Assessing Environmental Control on Temporal and Spatial Patterns of Larval Fish Assemblages in a Marine Protected Area
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Data Collection
2.3. Laboratory Processing
2.4. Data Analyses
3. Results
3.1. Environmental Variables
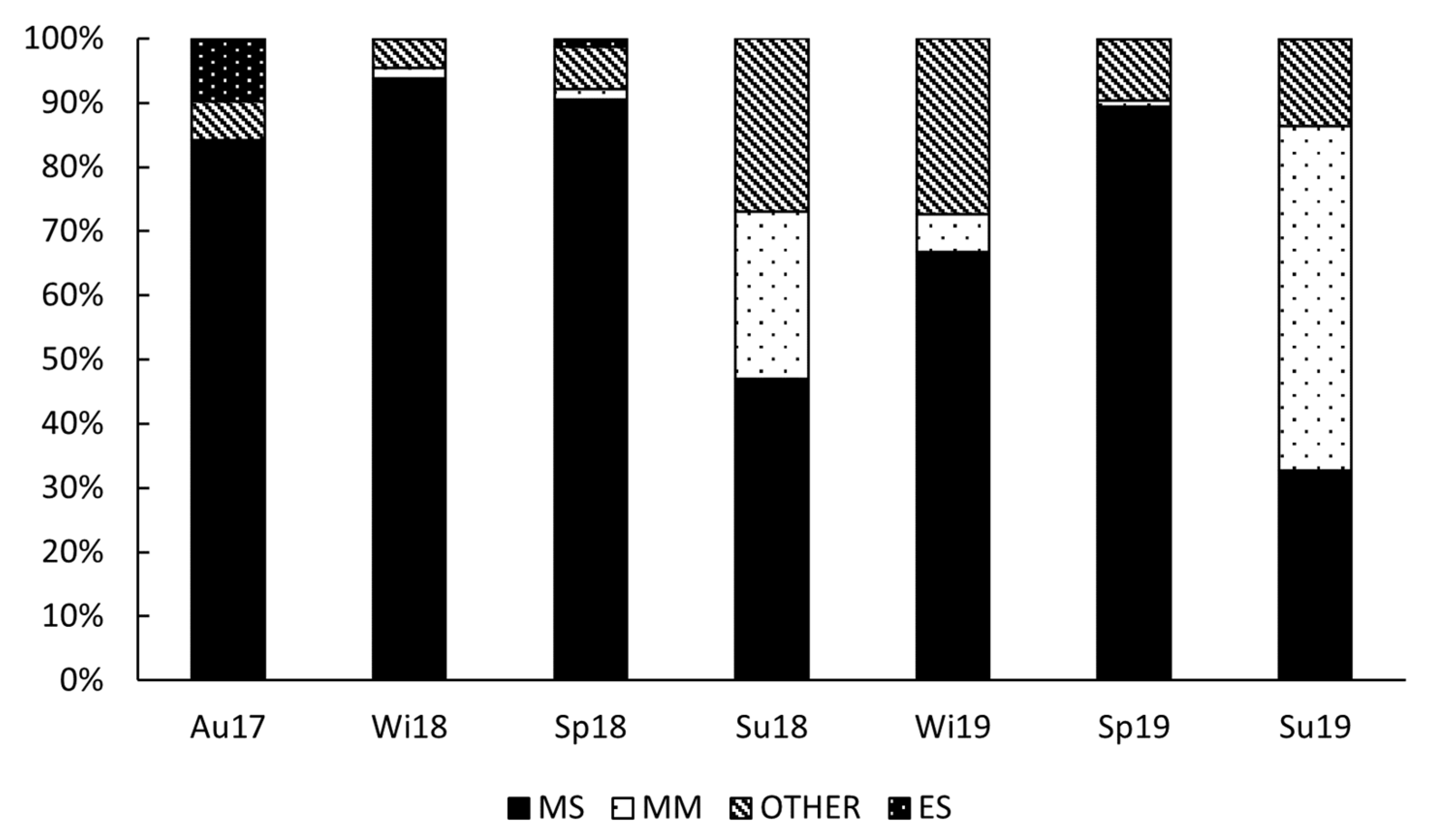
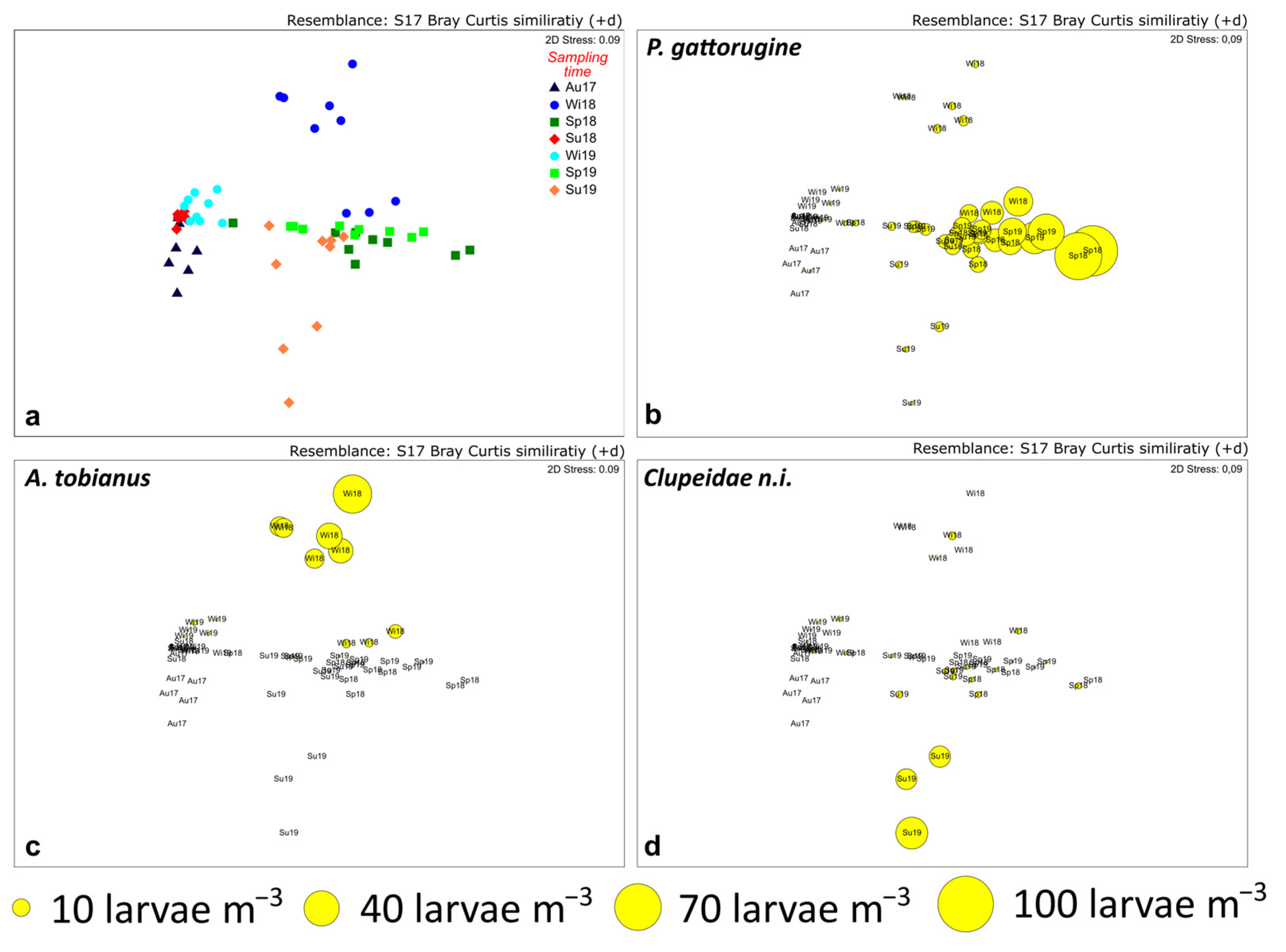
3.2. Larval Fish Assemblages
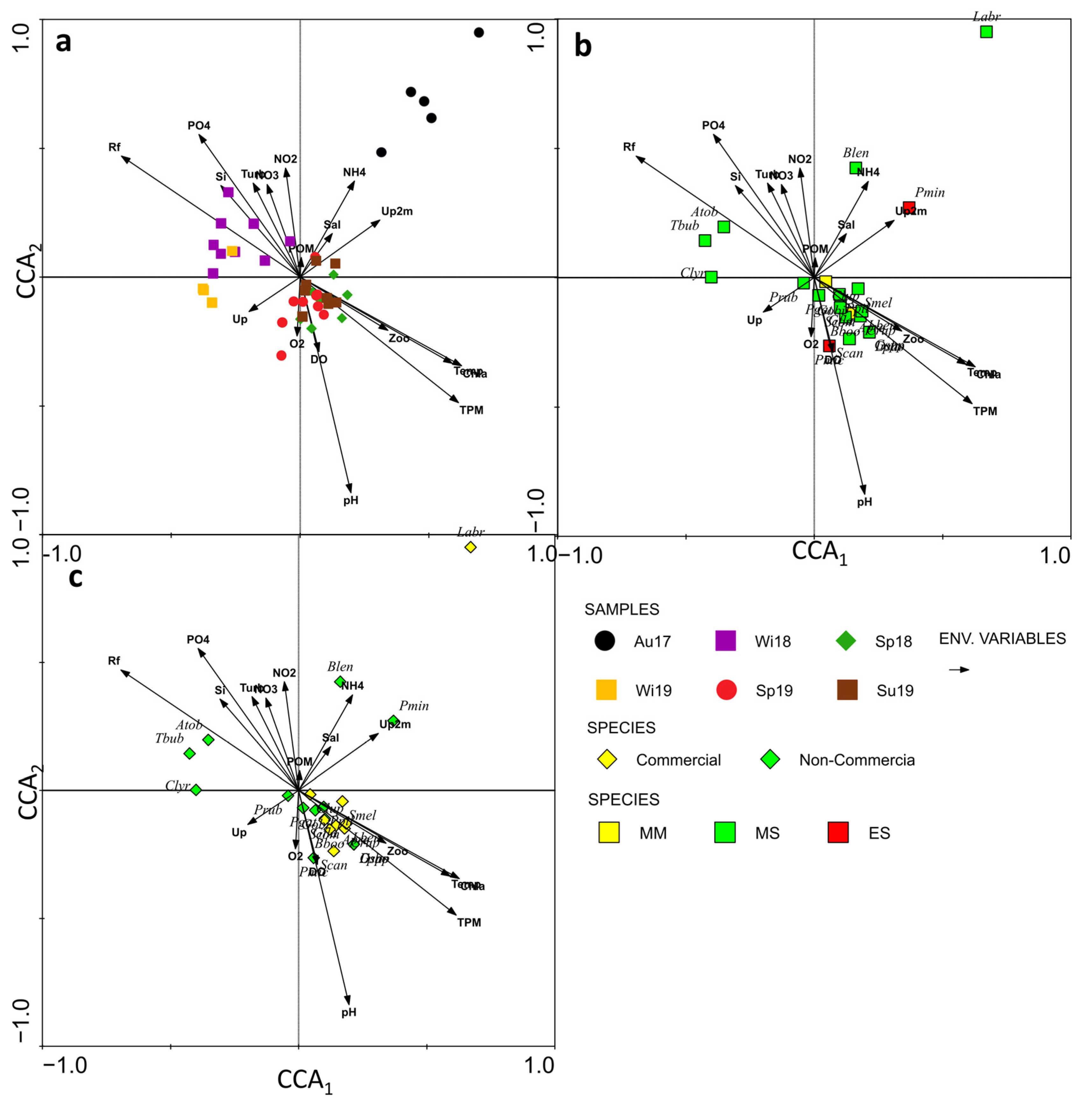
3.3. Environmental Control of Fish Larval Assemblages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fenberg, P.B.; Caselle, J.E.; Claudet, J.; Clemence, M.; Gaines, S.D.; García-Charton, J.A.; Gonçalves, E.J.; Grorud-Colvert, K.; Guidetti, P.; Jenkins, S.R.; et al. The science of European marine reserves: Status, efficacy, and future needs. Mar. Policy 2012, 36, 1012–1021. [Google Scholar] [CrossRef]
- Claudet, J.; Osenberg, C.W.; Benedetti-Cecchi, L.; Domenici, P.; Garcia-Charton, J.A.; Perez-Ruzafa, A.; Badalamenti, F.; Bayle-Sempere, J.; Brito, A.; Bulleri, F.; et al. Marine reserves: Size and age do matter. Ecol. Lett. 2008, 11, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Halpern, B.S. The impact of marine reserves: Do reserves work and does reserve size matter? Ecol. Appl. 2003, 13, 117–137. [Google Scholar] [CrossRef]
- Batista, M.I.; Cabral, H.N. An overview of Marine Protected Areas in SW Europe: Factors contributing to their management effectiveness. Ocean Coast. Manag. 2016, 132, 15–23. [Google Scholar] [CrossRef]
- e Costa, B.H.; Claudet, J.; Franco, G.; Erzini, K.; Caro, A.; Gonçalves, E.J. A regulation-based classification system for Marine Protected Areas (MPAs). Mar. Policy 2016, 72, 192–198. [Google Scholar] [CrossRef]
- Claudet, J.; Osenberg, C.W.; Domenici, P.; Badalamenti, F.; Milazzo, M.; Falcón, J.M.; Bertocci, I.; Benedetti-Cecchi, L.; García-Charton, J.-A.; Goñi, R.; et al. Marine reserves: Fish life history and ecological traits matter. Ecol. Appl. 2010, 20, 830–839. [Google Scholar] [CrossRef]
- Pipitone, C.; Agnetta, D.; Zenone, A.; Giacalone, V.M.; Badalamenti, F.; Fiorentino, F.; Rinelli, P.; Sinopoli, M.; Fernández, T.V.; D’Anna, G. When the Trawl Ban Is a Good Option: Opportunities to Restore Fish Biomass and Size Structure in a Mediterranean Fisheries Restricted Area. Sustainability 2023, 15, 2425. [Google Scholar] [CrossRef]
- Planes, S.; Galzin, R.; Rubies, A.G.; GoÑI, R.; Harmelin, J.G.; DirÉAch, L.L.; Lenfant, P.; Quetglas, A. Effects of marine protected areas on recruitment processes with special reference to Mediterranean littoral ecosystems. Environ. Conserv. 2000, 27, 126–143. [Google Scholar] [CrossRef]
- Andrello, M.; Mouillot, D.; Somot, S.; Thuiller, W.; Manel, S.; Bode, M. Additive effects of climate change on connectivity between marine protected areas and larval supply to fished areas. Divers. Distrib. 2015, 21, 139–150. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Claudet, J.; Guidetti, P. Spillover from marine protected areas to adjacent fisheries has an ecological and a fishery component. J. Nat. Conserv. 2016, 32, 62–66. [Google Scholar] [CrossRef]
- Edgar, G.J.; Stuart-Smith, R.D.; Willis, T.J.; Kininmonth, S.; Baker, S.C.; Banks, S.; Barrett, N.S.; Becerro, M.A.; Bernard, A.T.F.; Berkhout, J.; et al. Global conservation outcomes depend on marine protected areas with five key features. Nature 2014, 506, 216–220. [Google Scholar] [CrossRef] [PubMed]
- IUCN. Marine Protected Areas and Climate Change, Issues Brief. Available online: https://www.iucn.org/sites/dev/files/mpas_and_climate_change_issues_brief.pdf (accessed on 1 October 2020).
