Response of the Ciliates Fabrea salina and Condylostoma sp. to Different Salinities and Microalgal Feeds
Abstract
:1. Introduction
2. Materials and Methods
3. Results
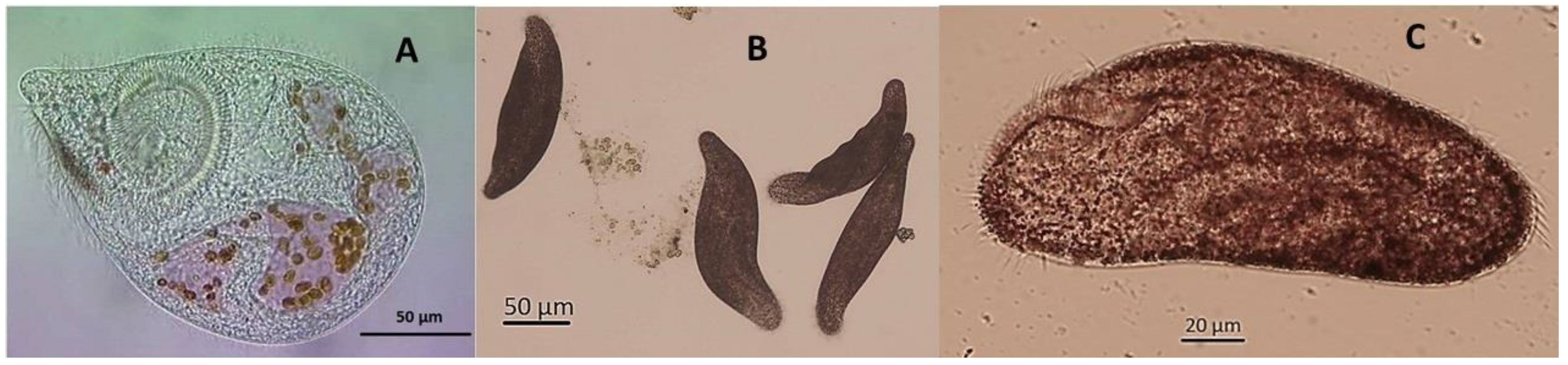
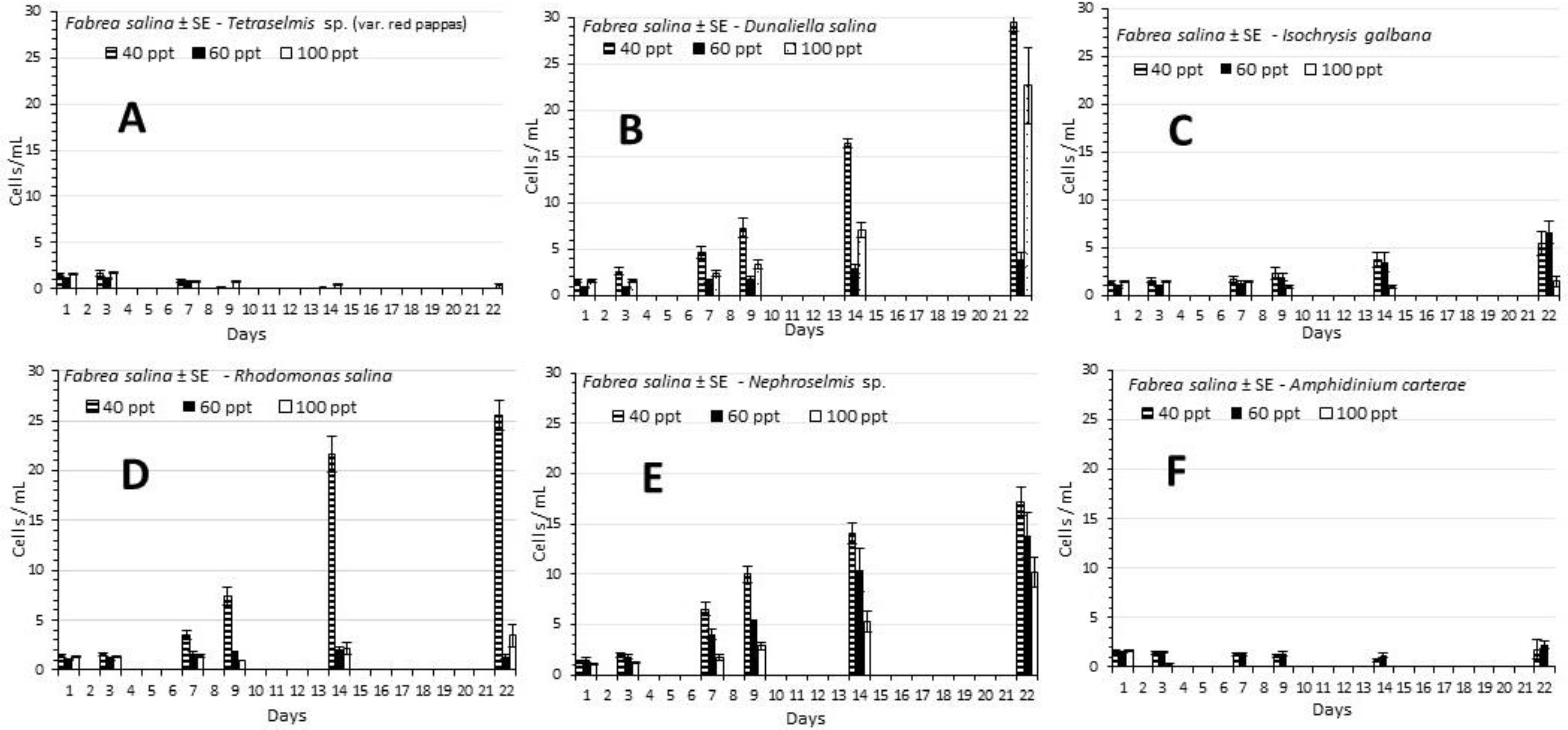
3.1. Fabrea Salina
3.2. Condylostoma sp.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porter, K.G.; Sherr, E.B.; Sherr, B.E.; Pace, M.; Sanders, R.W. Protozoa in planktonic food webs. J. Protoz. 1985, 32, 409–415. [Google Scholar] [CrossRef]
- Fenchel, T. Ecology of Protozoa. The Biology of Free-Living Phagotrophic Protists; Springer-Verlag: Berlin, Germany, 1987; 197p. [Google Scholar]
- Kamiyama, T. The impact of grazing by microzooplankton in northern Hiroshima Bay, the Seto Inland Sea, Japan. Mar. Biol. 1994, 119, 77–88. [Google Scholar] [CrossRef]
- Pace, M.L.; Orcutt, J.D., Jr. The relative importance of protozoans, rotifers, and crustaceans in a freshwater zooplankton community. Limnol. Ocean. 1981, 26, 822–830. [Google Scholar] [CrossRef] [Green Version]
- Pierce, R.W.; Turner, J.T. Ecology of planktonic ciliates in marine food webs. Rev. Aquat. Sci. 1992, 6, 139–181. [Google Scholar]
