Wild Felid Diversity, Space Use and Activity Patterns in the Eastern Himalaya, India
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Area
2.2. Field Sampling and Camera Trapping
2.3. Data Analysis
3. Results
4. Discussion
4.1. Space Use
4.2. Activity Pattern
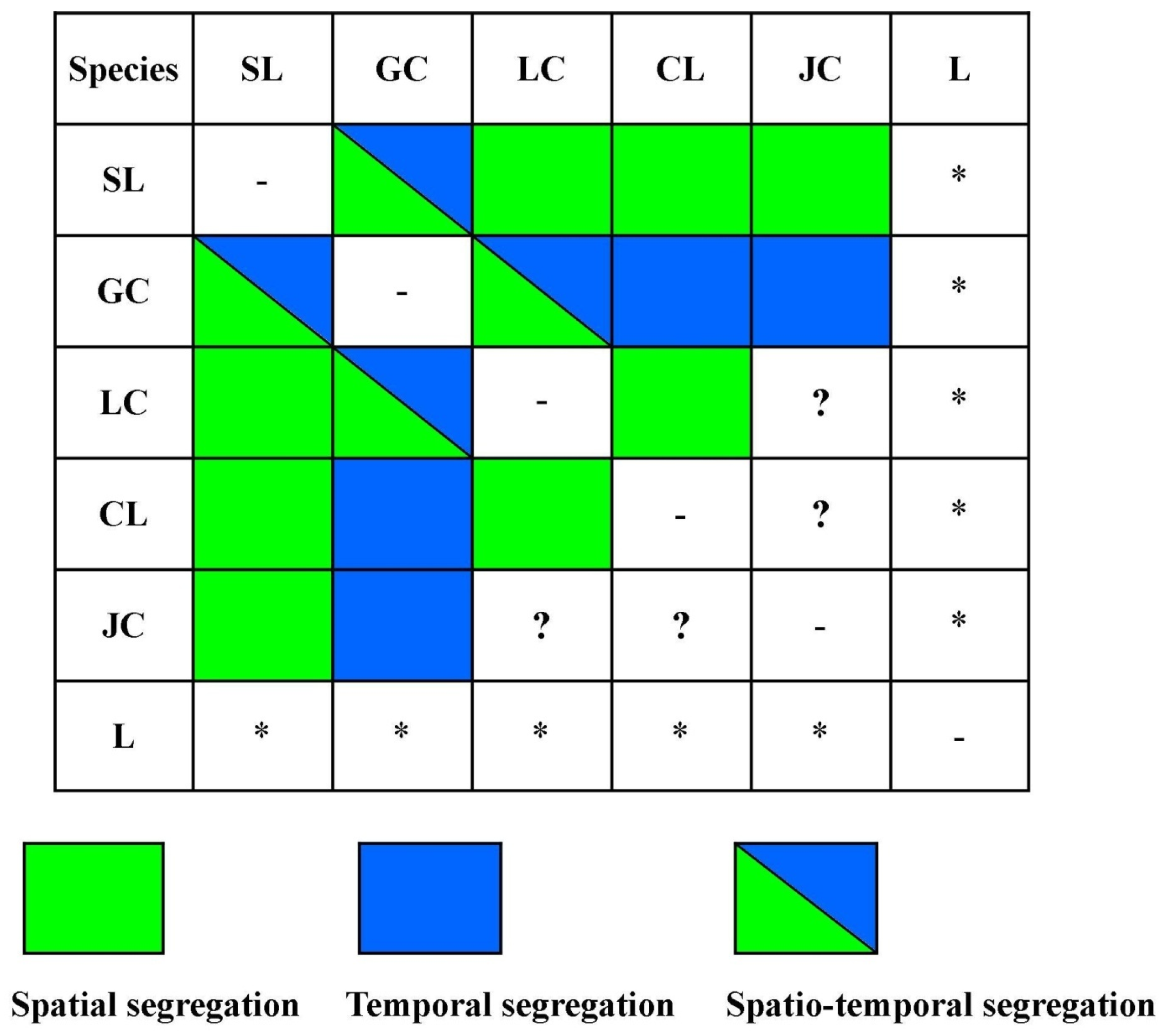
4.3. Spatio-Temporal Overlap and Segregation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Colorado-Zuluaga, G.J. How ecological communities are structured: A review on ecological assembly rules. Revista EIA 2015, 12, 27–53. [Google Scholar]
- Lockwood, J.; Powell, R.D.; Nott, M.P.; Pimm, S.L. Assembling ecological communities in time and space. Oikos 2017, 80, 549–553. [Google Scholar] [CrossRef]
- Fox, B.J.; Fox, M.D. Factors determining mammal species richness on habitat islands and isolates: Habitat diversity, disturbance, species interactions and guild assembly rules. Glob. Ecol. Biogeogr. 2000, 9, 19–37. [Google Scholar] [CrossRef]
- Zupo, V.; Alexander, T.J.; Edgar, G.J. Relating trophic resources to community structure: A predictive index of food availability. R. Soc. Open Sci. 2017, 4, 160515. [Google Scholar] [CrossRef] [Green Version]
- Kelt, D.A.; Taper, M.L.; Meserve, P.L. Assessing the impact of competition on the assembly of communities: A case study using small mammals. Ecology 1995, 76, 1283–1296. [Google Scholar] [CrossRef]
- Campbell, L.A. Distribution and Habitat Associations of Mammalian Carnivores in the Central and Southern Sierra Nevada. Ph.D. Thesis, University of California, Davis, CA, USA, 2004. [Google Scholar]
- Tadesse, S.A. Community structure and trophic level interactions in the terrestrial ecosystems: A review. Int. J. Avian. Wildl. Biol. 2017, 2, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Linnell, J.D.C.; Strand, O. Interference interactions, co-existence and conservation of mammalian carnivores. Divers. Distrib. 2000, 6, 169–176. [Google Scholar]
- De Satgé, J.; Teichman, K.; Cristescu, B. Competition and coexistence in a small carnivore guild. Oecologia 2017, 184, 873–884. [Google Scholar] [CrossRef]
- Schoener, T.W. Resource partitioning in ecological communities. Science 1974, 185, 27–39. [Google Scholar]
- Sunquist, M.E.; Sunquist, F.C. Ecological constraints on predation by large felids. In Carnivore Behavior, Ecology and Evolution; Gittleman, J.L., Ed.; Cornell University Press: Ithaca, NY, USA, 1989; pp. 283–301. [Google Scholar]
- Di Bitetti, M.S.; De Angelo, C.D.; Di Blanco, Y.E.; Paviolo, A. Niche partitioning and species coexistence in a Neotropical felid assemblage. Acta. Oecol. 2010, 36, 403–412. [Google Scholar]
- Sunarto, S.; Kelly, M.J.; Parakkasi, K.; Hutajulu, M.B. Cat coexistence in central Sumatra: Ecological characteristics, spatial and temporal overlap, and implications for management. J. Zool. 2015, 296, 104–115. [Google Scholar]
