Spatial Patterns in the Morphological Diversity of Madagascan Frogs
Abstract
1. Introduction
2. Materials and Methods
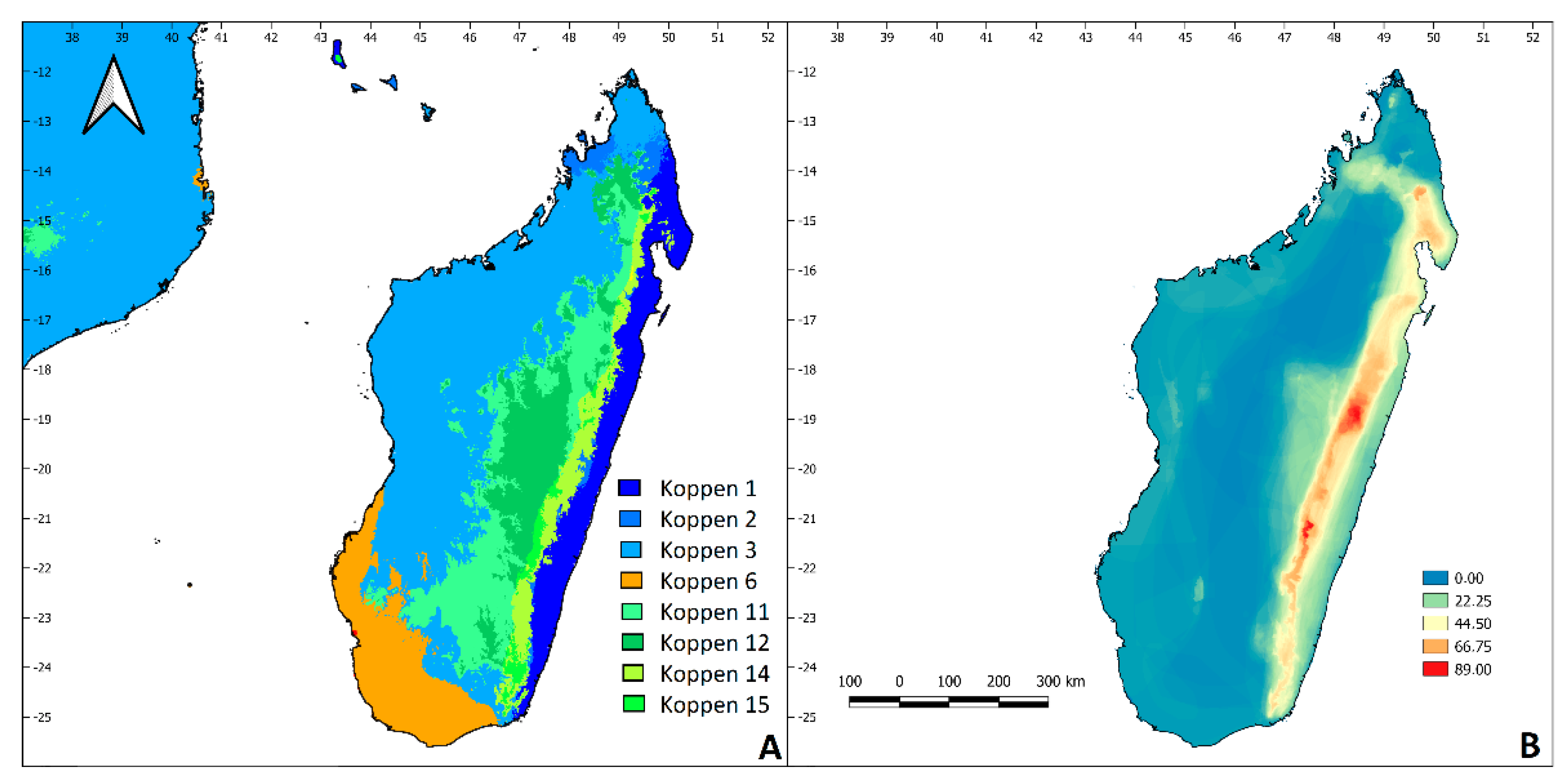
2.1. Study Region
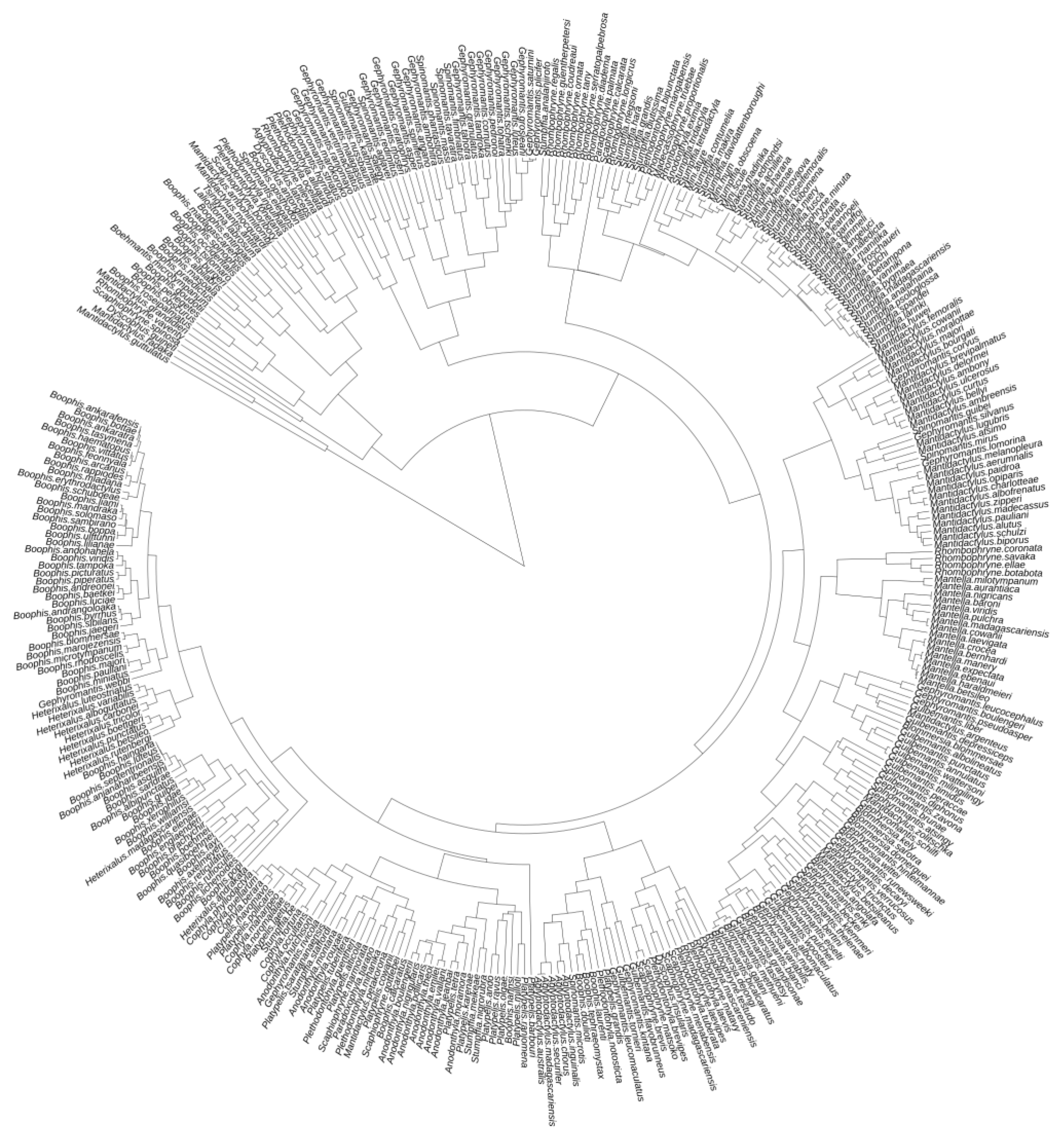
2.2. Species Data
2.3. Data Analyses
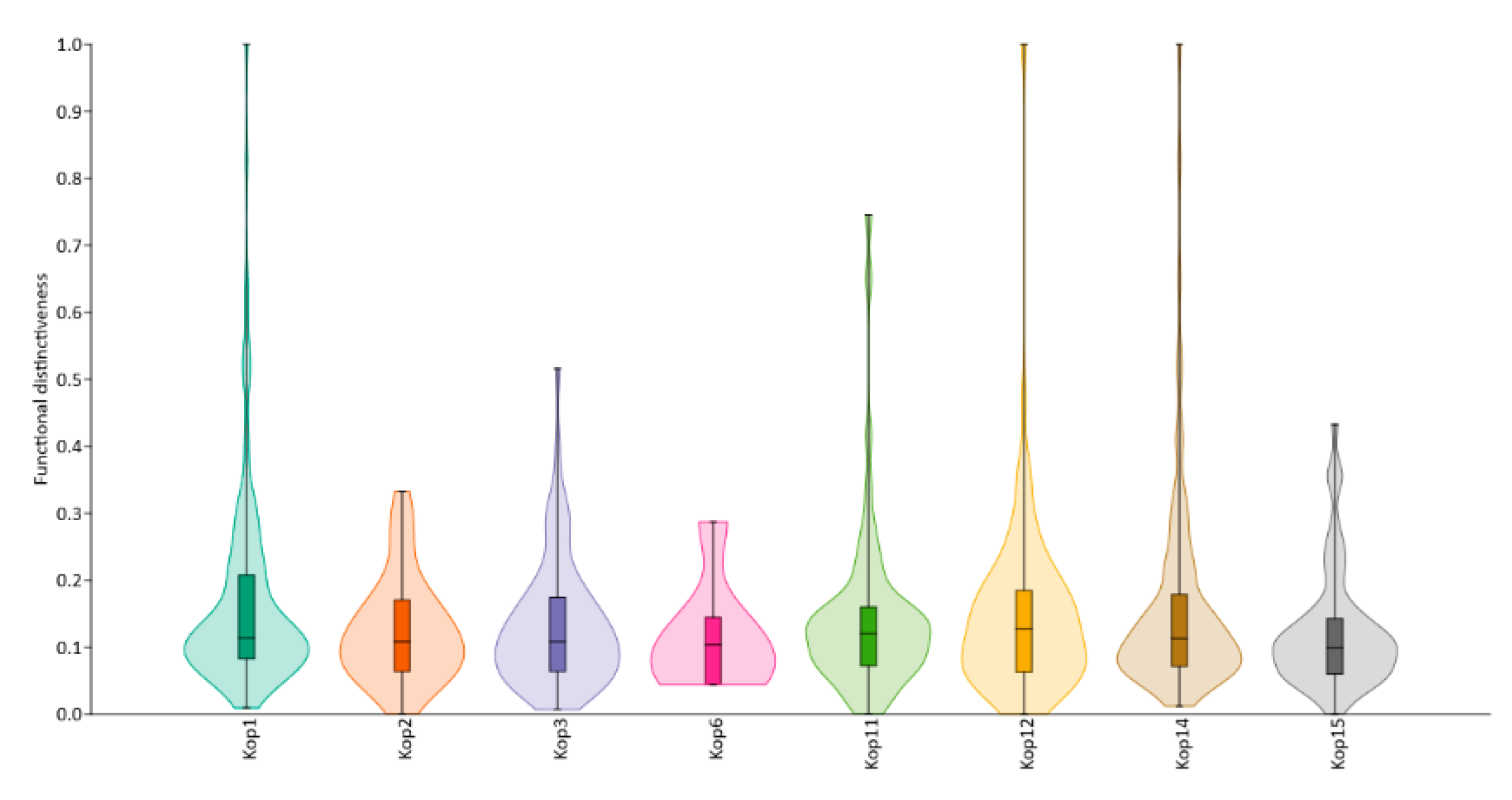
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ceballos, G.; Rodríguez, P.; Medellín, R.A. Assessing conservation priorities in megadiverse Mexico: Mammalian diversity, endemicity, and endangerment. Ecol. Appl. 1998, 8, 8–17. [Google Scholar] [CrossRef]
- Faith, D.P. Phylogenetic diversity, functional trait diversity and extinction: Avoiding tipping points and worst-case losses. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140011. [Google Scholar] [CrossRef]
- Loreau, M.; Naeem, S.; Inchausti, P.; Bengtsson, J.; Grime, J.P.; Hector, A.; Hooper, D.U.; Huston, M.A.; Raffaelli, D.; Schmid, B.; et al. Biodiversity and Ecosystem Functioning: Current Knowledge and Future Challenges. Science 2001, 294, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.U.; Chapin, F.S., III; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S.; et al. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Vaughn, C.C. Biodiversity Losses and Ecosystem Function in Freshwaters: Emerging Conclusions and Research Directions. Bioscience 2010, 60, 25–35. [Google Scholar] [CrossRef]
- Pimm, S.L. The complexity and stability of ecosystems. Nature 1984, 307, 321–326. [Google Scholar] [CrossRef]