- EEA. Marine Protected Areas in Europes Seas. An Overview and Perspectives for the Future; European Environmental Agengy Report; European Environmental Agency: Copenhagen, Denmark, 2015; p. 35. [Google Scholar]
- Borges, R.; Ben-Hamadou, R.; Chícharo, M.A.; Ré, P.; Gonçalves, E.J. Horizontal spatial and temporal distribution patterns of nearshore larval fish assemblages at a temperate rocky shore. Estuar. Coast. Shelf Sci. 2007, 71, 412–428. [Google Scholar] [CrossRef]
- Borges, R.; Vaz, J.; Serrao, E.; Gonçalves, E. Short-term Temporal Fluctuation of Very-nearshore Larval Fish Assemblages at the Arrábida Marine Park (Portugal). J. Coast. Res. 2009, 56, 376–380. [Google Scholar]
- Henriques, S.; Pais, M.P.; Costa, M.J.; Cabral, H.N. Seasonal variability of rocky reef fish assemblages: Detecting functional and structural changes due to fishing effects. J. Sea Res. 2013, 79, 50–59. [Google Scholar] [CrossRef]
- Pereira, T.J.; Manique, J.; Quintella, B.R.; Castro, N.; de Almeida, P.R.; Costa, J.L. Changes in fish assemblage structure after implementation of Marine Protected Areas in the south western coast of Portugal. Ocean Coast. Manag. 2017, 135, 103–112. [Google Scholar] [CrossRef]
- Klein, M.; Beveren, E.V.; Rodrigues, D.; Serrão, E.A.; Caselle, J.E.; Gonçalves, E.J.; Borges, R. Small scale temporal patterns of recruitment and hatching of Atlantic horse mackerel (L.) at a nearshore reef area. Fish. Oceanogr. 2018, 27, 505–516. [Google Scholar] [CrossRef]
- Chavez, F.P.; Messie, M.; Pennington, J.T. Marine primary production in relation to climate variability and change. Annu. Rev. Mar. Sci. 2011, 3, 227–260. [Google Scholar] [CrossRef]
- Álvarez, I.; Catalán, I.A.; Jordi, A.; Palmer, M.; Sabatés, A.; Basterretxea, G. Drivers of larval fish assemblage shift during the spring-summer transition in the coastal Mediterranean. Estuar. Coast. Shelf Sci. 2012, 97, 127–135. [Google Scholar] [CrossRef]
- Fanjul, A.; Iriarte, A.; Villate, F.; Uriarte, I.; Atkinson, A.; Cook, K. Zooplankton seasonality across a latitudinal gradient in the Northeast Atlantic Shelves Province. Cont. Shelf Res. 2018, 160, 49–62. [Google Scholar] [CrossRef]
- Bresnan, E.; Cook, K.B.; Hughes, S.L.; Hay, S.J.; Smith, K.; Walsham, P.; Webster, L. Seasonality of the plankton community at an east and west coast monitoring site in Scottish waters. J. Sea Res. 2015, 105, 16–29. [Google Scholar] [CrossRef]
- Rakocinski, C.F.; Lyczkowski-Shultz, J.; Richardson, S.L. Ichthyoplankton Assemblage Structure in Mississippi Sound as Revealed by Canonical Correspondence Analysis. Estuar. Coast. Shelf Sci. 1996, 43, 237–257. [Google Scholar] [CrossRef]
- Ramos, S.; Cowen, R.K.; Paris, C.; Ré, P.; Bordalo, A.A. Environmental forcing and larval fish assemblage dynamics in the Lima River estuary (northwest Portugal). J. Plankton Res. 2006, 28, 275–286. [Google Scholar] [CrossRef]
- Cushing, D.H. Plankton Production and Year-class Strength in Fish Populations: An Update of the Match/Mismatch Hypothesis. In Advances in Marine Biology; Blaxter, J.H.S., Southward, A.J., Eds.; Academic Press: Cambridge, MA, USA, 1990; Volume 26, pp. 249–293. [Google Scholar]
- Fortier, L.; Ponton, D.; Gilbert, M. The match/mismatch hypothesis and the feeding success of fish larvae in ice-covered southeastern Hudson Bay. Mar. Ecol. Prog. Ser. 1995, 120, 11–27. [Google Scholar] [CrossRef]
- Kristiansen, T.; Drinkwater, K.F.; Lough, R.G.; Sundby, S. Recruitment variability in North Atlantic cod and match-mismatch dynamics. PLoS ONE 2011, 6, e17456. [Google Scholar] [CrossRef] [PubMed]