- Davis, M.L.; Kelly, M.J.; Staufer, D.F. Carnivore co-existence and habitat use in the Mountain Pine Ridge Forest Reserve, Belize. Anim. Conserv. 2011, 14, 56–65. [Google Scholar] [CrossRef]
- Kneitel, J. Gause’s Competitive Exclusion Principle. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Academic Press: Cambridge, MA, USA, 2008; pp. 1731–1734. [Google Scholar]
- Karanth, K.U.; Sunquist, M.E. Behavioral correlates of predation by tiger (Panthera tigris), leopard (Panthera pardus) and dhole (Cuon alpinus) in Nagarahole, India. J. Zool. 2000, 250, 255–265. [Google Scholar] [CrossRef]
- Gittleman, J.L. Carnivore body size; ecological and taxonomical correlates. Oecologia 1985, 67, 540–554. [Google Scholar] [PubMed]
- Palomares, F.; Ferreras, P.; Fedriani, J.M.; Delibes, M. Spatial relationships between Iberian lynx and other carnivores in an area of south-western Spain. J. Appl. Ecol. 1996, 33, 5–13. [Google Scholar] [CrossRef]
- Fedriani, J.M.; Palomares, F.; Delibes, M. Niche relationships among three sympatric Mediterranean carnivores. Oecologia 1999, 121, 138–148. [Google Scholar] [CrossRef] [Green Version]
- Durant, S.M. Competition refuges and co-existence: An example from Serengeti carnivores. J. Anim. Ecol. 1998, 67, 370–386. [Google Scholar] [CrossRef] [Green Version]
- Simberlof, D.; Dayan, T. The guild concept and the structure of ecological communities. Annu. Rev. Ecol. Syst. 1991, 22, 115–143. [Google Scholar] [CrossRef]
- Karanth, K.U.; Srivathsa, A.; Vasudev, D.; Puri, M.; Parameshwaran, R.; Kumar, N.S. Spatio-temporal interactions facilitate large carnivore sympatry across a resource gradient. Proc. Biol. Sci. 2017, 284, 20161860. [Google Scholar] [CrossRef] [Green Version]
- Hoeks, S.; Huijbregts, M.A.J.; Busana, M.; Harfoot, M.B.J.; Svenning, J.-C.; Santini, L. Mechanistic insights into the role of large carnivores for ecosystem structure and functioning. Ecography 2020, 43, 1752–1763. [Google Scholar] [CrossRef]
- Ceballos, G.; Erlich, P.R.; Soberon, J.; Salazar, I.; Fay, J.P. Global mammal conservation: What must we manage? Science 2005, 309, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sepúlveda, J.; Martín, C.A. Conservation status of the world’s carnivorous mammals (order Carnivora). Mamm. Biol. 2022. [Google Scholar] [CrossRef]
- Kuemmerle, T.; Bluhm, H.; Ghoddousi, A.; Arakelyan, M.; Askerov, E.; Bleyhl, B.; Ghasabian, M.; Gavashelishvili, A.; Heidelberg, A.; Malkhasyan, A.; et al. Identifying priority areas for restoring mountain ungulates in the Caucasus ecoregion. Conserv. Sci. Pract. 2020, 2, e276. [Google Scholar]
- Macdonald, D.W.; Loveridge, A.J. (Eds.) Biology and Conservation of Wild Felids; Oxford University Press: Oxford, UK, 2010; p. 762. [Google Scholar]
- Brodie, J.F. Is research effort allocated efficiently for conservation? Felidae as a global case study. Biodivers. Conserv. 2009, 18, 2927–2939. [Google Scholar] [CrossRef]