- Liu, H.B.; Dagg, M.J.; Wu, C.-J.; Chiang, K.-P. Mesozooplankton consumption of microplankton in the Mississippi River plume, with special emphasis on planktonic ciliates. Mar. Ecol. Prog. 2005, 286, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.W. Mating types in Oxytricha and the significance of mating type systems in ciliates. Biol. Bull. 1956, 110, 352–357. [Google Scholar] [CrossRef]
- Lynn, D.H.; Gilron, G.L. A brief review of approaches using ciliated protists to assess aquatic ecosystem health. J. Aq. Ecos. Health 1992, 1, 263–270. [Google Scholar] [CrossRef]
- Gilron, G.L.; Lynn, D.H. Ciliated protozoa as test organisms in toxicity assessments. In Microscale Testing in Aquatic Toxicology: Advances, Techniques, and Practice; Wells, P.G., Lee, K., Blaise, C., Eds.; CRC Press: Boca Raton, FL, USA, 1998; pp. 323–336. [Google Scholar]
- Madoni, P. Benthic ciliates in Adriatic Sea lagoons. Eur. J. Protistol. 2006, 42, 165–173. [Google Scholar] [CrossRef]
- Sladecek, V. System of Water Quality from the Biological Point of View; Lubrecht & Cramer Ltd.: Devon, UK, 1973; pp. 1–218. [Google Scholar]
- Logar, R.M.; Vodovnik, M. The Applications of Microbes in Environmental Monitoring. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.555.7498&rep=rep1&type=pdf (accessed on 10 May 2022).