- Cowen, R.K.; Sponaugle, S. Larval dispersal and marine population connectivity. Annu. Rev. Mar. Sci. 2009, 1, 443–466. [Google Scholar] [CrossRef]
- Van der Veer, H.W.; Bolle, L.J.; Geffen, A.; Witte, J.I.J. Variability in transport of fish eggs and larvae. IV. Interannual variability in larval stage duration of immigrating plaice in the Dutch Wadden Sea. Mar. Ecol. Prog. Ser. 2009, 390, 213–223. [Google Scholar] [CrossRef]
- Vasconcelos, R.P.; Reis-Santos, P.; Cabral, H.N.; Costa, J.L.; Costa, M.J. River-Coast Connectivity, Estuarine Nursery Function and Coastal Fisheries. In Treatise on Estuarine and Coastal Science; Wolanski, E., McLusky, D., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 81–107. [Google Scholar]
- Ramos, S.; Paris, C.B.; Angélico, M.M. Larval fish dispersal along an estuarine–ocean gradient. Dispersal Dur. Early Life Hist. Fish 2017, 74, 1462–1473. [Google Scholar] [CrossRef]
- Gell, F.R.; Roberts, C.M. Benefits beyond boundaries: The fishery effects of marine reserves. Trends Ecol. Evol. 2003, 18, 448–455. [Google Scholar] [CrossRef]
- Sinopoli, M.; Cattano, C.; Chemello, R.; Timpanaro, A.; Milisenda, G.; Gristina, M. Nest-mediated parental care in a marine fish: Are large-scale nesting habitats selected and do these habitats respond to small-scale requirements? Mediterr. Mar. Sci. 2018, 19, 248–255. [Google Scholar] [CrossRef]
- Birkeland, C.; Dayton, P.K. The importance in fishery management of leaving the big ones. Trends Ecol. Evol. 2005, 20, 356–358. [Google Scholar] [CrossRef]
- Somarakis, S.; Tsoukali, S.; Giannoulaki, M.; Schismenou, E.; Nikolioudakis, N. Spawning stock, egg production and larval survival in relation to small pelagic fish recruitment. Mar. Ecol. Prog. Ser. 2019, 617–618, 113–136. [Google Scholar] [CrossRef]
- Sinopoli, M.; Cattano, C.; Chemello, R.; Timpanaro, A.; Timpanaro, V.; Gristina, M. Nest building in a Mediterranean wrasse (Symphodus ocellatus): Are the algae used randomly chosen or actively selected? Mar. Ecol. 2015, 36, 942–949. [Google Scholar] [CrossRef]
- Costa, M.D.P.; Muelbert, J.H.; Vieira, J.P.; Castello, J.P. Dealing with temporal variation and different life stages of whitemouth croaker Micropogonias furnieri (Actinopterygii, Sciaenidae) in species distribution modeling to improve essential estuarine fish habitat identification. Hydrobiologia 2015, 762, 195–208. [Google Scholar] [CrossRef]
- Pereira, R. Environmental Control of Planktonic Communities in a Coastal Marine Protected Area (PNLN). Master’s thesis, Universidade do Porto, Porto, Portugal, 2020. [Google Scholar]
- Relvas, P.; Barton, E.D.; Dubert, J.; Oliveira, P.B.; Peliz, Á.; da Silva, J.C.B.; Santos, A.M.P. Physical oceanography of the western Iberia ecosystem: Latest views and challenges. Prog. Oceanogr. 2007, 74, 149–173. [Google Scholar] [CrossRef]
- Alvarez, I.; Gomez-Gesteira, M.; deCastro, M.; Dias, J.M. Spatiotemporal evolution of upwelling regime along the western coast of the Iberian Peninsula. J. Geophys. Res. Ocean. 2008, 113, C07020. [Google Scholar] [CrossRef]
- Peliz, Á.; Dubert, J.; Santos, A.M.P.; Oliveira, P.B.; Le Cann, B. Winter upper ocean circulation in the Western Iberian Basin—Fronts, Eddies and Poleward Flows: An overview. Deep Sea Res. Part I Oceanogr. Res. Pap. 2005, 52, 621–646. [Google Scholar] [CrossRef]
- Amorim, E.; Ramos, S.; Elliott, M.; Bordalo, A.A. Immigration and early life stages recruitment of the European flounder (Platichthys flesus) to an estuarine nursery: The influence of environmental factors. J. Sea Res. 2016, 107, 56–66. [Google Scholar] [CrossRef]
