptobranchus allegheniensis. Biol. Bull. 1907, 13, 5–39. [Google Scholar] [CrossRef] [Green Version]
- Netting, M.G. The food of the hellbender Cryptobranchus alleganiensis (Daudin). Copeia 1929, 170, 23–24. [Google Scholar]
- Green, N.B. Further notes on the food habits of the water dog, Cryptobranchus alleganiensis Daudin. In Proceedings of the West Virginia Academy of Sciences, Montgomery, VA, USA, 3–4 May 1935; p. 36. [Google Scholar]
- Nickerson, M.A.; Mays, C.E. The Hellbenders: North American “Giant Salamanders”; Milwaukee Public Museum: Milwaukee, WI, USA, 1973. [Google Scholar]
- Peterson, C.L.; Reed, J.W.; Wilkinson, R.F. Seasonal food habits of Cryptobranchus alleganiensis (Caudata: Cryptobranchidae). Southwest. Nat. 1989, 34, 438–441. [Google Scholar] [CrossRef]
- Dierenfeld, E.S.; McGraw, K.J.; Firtsche, K.; Briggler, J.T.; Ettling, J. Herpetological husbandry-Nutrient Composition of Whole Crayfish (Orconectes and Procambarus Species) Consumed by Hellbender (Cryptobranchus alleganiensis). Herpetol. Rev. 2009, 40, 324. [Google Scholar]
- Nickerson, M.A.; Ashton, R.E., Jr.; Braswell, A.L. Lampreys in the diet of hellbender Cryptobranchus alleganiensis (Daudin), and the Neuse River waterdog Necturus lewisi (Brimley). Herpetol. Rev. 1983, 14. [Google Scholar]
- Nickerson, M.A.; Mays, C.E. A study of the Ozark hellbender Cryptobranchus alleganiensis bishopi. Ecology 1973, 54, 1164–1165. [Google Scholar] [CrossRef]
- Groves, J.D.; Williams, L.A. Cryptobranchus alleganiensis—Cannibalism. Herpetol. Rev. 2014, 45, 108–109. [Google Scholar]
- Petranka, J.W. Salamanders of the United States and Canada; Smithsonian Institute: Washington, DC, USA, 1998. [Google Scholar]
- Phillips, C.A.; Humphries, W.J. Caudata—Cryptobranchidae. In Amphibian Declines—The Conservation Status of the United States Species; Lannoo, M.J., Ed.; University of California Press: Berkely, CA, USA; Los Angeles, CA, USA, 2005; pp. 648–651. [Google Scholar]
- Unger, S.D. Adult Female Eastern hellbender Cryptobranchus alleganiensis (Cryptobranchidae) conspecific cannibalism confirmed via DNA barcoding. Herpetol. Notes 2020, 13, 169–170. [Google Scholar]
- Unger, S.D. Scavenging Behavior of the Aquatic Eastern Hellbender Salamander (Cryptobranchus alleganiensis) in North Carolina. J. N. C. Acad. Sci. 2018, 134, 1–2. [Google Scholar] [CrossRef]
- Wiggs, J.N. Food Habits, Starvation and Growth in the Hellbender, Cryptobranchus alleganiensis. Master’s Thesis, Missouri State University, Springfield, MO, USA, 1976. [Google Scholar]
- Lajtha, K.; Michener, R.H. Stable Isotopes in Ecology and Environmental Science; Blackwell Scientific Publications: Oxford, UK, 1994. [Google Scholar]
- Layman, C.A.; Araujo, M.S.; Boucek, R.; Hammerschlag-Peyer, C.M.; Harrison, E.; Jud, Z.R.; Matich, P.; Rosenblatt, A.E.; Vaudo, J.J.; Yeager, L.A. Applying stable isotopes to examine food-web structure: An overview of analytical tools. Biol. Rev. 2012, 87, 545–562. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.S.; Turner, T.F.; Sharp, Z.D. Short-and long-term effects of fixation and preservation on stable isotope values (δ13C, δ15N, δ34S) of fluid-preserved museum specimens. Copeia 2002, 2002, 1106–1112. [Google Scholar] [CrossRef]
- Christian, A.D.; Smith, B.N.; Berg, D.J.; Smoot, J.C.; Findlay, R.H. Trophic position and potential food sources of 2 species of unionid bivalves (Mollusca: Unionidae) in 2 small Ohio streams. J. N. Am. Benthol. Soc. 2004, 23, 101–113. [Google Scholar] [CrossRef]