- Rosenfeld, J.S. Functional redundancy in ecology and conservation. Oikos 2002, 98, 156–162. [Google Scholar] [CrossRef]
- Biggs, C.R.; Yeager, L.A.; Bolser, D.G.; Bonsell, C.; Dichiera, A.M.; Hou, Z.; Keyser, S.R.; Khursigara, A.J.; Lu, K.; Muth, A.F.; et al. Does functional redundancy affect ecological stability and resilience? A review and meta-analysis. Ecosphere 2020, 11, e03184. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hoey, A.S.; Choat, J.H. Limited functional redundancy in high diversity systems: Resilience and ecosystem function on coral reefs. Ecol. Lett. 2003, 6, 281–285. [Google Scholar] [CrossRef]
- Kruk, C.; Segura, A.M.; Costa, L.S.; Lacerot, G.; Kosten, S.; Peeters, E.T.H.M.; Huszar, V.L.M.; Mazzeo, N.; Scheffer, M. Functional redundancy increases towards the tropics in lake phytoplankton. J. Plankton Res. 2017, 39, 518–530. [Google Scholar] [CrossRef]
- Raine, E.H.; Gray, C.L.; Mann, D.J.; Slade, E.M. Tropical dung beetle morphological traits predict functional traits and show intraspecific differences across land uses. Ecol. Evol. 2018, 8, 8686–8696. [Google Scholar] [CrossRef] [PubMed]
- Almeida, R.P.S.; Teresa, F.B.; Camarota, F.; Izzo, T.J.; Silva, R.R.; Andrade-Silva, J.; de Arruda, F.V. The role of morphological traits in predicting the functional ecology of arboreal and ground ants in the Cerrado–Amazon transition. Oecologia 2023, 201, 199–212. [Google Scholar] [CrossRef]