- Macdonald, E.A.; Burnham, D.; Hinks, A.E.; Dickman, A.J.; Malhi, Y.; Macdonald, D.W. Conservation inequality and the charismatic cat: Felis felicis. Glob. Ecol. Conserv. 2015, 3, 851–866. [Google Scholar] [CrossRef] [Green Version]
- Myers, N.; Mittermier, R.A.; Mittermier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Tambe, S. Ecology and Management of the Alpine Landscape in the Khangchendzonga National Park, Sikkim Himalaya. Ph.D. Thesis, FRI University, Dehradun, India, 2007; p. 232. [Google Scholar]
- Sathyakumar, S.; Bashir, T.; Bhattacharya, T.; Poudyal, K. Assessing mammal distribution and abundance in intricate Eastern Himalayan habitats of Khangchendzonga, Sikkim, India. Mammalia 2011, 75, 257–268. [Google Scholar] [CrossRef]
- Carbone, C.; Christie, S.; Conforti, K.; Coulson, T.; Franklin, N.; Ginsberg, J.R.; Griffiths, M.; Holden, J.; Kawanishi, K.; Kinnaird, M.F.; et al. The use of photographic rates to estimate densities of tigers and other cryptic mammals. Anim. Conserv. 2001, 4, 75–79. [Google Scholar] [CrossRef] [Green Version]
- Kawanishi, K.; Sahak, A.M.; Sunquist, M. Preliminary analysis on abundance of large mammals at Sungai Relau, Taman Negara. J. Wildli. Parks. 1999, 17, 62–82. [Google Scholar]
- O’Brien, T.G.; Kinnaird, M.F.; Wibisono, H.T. Crouching tigers, hidden prey: Sumatran tiger and prey populations in a tropical forest landscape. Anim. Conserv. 2003, 6, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Bowkett, A.E.; Rovero, F.; Marshall, A.R. The use of camera-trap data to model habitat use by antelope species in the Udzungwa Mountain forests, Tanzania. Afr. J. Ecol. 2007, 46, 479–487. [Google Scholar] [CrossRef]
- Magguran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2004; p. 215. [Google Scholar]
- Lynam, A.J.; Jenks, K.E.; Tantipisanuh, N.; Chutipong, W.; Ngoprasert, D.; Steinmetz, R.; Sukmasuang, R.; Grassman, L.I., Jr.; Cutter, P.; Kitamura, S. Terrestrial activity patterns of wild cats from camera-trapping. Raffles Bull. Zool. 2013, 61, 407–415. [Google Scholar]
- Kovach, W.L. Oriana—Circular Statistics for Windows, ver. 4.; Kovach Computing Services: Pentraeth, Wale, 2011. [Google Scholar]
- Pianka, E.R. The structure of lizard communities. Annu. Rev. Ecol. Evol. Syst. 1973, 4, 53–74. [Google Scholar] [CrossRef] [Green Version]
- Castro-Arellano, I.; Lacher, T.E., Jr.; Willig, M.R.; Rangel, T. Assessment of assemblage-wide temporal niche segregation using null models. Methods Ecol. Evol. 2010, 1, 311–318. [Google Scholar]
- Davies, A.B.; Tambling, C.J.; Marneweck, D.G.; Ranc, N.; Druce, D.J.; Cromsigt, J.P.G.M.; le Roux, E.; Asner, G.P. Spatial heterogeneity facilitates carnivore coexistence. Ecology 2021, 102, e03319. [Google Scholar] [PubMed]
- Chattopadhayay, S.; Saha, S.S.; Ghosh, M.K.; Agrawal, V.C. Mammalia. In Fauna of Sikkim; State Fauna Series, 9 (Part1); Zoological Survey of India: Kolkata, India, 2006; pp. 33–76. [Google Scholar]