- Fry, B. Stable Isotope Ecology; Springer: New York, NY, USA, 2006. [Google Scholar]
- Arrington, D.-A.; Winemiller, K.-O. Preservation effects on stable isotope analysis of fish muscle. Trans. Am. Fish. Soc. 2002, 131, 337–342. [Google Scholar] [CrossRef]
- Junger, M.; Planas, D. Alteration of trophic interactions between periphyton and invertebrates in an acidified stream: A stable carbon isotope study. Hydrobiologia 1993, 262, 97–107. [Google Scholar] [CrossRef]
- Ponsard, S.; Amlou, M. Effects of several preservation methods on the isotopic content of Drosophila samples. Comptes Rendus L’académie Sci. Ser. III-Sci. Vie 1999, 322, 35–41. [Google Scholar] [CrossRef]
- Hobson, K.A.; Gloutney, M.L.; Gibbs, H.L. Preservation of blood and tissue samples for stable-carbon and stable-nitrogen isotope analysis. Can. J. Zool. 1997, 75, 1720–1723. [Google Scholar] [CrossRef]
- González-Bergonzoni, I.; Vidal, N.; Wang, B.; Ning, D.; Liu, Z.; Jeppesen, E.; Meerhoff, M. General validation of formalin-preserved fish samples in food web studies using stable isotopes. Methods Ecol. Evol. 2015, 6, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Moore, J.W.; Semmens, B.X. Incorporating uncertainty and prior information into stable isotope mixing models. Ecol. Lett. 2008, 11, 470–480. [Google Scholar] [CrossRef]
- Parnell, A.C.; Inger, R.; Bearhop, S.; Jackson, A.L. Source partitioning using stable isotopes: Coping with too much variation. PLoS ONE 2010, 5, e9672. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.L. Converting isotope values to diet composition: The use of mixing models. J. Mammal. 2012, 93, 342–352. [Google Scholar] [CrossRef]
- Phillips, D.L.; Inger, R.; Bearhop, S.; Jackson, A.L.; Moore, J.W.; Parnell, A.C.; Semmens, B.X.; Ward, E.J. Best practices for use of stable isotope mixing models in food-web studies. Can. J. Zool. 2014, 92, 823–835. [Google Scholar] [CrossRef] [Green Version]
- Peterson, C.L.; Metter, D.E.; Miller, B.T.; Wilkinson, R.F.; Topping, M.S. Demography of the hellbender Cryptobranchus alleganiensis in the Ozarks. Am. Midl. Nat. 1988, 119, 291–303. [Google Scholar] [CrossRef] [Green Version]
- Hiler, W.R. The Population Structure, Distribution, and Decline of the Ozark Hellbender in the Spring River, Arkansas. Master’s Thesis, Arkansas State University, Jonesboro, AR, USA, 2005. [Google Scholar]
- Pflieger, W.L.; Dryden, B. The Crayfishes of Missouri; Missouri Department of Conservation: Jefferson City, MO, USA, 1996. [Google Scholar]
- Boecklen, W.J.; Yarnes, C.T.; Cook, B.A.; James, A.C. On the use of stable isotopes in trophic ecology. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 411–440. [Google Scholar] [CrossRef] [Green Version]
- Stock, B.C.; Semmens, B.X. MixSIAR GUI User Manual, Version 1.0. 2013. Available online: http://conserver.iugo-cafe.org/user/brice.semmens/MixSIAR (accessed on 2 November 2016).
- De la Morinière, E.C.; Pollux, B.J.A.; Nagelkerken, I.; Hemminga, M.A.; Huiskes, A.H.L.; van der Velde, G. Ontogenetic dietary changes of coral reef fishes in the mangrove-seagrass-reef continuum: Stable isotopes and gut-content analysis. Mar. Ecol. Prog. Ser. 2003, 246, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, K.; Schwenk, K. Ingestion in reptiles and amphibians. In Encyclopedia of Life Sciences; Wiley Online Library: Hoboken, NJ, USA, 2002; pp. 1–7. [Google Scholar]