- Baraloto, C.; Hardy, O.J.; Paine, C.E.T.; Dexter, K.G.; Cruaud, C.; Dunning, L.T.; Gonzalez, M.-A.; Molino, J.-F.; Sabatier, D.; Savolainen, V.; et al. Using functional traits and phylogenetic trees to examine the assembly of tropical tree communities. J. Ecol. 2012, 100, 690–701. [Google Scholar] [CrossRef]
- Funk, J.L.; Wolf, A.A. Testing the trait-based community framework: Do functional traits predict competitive outcomes? Ecology 2016, 97, 2206–2211. [Google Scholar] [CrossRef]
- Martini, S.; Larras, F.; Boyé, A.; Faure, E.; Aberle, N.; Archambault, P.; Bacouillard, L.; Beisner, B.E.; Bittner, L.; Castella, E.; et al. Functional trait-based approaches as a common framework for aquatic ecologists. Limnol. Oceanogr. 2021, 66, 965–994. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Bernhardt-Römermann, M.; Römermann, C.; Nuske, R.; Parth, A.; Klotz, S.; Schmidt, W.; Stadler, J. On the identification of the most suitable traits for plant functional trait analyses. Oikos 2008, 117, 1533–1541. [Google Scholar] [CrossRef]
- Mason, N.W.; de Bello, F.; Mouillot, D.; Pavoine, S.; Dray, S. A guide for using functional diversity indices to reveal changes in assembly processes along ecological gradients. J. Veg. Sci. 2013, 24, 794–806. [Google Scholar] [CrossRef]
- Ganzhorn, J.U.; Lowry, P.P., II; Schatz, G.E.; Sommer, S. The biodiversity of Madagascar: One of the world’s hottest hotspots on its way out. Oryx 2001, 35, 346–348. [Google Scholar] [CrossRef]
- Goodman, S.M. The New Natural History of Madagascar; Princeton University Press: Princenton, NJ, USA, 2022. [Google Scholar]