- Mishra, C.; Madhusudanand, M.D.; Datta, A. Mammals of the high altitudes of western Arunachal Pradesh, Eastern Himalaya: An assessment of threats and conservation needs. Oryx 2006, 40, 29–35. [Google Scholar] [CrossRef] [Green Version]
- McCarthy, T.; Mallon, D.; Jackson, R.; Zahler, P.; McCarthy, K. Panthera Uncia; The IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2017; p. e.T22732A50664030. [Google Scholar] [CrossRef]
- Grassman, L.I.; Tewes, M.E.; Silvy, N.J.; Kreetiyutanont, K. Ecology of three sympatric felids in a mixed evergreen forest in north-central Thailand. J. Mammal 2005, 86, 29–38. [Google Scholar] [CrossRef]
- Nowell, K.; Jackson, P. Wild Cats: Status Survey and Conservation Action Plan; IUCN/SSC Cat Specialist Group, IUCN: Gland, Switzerland, 1996; p. 382. [Google Scholar]
- Choudhury, A. Sighting of Asiatic golden cat in the grasslands of Assam’s Manas National Park. Cat News 2007, 47, 29. [Google Scholar]
- Grassman, L.I.; Tewes, M.E.; Silvy, N.J.; Kreetiyutanont, K. Spatial organization and diet of the leopard cat (Prionailurus bengalensis) in north-central Thailand. J. Zool. 2005, 266, 45–54. [Google Scholar] [CrossRef]
- Rajaratnam, R.; Sunquist, M.; Rajaratnam, L.; Ambu, L. Diet and habitat selection of the leopard cat (Prionailurus bengalensis borneoensis) in an agricultural landscape in Sabah, Malaysian Borneo. J. Trop. Ecol. 2007, 23, 209–217. [Google Scholar] [CrossRef]
- Thapa, K.; Pradhan, N.; Barker, J.; Dahal, M.; Bhandari, A.R.; Gurung, G.S.; Rai, D.P.; Thapa, G.J.; Shrestha, S.; Singh, G.R. High elevation record of a leopard cat in the Kanchenjunga Conservation Area, Nepal. Cat News 2013, 58, 26–27. [Google Scholar]
- Duckworth, J.W.; Poole, C.M.; Tizard, R.J.; Walston, J.L.; Timmins, R.J. The Jungle Cat Felis chaus in Indochina: A threatened population of a widespread and adaptable species. Biodivers. Conserv. 2005, 14, 1263–1280. [Google Scholar] [CrossRef]
- McCarthy, T.M.; Fuller, T.K.; Munkhtsog, B. Movements and activities of snow leopard in South-western Mongolia. Biol. Conserv. 2005, 124, 527–537. [Google Scholar] [CrossRef]
- Mallon, D. The snow leopard in Ladakh. In International Pedigree Book of Snow Leopards; Helsinki Zoo: Helsinki, Finland, 1984; Volume 4, pp. 23–37. [Google Scholar]
- Schaller, G.B.; Tserendeleg, J.; Amarsanaa, G. Observations on snow leopards in Mongolia. Proc. Int. Snow Leopard Symp. 1994, 7, 33–42. [Google Scholar]
- Jackson, R.M. Home Range, Movements and Habitat Use of Snow Leopard (Uncia uncia) in Nepal. Ph.D. Thesis, University of London, London, UK, 1996; p. 233. [Google Scholar]
- Johansson, O. Unveiling the Ghost of the Mountain; Snow Leopard Ecology and Behaviour. Ph.D. Thesis, Faculty of Forest Science, Department of Ecology, Swedish University of Agricultural Science, Uppsala, Sweden, 2017; p. 51. [Google Scholar]