- Ramos, S.; Ré, P.; Bordalo, A.A. Recruitment of flatfish species to an estuarine nursery habitat (Lima estuary, NW Iberian Peninsula). J. Sea Res. 2010, 64, 473–486. [Google Scholar] [CrossRef]
- Ramos, S.; Amorim, E.; Elliott, M.; Cabral, H.; Bordalo, A.A. Early life stages of fishes as indicators of estuarine ecosystem health. Ecol. Indic. 2012, 19, 172–183. [Google Scholar] [CrossRef]
- Azeiteiro, U.M.; Bacelar-Nicolau, L.; Resende, P.; Gonçalves, F.; Pereira, M.J. Larval fish distribution in shallow coastal waters off North Western Iberia (NE Atlantic). Estuar. Coast. Shelf Sci. 2006, 69, 554–566. [Google Scholar] [CrossRef]
- Gonçalves, R.; Correia, A.D.; Atanasova, N.; Teodósio, M.A.; Ben-Hamadou, R.; Chícharo, L. Environmental factors affecting larval fish community in the salt marsh area of Guadiana estuary (Algarve, Portugal). Sci. Mar. 2015, 79, 25–34. [Google Scholar] [CrossRef]
- Russell, F.S. The Eggs and Planktonic Stages of British Marine Fishes; Academic Press: Cambridge, MA, USA, 1976. [Google Scholar]
- Ré, P.; Meneses, I. Early Stages of Marine Fishes Occurring in the Iberian Peninsula; IPIMAR: Matosinhos, Portugal, 2008. [Google Scholar]
- García, A.; Rodriguez, J.; Alemany, F. A Guide to the Eggs and Larvae of 100 Common Western Mediterranean Sea Bony Fish Species; FAO: Rome, Italy, 2018; p. 256. [Google Scholar]
- Munk, P.; Nielsen, J.G. Eggs and Larvae of North Sea Fishes; Biofolia: Frederiksberg, Denmark, 2005. [Google Scholar]
- Parsons, T.R.; Maita, Y.; Lalli, C.M. A Manual of Chemical and Biological Methods for Seawater Analysis; Pergamon: Elmsford, NY, USA, 1984; pp. 101–112. [Google Scholar]
- SCOR-UNESCO. Determination of photosynthetic pigments in seawater. Report of the SCOR-UNESCO Working Group 17. In Monographs on Oceanographic Methodology; UNESCO, Ed.; UNESCO: Paris, France, 1966; pp. 11–18. [Google Scholar]
- Grasshoff, K.; Ehrhardt, M.; Kremling, K. Methods of Seawater Analysis; Verlag Chemie: Weinheim, Germany, 1983; Volume Second revised and extended edition; p. 419. [Google Scholar]
- Jones, M.N. Nitrate reduction by shaking with cadmium: Alternative to cadmium columns. Water Res. 1984, 18, 643–646. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communications; University of Illinois Press: Urbana, IL, USA, 1963. [Google Scholar]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Franco, A.; Elliott, M.; Franzoi, P.; Torricelli, P. Life strategies of fishes in European estuaries: The functional guild approach. Mar. Ecol. Prog. Ser. 2008, 354, 219–228. [Google Scholar] [CrossRef]
- DGRM. Recursos da Pesca; Direção-Geral de Recursos Naturais, Segurança e Serviços Marítimos: Lisbon, Portugal, 2020; p. 181. [Google Scholar]
- Spjøtvoll, E.; Stoline, M.R. An Extension of the T-Method of Multiple Comparison to Include the Cases with Unequal Sample Sizes. J. Am. Stat. Assoc. 1973, 68, 975–978. [Google Scholar] [CrossRef]
- Clarke, K.; Warwick, R.; Clarke, K.R.; Warwick, R.M. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation; Primer-E, Ltd.: Plymouth, UK, 2001; p. 144. [Google Scholar]
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar] [CrossRef]
- Braak, C.J.F.T. Canonical Correspondence Analysis: A New Eigenvector Technique for Multivariate Direct Gradient Analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef]
- Braak, C.J.F.t.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca NY, USA, 2002. [Google Scholar]