- Andreone, F.; Carpenter, A., I; Cox, N.; du Preez, L.; Freeman, K.; Furrer, S.; Garcia, G.; Glaw, F.; Glos, J.; Knox, D.; et al. The Challenge of Conserving Amphibian Megadiversity in Madagascar. PLOS Biol. 2008, 6, e118. [Google Scholar] [CrossRef]
- Vieilledent, G.; Grinand, C.; Rakotomalala, F.A.; Ranaivosoa, R.; Rakotoarijaona, J.-R.; Allnutt, T.F.; Achard, F. Combining global tree cover loss data with historical national forest cover maps to look at six decades of deforestation and forest fragmentation in Madagascar. Biol. Conserv. 2018, 222, 189–197. [Google Scholar] [CrossRef]
- Morelli, T.L.; Smith, A.B.; Mancini, A.N.; Balko, E.A.; Borgerson, C.; Dolch, R.; Farris, Z.; Federman, S.; Golden, C.D.; Holmes, S.M.; et al. The fate of Madagascar’s rainforest habitat. Nat. Clim. Chang. 2020, 10, 89–96. [Google Scholar] [CrossRef]
- Valsangkar, A.B.; Radhakrishnamurty, K.; Subbarao, K.V.; Beckinsale, R.D. Paleomagnetism and potassium-argon age studies of acid igneous rocks from the St. Mary Islands. Mem. Geol. Soc. India 1981, 3, 265–376. [Google Scholar]
- Plummer, P.S. The Amirante ridge/trough complex: Response to rotational transform rift/drift between Seychelles and Madagascar. Terra Nova 1996, 8, 34–47. [Google Scholar] [CrossRef]
- Frost, D.R. Amphibian Species of the World: An Online Reference; Version 6.1; American Museum of Natural History: New York, NY, USA; Available online: https://amphibiansoftheworld.amnh.org/index.php (accessed on 1 December 2022).