- Cheyne, S.M.; Macdonald, D.W. Wild felid diversity and activity patterns in Sabangau peat-swamp forest, Indonesian Borneo. Oryx 2011, 45, 119–124. [Google Scholar]
- Rostro-García, S.; Kamler, J.F.; Minge, C.; Caragiulo, A.; Crouthers, R.; Groenenberg, M.; Gray, T.N.E.; In, V.; Pin, C.; Sovanna, P.; et al. Small cats in big trouble? Diet, activity, and habitat use of jungle cats and leopard cats in threatened dry deciduous forests, Cambodia. Ecol. Evol. 2021, 11, 4205–4217. [Google Scholar] [CrossRef]
- Grassman, L.I.; Haines, A.M.; Janečka, J.E.; Tewes, M.E. Activity periods of photo-captured mammals in north central Thailand. Mammalia 2006, 70, 306–309. [Google Scholar] [CrossRef]
- Austin, S.C.; Tewes, M.E.; Grassman, L.I., Jr.; Silvy, N.J. Ecology and conservation of leopard cat Prionailurus bengalensis and clouded leopard Neofelis Nebulosa in Khao Yai National Park, Thailand. Acta Zool. Sin. 2007, 53, 1–14. [Google Scholar]
- Saxena, A.; Rajvanshi, A. Diurnal activity of leopard cat in Rajaji National Park, India. Cat News 2014, 61, 21. [Google Scholar]
- Schmidt, K.; Nakanishi, N.; Izawa, M.; Okamura, M.; Watanabe, S.; Tanaka, S.; Doi, T. The reproductive tactics and activity patterns of solitary carnivores: The Iriomote cat. J. Ethol. 2009, 27, 165–174. [Google Scholar] [CrossRef]
- Bashir, T.; Bhattacharya, T.; Poudyal, K.; Sathyakumar, S.; Qureshi, Q. Integrating aspects of ecology and predictive modeling: Implications for the conservation of the leopard cat (Prionailurus bengalensis) in the Eastern Himalaya. Acta Theriol. 2014, 59, 35–47. [Google Scholar] [CrossRef]
- Kawanishi, K.; Sunquist, M.E. Food habits and activity patterns of the Asiatic golden cat (Catopuma temminckii) and dhole (Cuon alpinus) in a primary rainforest of Peninsular Malaysia. Mamm. Stud. 2008, 33, 173–177. [Google Scholar] [CrossRef]
- Azlan, J.M.; Sharma, D.S.K. The diversity and activity patterns of wild felids in a secondary forest in Peninsular Malaysia. Oryx 2006, 40, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Gittleman, J.L. Carnivore Behavior, Ecology and Evolution; Cornell University Press: Ithaca, NY, USA; London, UK, 1996; Volume 2, p. 664. [Google Scholar]
- Cornell, H. Niche Overlap. In Encyclopedia of Theoretical Ecology; Hastings, A., Gross, L.J., Eds.; University of California: Berkeley, CA, USA, 2011; pp. 489–498. [Google Scholar]
- Mittelbach, G.G.; McGill, B.J. Species coexistence and niche theory. In Community Ecology, 2nd ed.; Oxford Academic: Oxford, UK, 2019; pp. 141–157. [Google Scholar]
- Pringle, R.M. Ecology: A revolution in resource partitioning. Curr. Biol. 2021, 31, R1474–R1476. [Google Scholar] [CrossRef]
- Curras, M.R.; Donadio, E.; Middleton, A.D.; Pauli, J.N. Carnivore Niche Partitioning in a Human Landscape. Am. Nat. 2022, 199, 496–509. [Google Scholar] [CrossRef]
- Sunquist, M.; Sunquist, F. Wild Cats of the World; University of Chicago Press: Chicago, IL, USA, 2000; p. 452. [Google Scholar]