- Dias, N.; Mortara, R.A.; Lima, N. Morphological and physiological changes in Tetrahymena pyriformis for the in vitro cytotoxicity assessment of Triton X-100. Toxicol. Vitr. 2003, 17, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Nałecz-Jawecki, G. Spirotox-Spirostomum ambiguum acute toxicity test-10 years of experience. Environ. Toxicol. 2004, 19, 359–364. [Google Scholar] [CrossRef]
- Rao, J.V.; Srikanth, K.; Arepalli, S.K.; Gunda, V.G. Toxic effects of acephate on Paramecium caudatum with special emphasis on morphology, behaviour, and generation time. Pestic. Bioch. Physiol. 2006, 86, 131–137. [Google Scholar] [CrossRef]
- Vilas-Boas, J.A.; Senra, M.V.X.; Dias, R.J.P. Ciliates in ecotoxicological studies: A minireview. Acta Limnol. Brasil. 2020, 32, e202. [Google Scholar] [CrossRef]
- Mansano, A.S.; Moreira, R.A.; Pierozzi, M.; Oliveira, T.M.A.; Vieira, E.M.; Rocha, O.; Regali–Selechim, M.H. Effects of diuron and carbofuran pesticides in their pure and commercial forms on Paramecium caudatum: The use of protozoan in ecotoxicology. Environ. Poll. 2016, 213, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Soldo, A.T.; Brickson, S.A. Isolation, cloning, and axenic cultivation of marine ciliates. In Handbook of Methods in Aquatic Microbial Ecology; Kemp, P.F., Cole, J.J., Sherr, B.F., Sherr, E.B., Eds.; CRC Press: Boca Raton, FL, USA, 1993; p. 97. [Google Scholar]
- Duff, R.J.; Ball, H.; Lavrentyev, P.J. Application of combined morphological-molecular approaches to the identification of planktonic protists from environ-mental samples. J. Eukaryot. Microbiol. 2008, 55, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Carney, L.T.; Wilkenfeld, J.S.; Lane, P.D.; Solberg, O.D.; Fuqua, Z.B.; Cornelius, N.G.; Gillespie, S.; Williams, K.P.; Samocha, T.M.; Lane, T.W.; et al. Pond crash forensics: Presumptive identification of pond crash agents by next generation sequencing in replicate raceway mass cultures of Nannochloropsis salina. Algal Res. 2016, 17, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Hue, N.; Kim, T.; Khang, D.T.; Men, T.T.; Vanoverberghe, I.; Callens, M.; Muylaert, K. Isolation and identification of herbivorous ciliates from contaminated microalgal cultures. Europ. J. Protist. 2020, 76, 125743. [Google Scholar] [CrossRef]
- Hotos, G.N. A preliminary survey on the planktonic biota in a hypersaline pond of Messolonghi saltworks (W. Greece). Diversity 2021, 13, 270. [Google Scholar] [CrossRef]
- Renè, F. Rearing of gilt-head (Sparus aurata). In The Early Life History of Fish; Blaxter, J.H.S., Ed.; Springer-Verlag: Berlin, Germany, 1974; p. 747. [Google Scholar]
- Harvey, H.R.; Ederington, M.C.; MacManus, G.B. Lipid composition of the marine ciliates Pleuronema sp. and Fabrea salina: Shifts in response to changes in diet. J. Euk. Microb. 1997, 44, 189–193. [Google Scholar] [CrossRef]
- Pandey, B.D.; Yeragi, S.G.; Pal, A.K. Nutritional value of a heterotrichous ciliate, Fabrea salina with emphasis on its fatty acid profile. Asian-Austrailian J. An. Sci. 2004, 17, 995–999. [Google Scholar] [CrossRef]
- Rhodes, M.A.; Phelps, R.P. Ciliated protozoans as alternative live food for first feeding red snapper Lutjanus campechanus larvae. Proc. Gulf Caribb. Fish. Inst. 2006, 57, 963–974. [Google Scholar]