- Irwin, M.T.; Wright, P.C.; Birkinshaw, C.; Fisher, B.L.; Gardner, C.J.; Glos, J.; Goodman, S.M.; Loiselle, P.; Rabeson, P.; Raharison, J.-L.; et al. Patterns of species change in anthropogenically disturbed forests of Madagascar. Biol. Conserv. 2010, 143, 2351–2362. [Google Scholar] [CrossRef]
- Lehtinen, R.; Ramanamanjato, J.-B. Effects of rainforest fragmentation and correlates of local extinction in a herpetofauna from Madagascar. Appl. Herpetol. 2006, 3, 95–110. [Google Scholar] [CrossRef]
- Kolby, J.E.; Skerratt, L.F. Amphibian Chytrid Fungus in Madagascar neither Shows Widespread Presence nor Signs of Certain Establishment. PLoS ONE 2015, 10, e0139172. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.; Solofo NiainaFidy, J.F.; Edmonds, D. The New Toad in Town: Distribution of the Asian Toad, Duttaphrynus melanostictus, in the Toamasina Area of Eastern Madagascar. Trop. Conserv. Sci. 2015, 8, 440–455. [Google Scholar] [CrossRef]
- Edwards, W.M.; Griffiths, R.A.; Bungard, M.J.; Rakotondrasoa, E.F.; Razafimanahaka, J.H.; Andriantsimanarilafy, P.R.R.R.; Randrianantoandro, J.C. Microhabitat preference of the critically endangered Golden mantella frog in Madagascar. Herpetol. J. 2019, 29, 207–213. [Google Scholar] [CrossRef]
- Köppen, W.; Geiger, R. Klima der Erde (Climate of the Earth). Wall Map 1:16 Mill; Klett-Perthes: Stuttgart, Germany, 1954. [Google Scholar]
- Baker, B.; Diaz, H.; Hargrove, W.; Hoffman, F. Use of the Köppen–Trewartha climate classification to evaluate climatic refugia in statistically derived ecoregions for the People’s Republic of China. Clim. Chang. 2010, 98, 113–131. [Google Scholar] [CrossRef]
- Carroll, C.; Lawler, J.J.; Roberts, D.; Hamann, A. Biotic and Climatic Velocity Identify Contrasting Areas of Vulnerability to Climate Change. PLoS ONE 2015, 10, e0140486. [Google Scholar] [CrossRef] [PubMed]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [PubMed]
- Yoder, A.D.; Nowak, M.D. Has Vicariance or Dispersal Been the Predominant Biogeographic Force in Madagascar? Only Time Will Tell. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 405–431. [Google Scholar] [CrossRef]
- Amado, T.F.; Bidau, C.J.; Olalla-Tárraga, M.Á. Geographic variation of body size in New World anurans: Energy and water in a balance. Ecography 2019, 42, 456–466. [Google Scholar] [CrossRef]
- Wells, K.D. The Ecology and Behavior of Amphibians; University of Chicago Press: Chicago, IL, USA, 2010. [Google Scholar]
- Monadjem, A.; Kane, A.; Taylor, P.; Richards, L.; Hall, G.; Woodborne, S. Morphology and stable isotope analysis demonstrate different structuring of bat communities in rain-forest and savannah habitats. R. Soc. Open Sci. 2018, 5, 180849. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available online: http://qgis.osgeo.org (accessed on 5 March 2023).
- International Union for Conservation of Nature (IUCN). The IUCN Red List of Threatened Species. Version 2021-3. Available online: http://www.iucnredlist.org (accessed on 12 May 2023).