- Rodriguez, J.M.; Gonzalez-Nuevo, G.; Gonzalez-Pola, C.; Cabal, J. The ichthyoplankton assemblage and the environmental variables off the NW and N Iberian Peninsula coasts, in early spring. Cont. Shelf Res. 2009, 29, 1145–1156. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Almeida, C.M.R.; Silva, D.; Cunha, J.; Antunes, C.; Freitas, V.; Ramos, S. Microplastic contamination in an urban estuary: Abundance and distribution of microplastics and fish larvae in the Douro estuary. Sci. Total Environ. 2019, 659, 1071–1081. [Google Scholar] [CrossRef]
- Bernal, M.; Stratoudakis, Y.; Coombs, S.; Angelico, M.M.; de Lanzós, A.L.; Porteiro, C.; Sagarminaga, Y.; Santos, M.; Uriarte, A.; Cunha, E.; et al. Sardine spawning off the European Atlantic coast: Characterization of and spatio-temporal variability in spawning habitat. Prog. Oceanogr. 2007, 74, 210–227. [Google Scholar] [CrossRef]
- Ré, P.; Silva, R.; Cunha, E.; Farinha, A.; Meneses, I.; Moita, T. Sardine spawning off Portugal. Bol. Do Inst. Nac. De Investig. Das Pescas 1990, 15, 31–44. [Google Scholar]
- Santos, A.M.P.; Nieblas, A.E.; Verley, P.; Teles-Machado, A.; Bonhommeau, S.; Lett, C.; Garrido, S.; Peliz, A. Sardine (Sardina pilchardus) larval dispersal in the Iberian upwelling system, using coupled biophysical techniques. Prog. Oceanogr. 2018, 162, 83–97. [Google Scholar] [CrossRef]
- Ramos, S.; Ré, P.; Bordalo, A.A. New insights into the early life ecology of Sardina pilchardus (Walbaum, 1792) in the northern Iberian Atlantic. Sci. Mar. 2009, 73, 449–459. [Google Scholar] [CrossRef]
- Beldade, R.; Borges, R.; Gonçalves, E.J. Depth distribution of nearshore temperate fish larval assemblages near rocky substrates. J. Plankton Res. 2006, 28, 1003–1013. [Google Scholar] [CrossRef]
- Garrido, S.; Santos, A.M.P.; dos Santos, A.; Ré, P. Spatial distribution and vertical migrations of fish larvae communities off Northwestern Iberia sampled with LHPR and Bongo nets. Estuar. Coast. Shelf Sci. 2009, 84, 463–475. [Google Scholar] [CrossRef]
- Santos, A.M.P.; Peliz, A.; Dubert, J.; Oliveira, P.B.; Angélico, M.M.; Ré, P. Impact of a winter upwelling event on the distribution and transport of sardine (Sardina pilchardus) eggs and larvae off western Iberia: A retention mechanism. Cont. Shelf Res. 2004, 24, 149–165. [Google Scholar] [CrossRef]
- Rodriguez, J.M. Assemblage structure of ichthyoplankton in the NE Atlantic in spring under contrasting hydrographic conditions. Sci. Rep. 2019, 9, 8636. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Morais, P.; Chícharo, M.A. Ichthyoplankton dynamics in the Guadiana estuary and adjacent coastal area, South-East Portugal. Estuar. Coast. Shelf Sci. 2006, 70, 85–97. [Google Scholar] [CrossRef]
- Primo, A.L.; Azeiteiro, U.M.; Marques, S.C.; Ré, P.; Pardal, M.A. Vertical patterns of ichthyoplankton at the interface between a temperate estuary and adjacent coastal waters: Seasonal relation to diel and tidal cycles. J. Mar. Syst. 2012, 95, 16–23. [Google Scholar] [CrossRef]
- Santos, A.M.P.; Ré, P.; dos Santos, A.; Peliz, Á. Vertical distribution of the European sardine (Sardina pilchardus) larvae and its implications for their survival. J. Plankton Res. 2006, 28, 523–532. [Google Scholar] [CrossRef]
- ICES. Bay of Biscay and Iberian Coast ecoregion—Fisheries overview, including mixed-fisheries considerations. In Report of the ICES Advisory Committee; ICES: Kopenhagen, Denmark, 2019. [Google Scholar] [CrossRef]
- Borges, R.; Beldade, R.; Gonçalves, E.J. Vertical structure of very nearshore larval fish assemblages in a temperate rocky coast. Mar. Biol. 2006, 151, 1349–1363. [Google Scholar] [CrossRef]