- Flinders, C.A. The Ecology of Lotic System Crayfish in the Spring River Watershed in Northern Arkansas and Southern Missouri, University of Central Arkansas. Ph.D. Thesis, University of Central Arkansas, Conway, AR, USA, 2000. [Google Scholar]
- Betrus, C.J.; Fleishman, E.; Blair, R.B. Cross-taxonomic potential and spatial transferability of an umbrella species index. J. Environ. Manag. 2005, 74, 79–87. [Google Scholar] [CrossRef]

| River | Station | Species | n | Percent of Total |
|---|---|---|---|---|
| Eleven Point | EP1 | Cambarus hubbsi | 48 | 52.17 |
| Orconectes eupunctus | 41 | 44.57 | ||
| Orconectes ozarkae | 3 | 3.26 | ||
| EP2 | Cambarus hubbsi | 10 | 41.67 | |
| Orconectes eupunctus | 10 | 41.67 | ||
| Orconectes ozarkae | 4 | 16.67 | ||
| EP3 | Cambarus hubbsi | 22 | 56.41 | |
| Orconectes eupunctus | 15 | 38.46 | ||
| Orconectes ozarkae | 2 | 5.13 | ||
| Spring | SR1 | Cambarus hubbsi | 40 | 49.38 |
| Orconectes eupunctus | 18 | 22.22 | ||
| Orconectes ozarkae | 23 | 28.40 |
| Species | River | Station | n | Mean δ13C | SD | Mean δ15N | SD |
|---|---|---|---|---|---|---|---|
| C. hubbsi | Eleven Point | EP1 | 10 | −30.02 | 1.04 | +5.44 | 0.56 |
| O. eupunctus | Eleven Point | EP1 | 10 | −31.08 | 1.14 | +4.92 | 0.33 |
| O. ozarkae | Eleven Point | EP1 | 4 | −30.05 | 0.59 | +5.39 | 0.33 |
| C. a. bishopi | Eleven Point | EP1 | 2 | −30.69 | 0.75 | +9.79 | 0.68 |
| C. hubbsi | Eleven Point | EP2 | 10 | −28.94 | 0.62 | +5.80 | 0.42 |
| O. eupunctus | Eleven Point | EP2 | 10 | −30.28 | 0.77 | +5.51 | 0.37 |
| O. ozarkae | Eleven Point | EP2 | 4 | −29.16 | 0.94 | +5.16 | 0.23 |
| C. a. bishopi | Eleven Point | EP2 | 5 | −28.88 | 0.75 | +10.99 | 0.39 |
| C. hubbsi | Eleven Point | EP3 | 10 | −29.81 | 0.75 | +5.87 | 0.40 |
| O. eupunctus | Eleven Point | EP3 | 10 | −31.24 | 0.34 | +4.99 | 0.34 |
| O. ozarkae | Eleven Point | EP3 | 2 | −30.40 | 1.62 | +4.86 | 0.47 |
| C. a. bishopi | Eleven Point | EP3 | 2 | −28.35 | 0.21 | +11.91 | 0.22 |
| C. hubbsi | Eleven Point | Combined | 30 | −29.59 | 0.57 | +5.70 | 0.09 |
| O. eupunctus | Eleven Point | Combined | 30 | −30.87 | 0.51 | +5.14 | 0.02 |
| O. ozarkae | Eleven Point | Combined | 10 | −29.87 | 0.64 | +5.14 | 0.12 |
| N. albater | Eleven Point | EP4 | 6 | −30.07 | 1.62 | +10.29 | 0.05 |
| Etheostoma sp. | Eleven Point | EP4 | 16 | −30.70 | 0.79 | +10.25 | 0.21 |
| C. carolinae | Eleven Point | EP4 | 2 | −27.85 | 1.69 | +11.30 | 0.19 |
| C. hypselurus | Eleven Point | EP4 | 14 | −32.36 | 0.90 | +9.61 | 0.13 |
| C. a. bishopi | Eleven Point | Combined | 9 | −29.31 | 1.23 | +10.90 | 1.07 |
| C. hubbsi | Spring | SR1 | 15 | −29.01 | 1.19 | +6.65 | 0.55 |
| O. eupunctus | Spring | SR1 | 10 | −29.44 | 1.30 | +6.05 | 0.50 |
| O. ozarkae | Spring | SR1 | 10 | −28.60 | 0.93 | +6.03 | 0.96 |
| Cottus sp. | Spring | SR1 | 3 | −31.07 | 0.53 | +8.15 | 0.13 |
| C. a. bishopi | Spring | SR1 | 3 | −29.98 | 2.77 | +11.31 | 0.52 |
| C. a. bishopi 1970’s | Spring | SR1 | 39 | −31.91 | 0.93 | +11.37 | 0.61 |
| C. a. bishopi 2000’s | Spring | SR1 | 10 | −31.06 | 1.61 | +12.15 | 3.38 |
| River | Station | Time | Prey | Mean Contribution (%) | SD | 95% CI | |
|---|---|---|---|---|---|---|---|
| Eleven Point | EP1 | Present | Crayfish | Cambarus hubbsi | 14.2 | 10.0 | 32.1 |