- Hurlbert, A.H.; Jetz, W. Species richness, hotspots, and the scale dependence of range maps in ecology and conservation. Proc. Natl. Acad. Sci. USA 2007, 104, 13384–13389. [Google Scholar] [CrossRef]
- Glaw, F.; Vences, M. A Field Guide to the Amphibians and Reptiles of Madagascar; Moos Druck: Köln, Germany, 1994. [Google Scholar]
- Vences, M.; Glaw, F.; Andreone, F.; Jesu, R.; Schimmenti, G. Systematic revision of the enigmatic Malagasy broad-headed frogs (Laurentomantis Dubois, 1980), and their phylogenetic position within the endemic mantellid radiation of Madagascar. Contrib. Zool. 2002, 70, 191–212. [Google Scholar] [CrossRef]
- vences, m.; glaw, f. Systematic review and molecular phylogenetic relationships of the direct developing Malagasy anurans of the Mantidactylus asper group (Amphibia, Mantellidae). Alytes 2001, 19, 107–139. [Google Scholar]
- Rakotoarison, A.; Crottini, A.; Müller, J.; Rödel, M.-O.; Glaw, F.; Vences, M. Revision and phylogeny of narrow-mouthed treefrogs (Cophyla) from northern Madagascar: Integration of molecular, osteological, and bioacoustic data reveals three new species. Zootaxa 2015, 3937, 61–89. [Google Scholar] [CrossRef] [PubMed]
- Amat, F.; Wollenberg, K.C.; Vences, M. Correlates of eye colour and pattern in mantellid frogs. Salamandra 2013, 49, 7–17. [Google Scholar]
- Rosa, G.M.; Penny, S.G.; Andreone, F.; Crottini, A.; Holderied, M.W.; Rakotozafy, L.S.; Schwitzer, C. A new species of the Boophis rappiodes group (Anura, Mantellidae) from the Sahamalaza Peninsula, northwest Madagascar, with acoustic monitoring of its nocturnal calling activity. Zookeys 2014, 435, 111–132. [Google Scholar] [CrossRef]
- Vences, M.; Rakotoarison, A.; Rakotondrazafy, A.M.A.; Ratsoavina, F.M.; Randrianiaina, R.D.; Glaw, F.; Lehtinen, R.M.; Raxworthy, C.J. Assessing the diversity of phytotelmic frogs along Madagascar’s east coast: Redefinition of Guibemantis bicalcaratus (Boettger, 1913) and revalidation of Guibemantis methueni (Angel, 1929). Vertebr. Zool. 2013, 63, 193–205. [Google Scholar] [CrossRef]
- Hillman, S.S.; Withers, P.C.; Drewes, R.C.; Hillyard, S.D. Ecological and Environmental Physiology of Amphibians; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Bowman, A.W.; Azzalini, A. Applied Smoothing Techniques for Data Analysis: The Kernel Approach with S-Plus Illustrations; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Bonferroni, C. Teoria statistica delle classi e calcolo delle probabilita. Pubbl. R Ist. Sup. Sci. Econ. Commer. Fir. 1936, 8, 3–62. [Google Scholar]
- Bowman, A.W.; Azzalini, A. R Package ‘Sm’: Nonparametric Smoothing Methods (version 2.2-5.7). Available online: http://www.stats.gla.ac.uk/~adrian/sm (accessed on 18 July 2021).
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, Austria. Available online: https://www.r-project.org/ (accessed on 15 October 2022).
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Davies, T.J. Phylogenies in Ecology: A Guide to Concepts and Methods; Princeton University Press: Princeton, NJ, USA, 2016. [Google Scholar]
- Violle, C.; Thuiller, W.; Mouquet, N.; Munoz, F.; Kraft, N.J.; Cadotte, M.W.; Livingstone, S.W.; Mouillot, D. Functional Rarity: The Ecology of Outliers. Trends Ecol. Evol. 2017, 32, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Grenié, M.; Denelle, P.; Tucker, C.M.; Munoz, F.; Violle, C. Funrar: An R package to characterize functional rarity. Divers. Distrib. 2017, 23, 1365–1371. [Google Scholar] [CrossRef]
- Gower, J.C. Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 1966, 53, 325–338. [Google Scholar] [CrossRef]