- Garrido, S.; Ben-Hamadou, R.; Santos, A.M.; Ferreira, S.; Teodosio, M.A.; Cotano, U.; Irigoien, X.; Peck, M.A.; Saiz, E.; Re, P. Born small, die young: Intrinsic, size-selective mortality in marine larval fish. Sci. Rep. 2015, 5, 17065. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.V.S.; Ramos, S.; Bonecker, A.C.T. Environmental control on larval stages of fish subject to specific salinity range in tropical estuaries. Reg. Stud. Mar. Sci. 2017, 13, 42–53. [Google Scholar] [CrossRef]
- Garrido, S.; Silva, A.; Marques, V.; Figueiredo, I.; Bryère, P.; Mangin, A.; Santos, A.M.P. Temperature and food-mediated variability of European Atlantic sardine recruitment. Prog. Oceanogr. 2017, 159, 267–275. [Google Scholar] [CrossRef]
- Lecchini, D.; Shima, J.; Banaigs, B.; Galzin, R. Larval sensory abilities and mechanisms of habitat selection of a coral reef fish during settlement. Oecologia 2005, 143, 326–334. [Google Scholar] [CrossRef]
- Arvedlund, M.; Kavanagh, K. The Senses and Environmental Cues Used by Marine Larvae of Fish and Decapod Crustaceans to Find Tropical Coastal Ecosystems. In Ecological Connectivity among Tropical Coastal Ecosystems; Nagelkerken, I., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 135–184. [Google Scholar]
- Hu, Y.; Majoris, J.E.; Buston, P.M.; Webb, J.F. Potential roles of smell and taste in the orientation behaviour of coral-reef fish larvae: Insights from morphology. J. Fish Biol. 2019, 95, 311–323. [Google Scholar] [CrossRef]
- Ramos, S.; Cabral, H.; Elliott, M. Do fish larvae have advantages over adults and other components for assessing estuarine ecological quality? Ecol. Indic. 2015, 55, 74–85. [Google Scholar] [CrossRef]
- Cabrita, M.T.; Silva, A.; Oliveira, P.B.; Angélico, M.M.; Nogueira, M. Assessing eutrophication in the Portuguese continental Exclusive Economic Zone within the European Marine Strategy Framework Directive. Ecol. Indic. 2015, 58, 286–299. [Google Scholar] [CrossRef]
- Lee, O.; Nash, R.D.M.; Danilowicz, B.S. Small-scale spatio-temporal variability in ichthyoplankton and zooplankton distribution in relation to a tidal-mixing front in the Irish Sea. ICES J. Mar. Sci. 2005, 62, 1021–1036. [Google Scholar] [CrossRef]
- Esteves, E.; Pina, T.; Chícharo, M.A.; Andrade, J.P. The distribution of estuarine fish larvae: Nutritional condition andco-occurrence with predators and prey. Acta Oecologica 2000, 21, 161–173. [Google Scholar] [CrossRef]
- Fiksen, Ø.; Jørgensen, C.; Kristiansen, T.; Vikebø, F.; Huse, G. Linking behavioural ecology and oceanography: Larval behaviour determines growth, mortality and dispersal. Mar. Ecol. Prog. Ser. 2007, 347, 195–205. [Google Scholar] [CrossRef]
- Stanley, R.; Snelgrove, P.V.; Deyoung, B.; Gregory, R.S. Dispersal patterns, active behaviour, and flow environment during early life history of coastal cold water fishes. PLoS ONE 2012, 7, e46266. [Google Scholar] [CrossRef] [PubMed]
- Teodósio, M.A.; Paris, C.B.; Wolanski, E.; Morais, P. Biophysical processes leading to the ingress of temperate fish larvae into estuarine nursery areas: A review. Estuar. Coast. Shelf Sci. 2016, 183, 187–202. [Google Scholar] [CrossRef]
- Wolanski, E. Bounded and unbounded boundaries–Untangling mechanisms for estuarine-marine ecological connectivity: Scales of m to 10,000 km–A review. Estuar. Coast. Shelf Sci. 2017, 198, 378–392. [Google Scholar] [CrossRef]




| Mean ± SD | MAX | MIN | |
|---|---|---|---|
| Temperature (°C) | 14.091 ± 1.204 | 16.778 | 11.999 |
| Salinity | 34.646 ± 0.484 | 35.503 | 33.521 |
| sat O2 (%) | 97.640 ± 8.536 | 130.650 | 84.958 |