- Rhodes, A.M.; Phelps, P.R. Evaluation of the Ciliated Protozoa, Fabrea salina as a First Food for Larval Red Snapper, Lutjanus campechanus in a Large Scale Rearing Experiment. J. Appl. Aquac. 2008, 20, 120–133. [Google Scholar] [CrossRef]
- Repak, A.J. The suitability of selected marine algae on the growth of Fabrea salina. J. Protozool. 1983, 30, 52–54. [Google Scholar] [CrossRef]
- Brown, M.R.; Jeffrey, S.W.; Volkman, J.K.; Dunstan, G.A. Nutritional properties of microalgae for mariculture. Aquaculture 1997, 151, 315–331. [Google Scholar] [CrossRef]
- Rattan, P.; Ansari, Z.A.; Chatterji, A. Studies on experimental culture of a marine ciliate Fabrea salina. J. Aquat. Trop. 1999, 14, 299–308. [Google Scholar]
- Pandey, B.D.; Yeragi, S.G. Preliminary and mass culture experiments on a heterotrichous ciliate, Fabrea salina. Aquaculture 2004, 232, 241–254. [Google Scholar] [CrossRef]
- Guermazi, W.; Elloumi, J.; Ayadi, H.; Bouain, A.; Aleya, L. Rearing of Fabrea salina Henneguy (Ciliophora, Heterotrichida) with three unicellular feeds. CR Biol. 2008, 331, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Hotos, G.N. Feeding with various microalgae the salt “loving” ciliate Fabrea salina in normal salinity of 35 ppt. Intern. J. Food Sci. Agric. 2019, 3, 150–152. [Google Scholar] [CrossRef]
- Li, C.; Xu, K.; Lei, Y. Growth and grazing responses to temperature and prey concentration of Condylostoma spatiosum, a large benthic ciliate, feeding on Oxyrrhis marina. Aquat. Microb. Ecol. 2011, 64, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Fenchel, T. The ecology of marine microbenthos II. The food of marine benthic ciliates. Ophelia 1968, 5, 73–121. [Google Scholar] [CrossRef]
- Lei, Y.; Stumm, K.; Volkenborn, N.; Wickham, S.; Berninger, U.G. Impact of Arenicola marina (Polychaeta) on the microbial assemblages and meiobenthos in a marine intertidal flat. Mar. Biol. 2010, 157, 1271–1282. [Google Scholar] [CrossRef]
- Hotos, G.N.; Avramidou, D. The effect of various salinities and light intensities on the growth performance of five locally isolated microalgae [Amphidinium carterae, Nephroselmis sp., Tetraselmis sp. (var. red pappas), Asteromonas gracilis and Dunaliella sp.] in laboratory batch cultures. J. Mar. Sci. Eng. 2021, 9, 1275. [Google Scholar] [CrossRef]
- Pagliara, P.; Caroppo, C. Toxicity assessment of Amphidinium carterae, Coolia cfr. monotis and Ostreopsis cfr. ovata (Dinophyta) isolated from the northern Ionian Sea (Mediterranean Sea). Toxicon Off. J. Int. Soc. Toxinol. 2012, 60, 1203–1214. [Google Scholar] [CrossRef]
- Moreira-González, Á.R.; Fernandes, L.F.; Uchida, H.; Uesugi, A.; Suzuki, T.; Chomérat, N.; Bilien, G.; Pereira, A.T.; Mafra, L.L. Morphology, growth, toxin production, and toxicity of cultured marine benthic dinoflagellates from Brazil and Cuba. J. Appl. Phyc. 2019, 31, 3699–3719. [Google Scholar] [CrossRef]
- Kong, X.; Han, X.; Gao, M.; Su, R.; Wang, K.; Li, X.; Lu, W. Antialgal and antilarval activities of bioactive compounds extracted from the marine dinoflagellate Amphidinium carterae. J. Ocean. Univ. China 2016, 15, 1014–1020. [Google Scholar] [CrossRef]
- Murray, S.A.; Kohli, G.S.; Farrell, H.; Spiers, Z.B.; Place, A.R.; Dorantes-Aranda, J.J.; Ruszczyk, J. A fish kill associated with a bloom of Amphidinium carterae in a coastal lagoon in Sydney, Australia. Harmful Algae 2015, 49, 19–28. [Google Scholar] [CrossRef]