- Pavoine, S.; Vallet, J.; Dufour, A.B.; Gachet, S.; Daniel, H. On the challenge of treating various types of variables: Application for improving the measurement of functional diversity. Oikos 2009, 118, 391–402. [Google Scholar] [CrossRef]
- Harmon, L.J.; Losos, J.B. The effect of intraspecific sample size on type I and type II error rates in comparative studies. Evolution 2005, 59, 2705–2710. [Google Scholar]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
- Spearman, C. The Proof and Measurement of Association between Two Things. Am. J. Psychol. 1904, 15, 72–101. [Google Scholar] [CrossRef]
- Tobar-Suárez, C.; Urbina-Cardona, N.; Villalobos, F.; Pineda, E. Amphibian species richness and endemism in tropical montane cloud forests across the Neotropics. Biodivers. Conserv. 2021, 31, 295–313. [Google Scholar] [CrossRef]
- Salick, J.; Pong, T.Y. An Analysis of Termite Faunae in Malayan Rainforests. J. Appl. Ecol. 1984, 21, 547–561. [Google Scholar] [CrossRef]
- Heying, H.E. Social and reproductive behaviour in the Madagascan poison frog, Mantella laevigata, with comparisons to the dendrobatids. Anim. Behav. 2001, 61, 567–577. [Google Scholar] [CrossRef]
- Lehtinen, R. The use of screw pines (Pandanus spp.) by amphibians and reptiles in Madagascar. Herpetol. Bull. 2002, 82, 20–25. [Google Scholar]
- Wells, N.A. Some Hypothesis on the Mesozoic and Cenozoic Paleoenvironmental History of Madagascar. In The Natural History of Madagascar; Goodman, S.M., Benstead, J.P., Eds.; Chicago Press: Chicago, IL, USA, 2003; pp. 16–33. [Google Scholar]
- Strauß, A.; Reeve, E.; Randrianiaina, R.D.; Vences, M.; Glos, J. The world’s richest tadpole communities show functional re-dundancy and low functional diversity: Ecological data on Madagascar’s stream-dwelling amphibian larvae. BMC Ecol. 2010, 10, 12. [Google Scholar] [CrossRef]
- Rakotoarinivo, M.; Blach-Overgaard, A.; Baker, W.J.; Dransfield, J.; Moat, J.; Svenning, J.-C. Palaeo-precipitation is a major determinant of palm species richness patterns across Madagascar: A tropical biodiversity hotspot. Proc. R. Soc. B Boil. Sci. 2013, 280, 20123048. [Google Scholar] [CrossRef]
- Wilson, K.A.; Underwood, E.C.; Morrison, S.A.; Klausmeyer, K.R.; Murdoch, W.W.; Reyers, B.; Wardell-Johnson, G.; Marquet, P.A.; Rundel, P.W.; McBride, M.F.; et al. Conserving Biodiversity Efficiently: What to Do, Where, and When. PLoS Biol. 2007, 5, e223. [Google Scholar] [CrossRef]
- Andreone, F. A Conservation Strategy for the Amphibians of Madagascar; Museo Regionale di Scienze Naturali: Torino, Italy, 2008. [Google Scholar]
- Scherz, M.D.; Hutter, C.; Rakotoarison, A.; Riemann, J.C.; Rödel, M.-O.; Ndriantsoa, S.H.; Glos, J.; Roberts, S.H.; Crottini, A.; Vences, M.; et al. Morphological and ecological convergence at the lower size limit for vertebrates highlighted by five new miniaturised microhylid frog species from three different Madagascan genera. PLoS ONE 2019, 14, e0213314. [Google Scholar] [CrossRef]
- Hooper, D.U.; Dukes, J.S. Functional composition controls invasion success in a California serpentine grassland. J. Ecol. 2010, 98, 764–777. [Google Scholar] [CrossRef]
- Jenkins, R.K.; Brady, L.D.; Bisoa, M.; Rabearivony, J.; Griffiths, R.A. Forest disturbance and river proximity influence cha-meleon abundance in Madagascar. Biol. Conserv. 2003, 109, 407–415. [Google Scholar] [CrossRef]
- Campera, M.; Santini, L.; Balestri, M.; Nekaris, K.A.I.; Donati, G. Elevation gradients of lemur abundance emphasise the importance of Madagascar’s lowland rainforest for the conservation of endemic taxa. Mammal Rev. 2019, 50, 25–37. [Google Scholar] [CrossRef]