- Bashir, T.; Bhattacharya, T.; Poudyal, K.; Sathyakumar, S. Notable observations on the melanistic Asiatic Golden cat (Pardofelis temminckii) of Sikkim, India. NeBIO 2011, 2, 1–4. [Google Scholar]




| Species | N | Temperate | Sub-Alpine | Alpine | Overall CR | MW-U χ2 | p |
|---|---|---|---|---|---|---|---|
| Snow leopard | 24 | - | - | 0.85 (0.35) | 0.85 (0.35) | - | - |
| Clouded leopard | 6 | 0.15 (0.15) | 0.04 (0.03) | - | 0.09 (0.06) | 114.5 | 0.73 |
| Leopard | 1 | 0.04 (0.04) | - | - | 0.04 (0.04) | - | - |
| Asiatic golden cat | 25 | 0.40 (0.24) | 0.43 (0.13) | - | 0.41 (0.13) | 95.5 | 0.28 |
| Jungle cat | 6 | 0.13 (0.09) | 0.11 (0.07) | - | 0.10 (0.05) | 116 | 0.85 |
| Leopard cat | 62 | 2.86 (0.87) | - | - | 2.86 (0.87) | - | - |
| Species 1 | Species 2 | S-Index | % Altitudinal Range Overlap | |
|---|---|---|---|---|
| Species 1 | Species 2 | |||
| Snow leopard | All others | 0 | - | - |
| Clouded leopard | Leopard | 0.333 | 7.40 | 4.00 |
| Clouded leopard | Leopard cat | 0.083 | 25.92 | 35.00 |
| Clouded leopard | Golden cat | 0.681 | 100.00 | 71.43 |
| Clouded leopard | Jungle cat | 0.733 | 100.00 | 59.73 |
| Leopard | Leopard cat | 0.100 | 30.00 | 75.00 |
| Leopard | Golden cat | 0 | 16.00 | 21.16 |
| Leopard | Jungle cat | 0 | 30.00 | 33.18 |
| Leopard cat | Golden cat | 0.111 | 65.00 | 34.39 |
| Leopard cat | Jungle cat | 0.538 | 100.00 | 44.25 |
| Golden cat | Jungle cat | 0.812 | 100.00 | 83.63 |
| Species | Mean Vector | S.E | 95% CI | Circular Variance |
|---|---|---|---|---|
| Snow leopard | 20:06 | 02:04 | 16:00–00:09 | 0.729 |
| Golden cat | 08:38 | 01:41 | 05:19–11:56 | 0.688 |
| Leopard cat | 00:51 | 00:30 | 23:48–01:47 | 0.359 |
| Pairwise | Watson’s U2 | p | Pianka’s index | p |
| Snow leopard–Golden cat | 0.292 | <0.01 | 0.346 | 0.618 |
| Snow leopard–Leopard cat | 0.226 | <0.05 | 0.422 | 0.352 |
| Golden cat–Leopard cat | 0.865 | <0.001 | 0.298 | 0.724 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashir, T.; Bhattacharya, T.; Poudyal, K.; Sathyakumar, S. Wild Felid Diversity, Space Use and Activity Patterns in the Eastern Himalaya, India. Ecologies 2023, 4, 41-54. https://doi.org/10.3390/ecologies4010005
Bashir T, Bhattacharya T, Poudyal K, Sathyakumar S. Wild Felid Diversity, Space Use and Activity Patterns in the Eastern Himalaya, India. Ecologies. 2023; 4(1):41-54. https://doi.org/10.3390/ecologies4010005
Chicago/Turabian StyleBashir, Tawqir, Tapajit Bhattacharya, Kamal Poudyal, and Sambandam Sathyakumar. 2023. "Wild Felid Diversity, Space Use and Activity Patterns in the Eastern Himalaya, India" Ecologies 4, no. 1: 41-54. https://doi.org/10.3390/ecologies4010005
APA StyleBashir, T., Bhattacharya, T., Poudyal, K., & Sathyakumar, S. (2023). Wild Felid Diversity, Space Use and Activity Patterns in the Eastern Himalaya, India. Ecologies, 4(1), 41-54. https://doi.org/10.3390/ecologies4010005