- Chen, B.Z.; Liu, H.B.; Lau, M.T.S. Grazing and growth responses of a marine oligotrichous ciliate fed with two nanoplankton: Does food quality matter for micrograzers? Aquat. Ecol. 2010, 44, 113–119. [Google Scholar] [CrossRef]
- Hansen, P.J. Growth and grazing responses of a ciliate feeding on the red-tide dinoflagellate Gyrodinium aureolum in monoculture and in mixture with a non-toxic alga. Mar. Ecol. Prog. Ser. 1995, 121, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Buskey, E.J.; Hyatt, C.J. Effects of the Texas (USA) brown tide alga on planktonic grazers. J. Plankton Res. 1995, 126, 285–292. [Google Scholar] [CrossRef]
- Javor, B.J. Hypersaline Environments; Springer-Verlag: Berlin, Germany, 1989; pp. 159–162. [Google Scholar]
- Gunde-Cimerman, N.; Oren, A.; Plemenitas, A. Adaptation to Life at High Salt Concentrations in Archaea, Bacteria and Eukarya; Springer: Berlin/Heidelberg, Germany, 2005; pp. 517–541. [Google Scholar] [CrossRef]
- De Winter, F.; Persoone, G. Preliminary experiments with the ciliate Fabrea salina as a potential live food for mariculture purposes. In Proceedings of the 10th European Symposium on Marine Biology, Osterid, Belgium, 17–23 September 1975; Research in Mariculture at Laboratory and Pilot Scale; Persoone, G., Jaspers, E., Eds.; IZWO: Wetteren, Belgium, 1975; pp. 37–48. [Google Scholar]
- De Winter, F.; Persoone, G.; Benijts-Claus, C. Fabrea salina A Promising Live Food for Mariculture Purposes. World Maric. Soc. 1975, 6, 429–439. [Google Scholar] [CrossRef]
- Fenchel, T. Ecology of heterotrophic microflagellates: III. Adaptations to heterogenous environments. Mar. Ecol. Prog. Ser. 1982, 9, 25–33. [Google Scholar] [CrossRef]
- Pierce, E.; Isquith, I.R.; Repak, A.J. Quantitative study of cannibal-gigantism in Blepharisma. Acta Protozool. 1978, 17, 493–501. [Google Scholar]
- Giffered, D.J. Laboratory culture of marine planktonic oligotrichs (Ciliophora, Oligotrichida). Mar. Ecol. Prog. Ser. 1985, 23, 257–267. [Google Scholar] [CrossRef]
- Marangoni, R.; Batistini, A.; Puntoni, S.; Colombetti, G. Temperature effects on motion parameters and phototactic reaction of the marine ciliate Fabrea salina. J. Photochem. Photobiol. B Biol. 1995, 30, 123–127. [Google Scholar] [CrossRef]
- Henrique Cezar, A.; Javaroti, D.C.D.; Ferreira, R.J.; Seleghim, H.R.M. Optimized culture and growth curves of two ciliated protozoan strains of Paramecium caudatum Ehrenberg, 1833 to use in ecotoxicologycal assays. Rev. Brasil. Zooc. 2016, 17, 77–90. [Google Scholar]
- Takamura, N.; Yasuno, M. Food selection of the ciliated protozoa, Condylostoma vorticella (Ehrenberg) in Lake Kasumigaura. Jap. J. Limnol. 1983, 44, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Fenchel, T. Suspension feeding in ciliated protozoa: Functional response and particle size selection. Microb. Ecol. 1980, 6, 1–11. [Google Scholar] [CrossRef]
- Fenchel, T. Suspension feeding in ciliated protozoa: Feeding rates and their ecological significance. Microb. Ecol. 1980, 6, 13–25. [Google Scholar] [CrossRef]
- Fenchel, T. Suspension feeding in ciliated protozoa: Structure and function of feeding organelles. Arch. Protistenk. 1980, 123, 230–260. [Google Scholar] [CrossRef]
- Fenchel, T. Relation between particle size selection and clearance in suspension feeding ciliates. Limnol. Oceanogr. 1980, 25, 733–738. [Google Scholar] [CrossRef]