| Trait | Range | Interpretation | Trait | Range | Interpretation |
|---|---|---|---|---|---|
| Snout–urostyle length | 8.0–125.6 | Body size (mm) | Number of toes | 3 4 5 | Miniaturization Miniaturization Ancestral |
| Finger–pads | 0 1 2 | Absent Slightly enlarged Distinctly enlarged | Dorsal skin | 0 1 2 | Smooth–finely granular Disseminated tubercles–ridges Roughly granular |
| Hand webbing | 0 1 2 | Absent Reduced Developed | Supra–ocular tubercles | 0 1 2 | Absent Small Large |
| Foot webbing | 0 1 2 3 | Absent Reduced Developed Fully webbed | Limb tubercles | 0 1 2 | Absent Heel–elbow Arms–legs |
| Tibiotarsal joint reaching | 0 1 2 3 4 | Forelimb Tympanum Eye Nostril Snout tip | Ventral coloration | 0 1 2 3 4 | White/unpigmented Dark spotted Grey–amber Dark Intensely colored |
| Outer metatarsal tubercle | 0 1 | Absent Present | Pupils | 0 1 2 | Vertical Horizontal Horizontal indistinct |
| Femoral glands | 0 1 | Absent Present |
| Kop1 | Kop2 | Kop3 | Kop6 | Kop11 | Kop12 | Kop14 | |
|---|---|---|---|---|---|---|---|
| Kop1 | |||||||
| Kop2 | 0.0 | ||||||
| Kop3 | 0.0 | 0.0 | |||||
| Kop6 | 0.700 | 0.196 | 1.00 | ||||
| Kop11 | 0.0 | 0.0 | 1.00 | 1.00 | |||
| Kop12 | 0.0 | 0.0 | 1.00 | 1.00 | 1.00 | ||
| Kop14 | 0.0 | 0.0 | 0.0 | 1.00 | 0.0 | 0.0 | |
| Kop15 | 0.0 | 0.0 | 0.0 | 0.308 | 0.0 | 0.0 | 0.0 |
| N Taxa | PD | SES PD | p < 0.05% | p > 0.95% | |
|---|---|---|---|---|---|
| Köppen 1 | 129 | 4.472 | 1.295 | 0.0 | 26.2 |
| Köppen 2 | 27 | 1.309 | −2.518 | 98.2 | 0.0 |
| Köppen 3 | 76 | 2.764 | −2.465 | 95.0 | 0.0 |
| Köppen 6 | 7 | 0.621 | −0.680 | 8.4 | 0.0 |
| Köppen 11 | 61 | 2.715 | −0.122 | 0.0 | 0.0 |
| Köppen12 | 82 | 3.412 | 0.915 | 0.0 | 6.2 |
| Köppen 14 | 150 | 4.815 | 0.996 | 0.0 | 7.8 |
| Köppen 15 | 65 | 2.684 | −1.085 | 15.3 | 0.0 |
| N Taxa | MPD | SES MPD | p < 0.05% | p > 0.95% | |
|---|---|---|---|---|---|
| Köppen 1 | 129 | 0.208 | 2.615 | 0.0 | 99.8 |
| Köppen 2 | 27 | 0.176 | −0.796 | 0.0 | 0.0 |
| Köppen 3 | 76 | 0.179 | −1.190 | 8.5 | 0.0 |
| Köppen 6 | 7 | 0.137 | −1.331 | 49.0 | 0.0 |
| Köppen 11 | 61 | 0.186 | −0.461 | 0.0 | 0.0 |
| Köppen12 | 82 | 0.191 | 0.014 | 0.0 | 0.0 |
| Köppen 14 | 150 | 0.195 | 0.766 | 0.0 | 0.0 |
| Köppen 15 | 65 | 0.172 | −1.749 | 65.7 | 0.0 |
| Temperature | Precipitation | Prec. Seasonality | ||
|---|---|---|---|---|
| N Taxa | Spearman’s rs | −0.405 | 0.670 | −0.524 |
| p | 0.329 | 0.069 | 0.197 | |
| Mean SUL | Spearman’s rs | −0.286 | −0.478 | 0.151 |
| p | 0.501 | 0.231 | 0.571 | |
| Mean FD | Spearman’s rs | −0.286 | 0.693 | −0.452 |
| p | 0.501 | 0.057 | 0.267 | |
| P95 FD | Spearman’s rs | −0.149 | 0.746 | −0.671 |
| p | 0.725 | 0.035 | 0.075 | |
| SES PD | Spearman’s rs | −0.495 | 0.278 | −0.524 |
| p | 0.213 | 0.504 | 0.197 | |
| SES MPD | Spearman’s rs | 0.021 | 0.740 | −0.476 |
| p | 0.961 | 0.036 | 0.243 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escoriza, D.; Poch, S.; Boix, D. Spatial Patterns in the Morphological Diversity of Madagascan Frogs. Ecologies 2023, 4, 499-511. https://doi.org/10.3390/ecologies4030032
Escoriza D, Poch S, Boix D. Spatial Patterns in the Morphological Diversity of Madagascan Frogs. Ecologies. 2023; 4(3):499-511. https://doi.org/10.3390/ecologies4030032
Chicago/Turabian StyleEscoriza, Daniel, Santiago Poch, and Dani Boix. 2023. "Spatial Patterns in the Morphological Diversity of Madagascan Frogs" Ecologies 4, no. 3: 499-511. https://doi.org/10.3390/ecologies4030032
APA StyleEscoriza, D., Poch, S., & Boix, D. (2023). Spatial Patterns in the Morphological Diversity of Madagascan Frogs. Ecologies, 4(3), 499-511. https://doi.org/10.3390/ecologies4030032