| Diss O2 (mg/L) | 8.048 ± 0.614 | 10.417 | 7.069 |
| pH | 8.131 ± 0.134 | 8.312 | 7.865 |
| Turbidity (NTU) | 1.061 ± 0.676 | 4.985 | 0.064 |
| TPM (mg L−1) | 0.042 ± 0.008 | 0.058 | 0.025 |
| POM (mg L−1) | 0.010 ± 0.004 | 0.025 | 0.003 |
| Chlorophyll a (µg/L) | 3.259 ± 3.727 | 22.139 | 0.817 |
| Zooplankton (ind/m3) | 3.9 × 1011 ± 3.2 × 1011 | 1.5 × 1012 | 3.0 × 1010 |
| NO3 (μM L−1) | 6.456 ± 3.575 | 19.842 | 0.964 |
| NO2 (μM L−1) | 0.334 ± 0.113 | 0.754 | 0.057 |
| Nh4 (μM L−1) | 2.208 ± 1.904 | 23.731 | 0.412 |
| PO4 (μM L−1) | 0.750 ± 0.451 | 1.706 | 0.161 |
| Si (μM L−1) | 4.586 ± 3.760 | 22.598 | 0.720 |
| Family | Species | Commercial | EG | Mean Abundance | SD | FO (%) | Abundance (%) |
|---|---|---|---|---|---|---|---|
| Blenniidae | Parablennius gattorugine | No | MS | 8.402 | 16.743 | 66.667 | 54.647 |
| Ammodytidae | Ammodytes tobianus | No | MS | 2.420 | 7.970 | 30.159 | 15.739 |
| Clupeidae | Clupeidae n.i. | Yes | MM | 1.352 | 5.215 | 46.032 | 8.791 |
| Blenniidae | Parablennius ruber | No | MS | 0.347 | 0.876 | 30.159 | 2.256 |
| Sparidae | Boops boops | Yes | MS | 0.327 | 0.964 | 15.873 | 2.128 |
| Labridae | Symphodus melops | Yes | MS | 0.211 | 0.557 | 26.984 | 1.370 |
| Blenniidae | Parablennius pilicornis | No | MS | 0.195 | 0.490 | 25.397 | 1.271 |
| Blenniidae | Blennidae n.i. | No | MS | 0.152 | 0.522 | 17.460 | 0.989 |
| Labridae | Labridae n.i. | Yes | MS | 0.100 | 0.357 | 12.698 | 0.654 |
| Blenniidae | Lipophrys pholis | No | MS | 0.068 | 0.185 | 20.635 | 0.443 |
| Gobiidae | Gobiusculus flavescens | No | MS | 0.059 | 0.291 | 7.937 | 0.381 |
| Gobiidae | Gobiidae n.i. | No | 0.057 | 0.346 | 7.937 | 0.368 | |
| Gobiidae | Pomatoschistus minutus | No | ES | 0.055 | 0.332 | 3.175 | 0.357 |
| Cottidae | Taurulus bubalis | No | MS | 0.043 | 0.128 | 12.698 | 0.277 |
| Labridae | Ctenolabrus rupestris | Yes | MS | 0.040 | 0.151 | 7.937 | 0.263 |
| Callionymidae | Callionymus lyra | No | MS | 0.039 | 0.177 | 7.937 | 0.256 |
| Sparidae | Spondyliosoma cantharus | Yes | MS | 0.039 | 0.200 | 4.762 | 0.255 |
| Gobiidae | Pomatoschistus microps | No | ES | 0.031 | 0.143 | 6.349 | 0.204 |
| Labridae | Labrus bergylta | Yes | MS | 0.030 | 0.100 | 9.524 | 0.194 |
| Scombridae | Scomber spp. | Yes | MS | 0.028 | 0.169 | 4.762 | 0.185 |
| Gobiidae | Gobius spp. | No | 0.026 | 0.161 | 4.762 | 0.171 | |
| Sparidae | Diplodus sargus | Yes | MM | 0.023 | 0.159 | 3.175 | 0.151 |
| Atherinidae | Atherina presbyter | Yes | MM | 0.023 | 0.115 | 6.349 | 0.148 |
| Sparidae | Sparidae n.i. | Yes | MS | 0.013 | 0.061 | 4.762 | 0.085 |
| Liparidae | Liparis montagui | No | MS | 0.010 | 0.060 | 3.175 | 0.065 |
| Gadidae | Trisopterus luscus | Yes | MM | 0.010 | 0.044 | 4.762 | 0.064 |
| Gobiidae | Pomatoschistus pictus | No | MS | 0.009 | 0.049 | 3.175 | 0.056 |
| Pleuronectiform | Pleuronectiforms | Yes | 0.007 | 0.054 | 1.587 | 0.044 | |
| Blenniidae | Blennius ocellaris | No | MS | 0.006 | 0.046 | 1.587 | 0.038 |
| Labridae | Centrolabrus exoletus | Yes | MS | 0.005 | 0.042 | 1.587 | 0.035 |
| Blenniidae | Lipophrys triigloides | No | ES | 0.004 | 0.032 | 1.587 | 0.027 |
| Argentinidae | Argentina sphyraena | Yes | 0.004 | 0.031 | 1.587 | 0.026 | |
| Gadidae | Gadidae n.i. | Yes | 0.004 | 0.031 | 1.587 | 0.026 | |
| Soleidae | Pegusa lascaris | Yes | MS | 0.004 | 0.031 | 1.587 | 0.026 |
| Gadidae | Ciliata mustela | Yes | MM | 0.003 | 0.027 | 1.587 | 0.023 |
| Gobiidae | Gobius niger | No | ES | 0.003 | 0.027 | 1.587 | 0.023 |
| Syngnathidae | Nerophis lumbriciformis | No | ES | 0.003 | 0.026 | 1.587 | 0.022 |