- Yang, J.P.; Loder, M.G.J.; Boersma, M.; Wiltshire, K.H. Factors influencing the grazing response of the marine oligotrichous ciliate Strombidium cf. sulcatum. Aquat. Microb. Ecol. 2015, 74, 59–71. [Google Scholar] [CrossRef]
- Cheng, S.H.; Aoki, S.; Maeda, M.; Hino, A. Competition between the rotifer Brachionus rotundiformis and the ciliate Euplotes vannus fed on two different algae. Aquaculture 2004, 241, 331–343. [Google Scholar] [CrossRef]
- Guilherme de Freitas, C.; Tsuzuki, Y.M.; Costa Melo, M.E. Monoculture of the ciliate protozoan Euplotes sp. (Ciliophora; Hypotrichia) fed with different diets. Acta Scientiarum. Biol. Sci. 2013, 35, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Sherr, E.B.; Sherr, B.F. High rates of consumption of bacteria by pelagic ciliates. Nature 1987, 325, 710–711. [Google Scholar] [CrossRef]
- Day, J.G.; Thomas, N.J.; Achilles-Day, U.E.M.; Leakey, R.J.G. Early detection of protozoan grazers inalgal biofuel cultures. Bioresour. Technol. 2012, 114, 715–719. [Google Scholar] [CrossRef]
- Erkelens, M.; Ball, A.S.; Lewis, D.M. The influence of protozoa with a filtered and non-filtered seawater culture of Tetraselmis sp., and effects to the bacterial and algal com-munities over 10 days. Bioresour. Technol. 2014, 173, 361–366. [Google Scholar] [CrossRef]
- Zhuang, L.-L.; Wu, Y.-H.; Espinosa, D.M.V.; Zhang, T.-Y.; Dao, G.-H.; Hu, H.-Y. Soluble Algal Products (SAPs) in large scale cultivation of microalgae for biomass/bioenergy production: A review. Renew. Sustain. Energy Rev. 2016, 59, 141–148. [Google Scholar] [CrossRef]

| SGR Tg | Fabrea salina | Condylostoma sp. | ||||
|---|---|---|---|---|---|---|
| 1st–7th Day (Days) | ||||||
| 7th–14th Day (Days) | ||||||
| Salinity (ppt) | 40 | 60 | 100 | 20 | 40 | 60 |
| Microalgae | ||||||
| Tetraselmis sp. | - | - | - | - | - | - |
| - | - | - | - | - | - | |
| Dunaliella salina | 0.18 (3.95) | 0.10 (6.99) | 0.06 (10.72} | 0.22 (3.17) | 0.16 (4.33) | - |
| 0.18 (3.83) | 0.08 (8.59) | 0.16 (4.37) | 0.11 (5.74) | 0.16 (4.28) | - | |
| Isochrysis galbana | 0.03 (22.8) | 0.06 (11.1) | - | 0.12 (5.57) | 0.08 (8.73) | - |
| 0.11 (6.04) | 0.11 (5.07) | - | 0.26 (2.66) | 0.14 (4.78) | - | |
| Rhodomonas salina | 0.16 (4.42) | 0.07 (10.05) | 0.01 67.2) | 0.10 (7.21) | 0.10 (6.86) | - |
| 0.26 (2.69) | 0.03 (20.22) | 0.06 (11.2) | 0.24 (2.86) | 0.24 (2.93) | - | |
| Nephroselmis sp. | 0.26 (2.62) | 0.17 (4.15) | 0.07 (10.2) | 0.17 (4.02) | 0.11 (6.41) | - |
| 0.11 (6.34) | 0.13 (5.17) | 0.17 (4.19) | 0.22 (3.15) | 0.23 (3.00) | - | |
| Amphidinium carterae | - | - | - | - | - | - |
| - | - | - | - | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hotos, G.N.; Touloupi, I. Response of the Ciliates Fabrea salina and Condylostoma sp. to Different Salinities and Microalgal Feeds. Ecologies 2022, 3, 225-234. https://doi.org/10.3390/ecologies3020017
Hotos GN, Touloupi I. Response of the Ciliates Fabrea salina and Condylostoma sp. to Different Salinities and Microalgal Feeds. Ecologies. 2022; 3(2):225-234. https://doi.org/10.3390/ecologies3020017
Chicago/Turabian StyleHotos, George N., and Ioanna Touloupi. 2022. "Response of the Ciliates Fabrea salina and Condylostoma sp. to Different Salinities and Microalgal Feeds" Ecologies 3, no. 2: 225-234. https://doi.org/10.3390/ecologies3020017