| Orconectes eupunctus | 10.8 | 8.3 | 27.1 | ||||
| Orconectes ozarkae | 14.0 | 10.0 | 32.5 | ||||
| Fish | Noturus albater | 15.7 | 12.9 | 41.9 | |||
| Etheostoma sp. | 12.2 | 10.3 | 33.0 | ||||
| Cottus carolinae | 24.1 | 11.8 | 42.5 | ||||
| Cottus hypselurus | 9.10 | 8.5 | 25.9 | ||||
| EP2 | Present | Crayfish | Cambarus hubbsi | 21.0 | 14.9 | 48.2 | |
| Orconectes eupunctus | 17.1 | 13.2 | 42.9 | ||||
| Orconectes ozarkae | 18.6 | 13.0 | 42.6 | ||||
| Fish | Noturus albater | 13.1 | 9.5 | 31.0 | |||
| Etheostoma sp. | 10.9 | 8.5 | 27.6 | ||||
| Cottus carolinae | 10.2 | 7.1 | 23.4 | ||||
| Cottus hypselurus | 9.20 | 7.3 | 23.0 | ||||
| EP3 | Present | Crayfish | Cambarus hubbsi | 15.0 | 10.8 | 35.3 | |
| Orconectes eupunctus | 11.3 | 8.6 | 27.9 | ||||
| Orconectes ozarkae | 12.5 | 8.9 | 28.9 | ||||
| Fish | Noturus albater | 15.2 | 12.4 | 40.2 | |||
| Etheostoma sp. | 12.6 | 10.4 | 33.2 | ||||
| Cottus carolinae | 23.7 | 11.8 | 42.6 | ||||
| Cottus hypselurus | 9.70 | 9.2 | 28.3 | ||||
| Spring | SR1 | Present | Crayfish | Cambarus hubbsi | 16.1 | 15.0 | 46.5 |
| Orconectes eupunctus | 13.7 | 13.4 | 41.0 | ||||
| Orconectes ozarkae | 12.8 | 12.3 | 38.0 | ||||
| Fish | Cottus sp. | 57.4 | 20.9 | 85.2 | |||
| Historical 2000’s | Crayfish | Cambarus hubbsi | 20.9 | 19.0 | 60.8 | ||
| Orconectes eupunctus | 31.5 | 21.2 | 68.8 | ||||
| Orconectes ozarkae | 22.3 | 18.2 | 57.6 | ||||
| Fish | Cottus sp. | 25.3 | 13.2 | 48.1 | |||
| Historical 1970’s | Crayfish | Cambarus hubbsi | 3.40 | 3.9 | 10.9 | ||
| Orconectes eupunctus | 21.9 | 10.4 | 38.1 | ||||
| Orconectes ozarkae | 4.4 | 4.9 | 15.2 | ||||
| Fish | Cottus sp. | 70.4 | 9.1 | 86.7 | |||
| Both Historical sets of samples | Crayfish | Cambarus hubbsi | 3.30 | 3.7 | 9.8 | ||
| Orconectes eupunctus | 33.4 | 9.6 | 47.1 | ||||
| Orconectes ozarkae | 3.80 | 4.6 | 12.7 | ||||
| Fish | Cottus sp. | 59.6 | 7.2 | 71.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hiler, W.; Trauth, S.E.; Wheeler, B.; Jimenez, A.; Radanovic, M.; Milanovich, J.R.; Christian, A.D. Stable Isotope Analysis of Ozark Hellbender (Cryptobranchus alleganiensis bishopi) Living and Preserved Museum Tissue Reveals a Shift in Their Generalist Diet Composition. Ecologies 2021, 2, 187-202. https://doi.org/10.3390/ecologies2020011
Hiler W, Trauth SE, Wheeler B, Jimenez A, Radanovic M, Milanovich JR, Christian AD. Stable Isotope Analysis of Ozark Hellbender (Cryptobranchus alleganiensis bishopi) Living and Preserved Museum Tissue Reveals a Shift in Their Generalist Diet Composition. Ecologies. 2021; 2(2):187-202. https://doi.org/10.3390/ecologies2020011
Chicago/Turabian StyleHiler, Waylon, Stanley E. Trauth, Benjamin Wheeler, Aimee Jimenez, Milica Radanovic, Joseph R. Milanovich, and Alan D. Christian. 2021. "Stable Isotope Analysis of Ozark Hellbender (Cryptobranchus alleganiensis bishopi) Living and Preserved Museum Tissue Reveals a Shift in Their Generalist Diet Composition" Ecologies 2, no. 2: 187-202. https://doi.org/10.3390/ecologies2020011
APA StyleHiler, W., Trauth, S. E., Wheeler, B., Jimenez, A., Radanovic, M., Milanovich, J. R., & Christian, A. D. (2021). Stable Isotope Analysis of Ozark Hellbender (Cryptobranchus alleganiensis bishopi) Living and Preserved Museum Tissue Reveals a Shift in Their Generalist Diet Composition. Ecologies, 2(2), 187-202. https://doi.org/10.3390/ecologies2020011