| Gobiidae | Pomatoschistus spp. | No | ES | 0.003 | 0.025 | 1.587 | 0.021 |
| Callionymidae | Callionymus reticulatus | No | MS | 0.003 | 0.021 | 1.587 | 0.018 |
| n.i. | n.i. | 1.216 | 2.113 | 65.079 | 7.906 |
| ANOSIM | SIMPER | ||||
|---|---|---|---|---|---|
| Sampling Times | R | P | Dissimilarity (%) | Discriminating Species | Contribution (%) |
| Au17 vs. Wi18 | 1.000 | 0.004 | 98.25 | Ammodytes tobianus | 41.97 |
| Au17 vs. Sp18 | 0.938 | 0.001 | 98.96 | Parablennius gattorugine | 42.77 |
| Au17 vs. Su18 | 0.370 | 0.013 | 98.95 | Labridae n.i. | 30.03 |
| Au17 vs. Wi19 | 0.642 | 0.002 | 98.28 | Clupeidae n.i. | 19.11 |
| Au17 vs. Sp19 | 1.000 | 0.001 | 97.22 | Parablennius gattorugine | 51.15 |
| Au17 vs. Su19 | 1.000 | 0.002 | 97.48 | Clupeidae n.i. | 29.37 |
| Wi18 vs. Sp18 | 0.938 | 0.001 | 72.88 | Ammodytes tobianus | 32.07 |
| Wi18 vs. Su18 | 1.000 | 0.002 | 99.35 | Ammodytes tobianus | 45.90 |
| Wi18 vs. Wi19 | 0.963 | 0.003 | 83.24 | Ammodytes tobianus | 43.09 |
| Wi18 vs. Sp19 | 0.654 | 0.001 | 60.88 | Ammodytes tobianus | 41.00 |
| Wi18 vs. Su19 | 0.926 | 0.003 | 71.02 | Ammodytes tobianus | 35.55 |
| Sp18 vs. Su18 | 0.926 | 0.001 | 98.76 | Parablennius gattorugine | 46.68 |
| Sp18 vs. Wi19 | 0.901 | 0.004 | 88.10 | Parablennius gattorugine | 40.97 |
| Sp18 vs. Sp19 | 0.617 | 0.003 | 56.00 | Parablennius gattorugine | 28.81 |
| Sp18 vs. Su19 | 0.765 | 0.002 | 62.35 | Clupeidae n.i. | 24.76 |
| Su18 vs. Wi19 | 0.605 | 0.002 | 90.91 | Clupeidae n.i. | 22.13 |
| Su18 vs. Sp19 | 1.000 | 0.001 | 97.83 | Parablennius gattorugine | 55.77 |
| Su18 vs. Su19 | 1.000 | 0.002 | 95.17 | Clupeidae n.i. | 30.19 |
| Wi19 vs. Sp19 | 0.975 | 0.001 | 80.22 | Parablennius gattorugine | 51.60 |
| Wi19 vs. Su19 | 0.963 | 0.002 | 81.78 | Clupeidae n.i. | 26.28 |
| Sp19 vs. Su19 | 0.815 | 0.004 | 57.12 | Clupeidae n.i. | 31.38 |
| Environmental Variables | CCA1 | CCA2 |
|---|---|---|
| Temperature (°C) | 0.55 * | −0.31 |
| Salinity | 0.12 | 0.15 |
| sat O2 (%) | 0.06 | −0.25 |
| Diss O2 (mg/L) | −0.02 | −0.20 |
| pH | 0.16 | −0.73 * |
| Turbidity (NTU) | −0.17 | 0.33 |
| Zooplankton | 0.31 | −0.19 |
| Chlorophyll a (mg m−3) | 0.59 * | −0.32 |
| TPM (mg L−1) | 0.57 * | −0.44 * |
| POM (mg L−1) | 0.01 | 0.07 |
| NO3 (μM L−1) | −0.12 | 0.32 |
| NO2 (μM L−1) | −0.04 | 0.37 |
| Nh4 (μM L−1) | 0.21 | 0.32 |
| PO4 (μM L−1) | −0.36 | 0.50 * |
| Si (μM L−1) | −0.29 | 0.32 |
| Upwelling2m (m3 s−1 km−1) | 0.29 | 0.19 |
| Cavado_Riverflow (m3/s) | −0.64 * | 0.43 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, R.; Rodrigues, S.M.; Silva, D.M.; Ramos, S. Assessing Environmental Control on Temporal and Spatial Patterns of Larval Fish Assemblages in a Marine Protected Area. Ecologies 2023, 4, 288-309. https://doi.org/10.3390/ecologies4020019
Pereira R, Rodrigues SM, Silva DM, Ramos S. Assessing Environmental Control on Temporal and Spatial Patterns of Larval Fish Assemblages in a Marine Protected Area. Ecologies. 2023; 4(2):288-309. https://doi.org/10.3390/ecologies4020019
Chicago/Turabian StylePereira, Rúben, Sabrina M. Rodrigues, Diogo M. Silva, and Sandra Ramos. 2023. "Assessing Environmental Control on Temporal and Spatial Patterns of Larval Fish Assemblages in a Marine Protected Area" Ecologies 4, no. 2: 288-309. https://doi.org/10.3390/ecologies4020019
APA StylePereira, R., Rodrigues, S. M., Silva, D. M., & Ramos, S. (2023). Assessing Environmental Control on Temporal and Spatial Patterns of Larval Fish Assemblages in a Marine Protected Area. Ecologies, 4(2), 288-309. https://doi.org/10.3390/ecologies4020019






