Preserving Microbial Biodiversity: The Case of Food-Associated Microorganisms
Abstract
:1. Introduction
2. Food-Associated Microorganisms
2.1. Beneficial Microorganisms
2.2. Spoilage Microorganisms
2.2.1. Spoilage of Meat and Meat Products
2.2.2. Spoilage of Milk and Dairy Products
2.2.3. Spoilage of Fruits and Vegetables
2.3. Pathogenic Microorganisms
3. The Concept of Native Food Microbiota
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Escalas, A.; Hale, L.; Coordeckers, J.W.; Yang, Y.; Firestone, M.K.; Alvarez-Cohen, L.; Zhou, J. Microbial functional diversity: From concepts to applications. Ecol. Evol. 2019, 9, 12000–12016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, S.; Kumar, G.; Chhabra, S.; Prasad, R. Role of soil microbes in biogeochemical cycle for enhancing soil fertility. In New and Future Developments in Microbial Biotechnology and Bioengineering. Phytomicrobiome for Sustainable Agriculture; Verma, J.P., Macdonald, C.A., Gupta, V.K., Podile, A.R., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2021; pp. 149–157. [Google Scholar]
- McKenney, E.A.; Koelle, K.; Dunn, R.R.; Yoder, A.D. The ecosystem services of animal microbiomes. Mol. Ecol. 2018, 27, 2164–2172. [Google Scholar] [CrossRef] [PubMed]
- Byrd, A.L.; Belkaid, Y.; Serge, J.A. The human skin microbiome. Nat. Rev. Microbiol. 2018, 16, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Bull, A.T.; Goodfellow, M.; Slater, J.H. Biodiversity as a source of innovation in biotechnology. Annu. Rev. Microbiol. 1992, 46, 219–252. [Google Scholar] [CrossRef]
- Bodelier, P.L.E. Towards understanding, managing, and protecting microbial ecosystems. Front. Microbiol. 2011, 2, 80. [Google Scholar] [CrossRef] [Green Version]
- Vuong, P.; Chong, S.; Kaur, P. The little things that matter: How bioprospecting microbial biodiversity can build towards the realization of United Nations Sustainable Development Goals. npj Biodivers. 2020, 1, 4. [Google Scholar] [CrossRef]
- Colwell, R.R. Microbial diversity: The importance of exploration and conservation. J. Ind. Microbiol. Biotechnol. 1997, 18, 302–307. [Google Scholar] [CrossRef]
- Drosinos, E.H.; Paramithiotis, S. Trends in lactic acid fermentation. In Food Microbiology Research Trends; Palino, M.V., Ed.; Nova Publishers: Hauppauge, NY, USA, 2007; pp. 39–92. [Google Scholar]
- Paramithiotis, S.; Drosinos, E.H.; Sofos, J.; Nychas, G.J.E. Fermentation: Microbiology and biochemistry. In Handbook of Meat Processing; Toldra, F., Ed.; Springer: New York, NY, USA, 2010; pp. 185–198. [Google Scholar]
- Paramithiotis, S.; Papoutsis, G.; Drosinos, E.H. Lactic acid fermentation of fruits and vegetables; an overview. In Lactic Acid Fermentation of Fruits and Vegetables; Paramithiotis, S., Ed.; CRC Science: Cleveland, OH, USA, 2017; pp. 1–18. [Google Scholar]
- Paramithiotis, S. Microorganisms associated with food fermentation. In Bioactive Compounds in Fermented Foods: Health Aspects; Rai, A.K., Anu Appaiah, K.A., Eds.; CRC Press: Cleveland, OH, USA, 2021; pp. 3–47. [Google Scholar]
- Lee, J.S.; Heo, G.Y.; Lee, J.W.; Oh, Y.J.; Park, J.A.; Park, Y.H.; Pyun, Y.R.; Ahn, J.S. Analysis of kimchi microflora using denaturing gradient gel electrophoresis. Int. J. Food Microbiol. 2005, 102, 143–150. [Google Scholar] [CrossRef]
- Cho, J.; Lee, D.; Yang, C.; Jeon, J.; Kim, J.; Han, H. Microbial population dynamics of kimchi, a fermented cabbage product. FEMS Microbiol. Lett. 2006, 257, 262–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.W.; Kim, K.H.; Nam, Y.D.; Roh, S.W.; Kim, M.S.; Jeon, C.O.; Oh, H.M.; Bae, J.W. Analysis of yeast and archaeal population dynamics in kimchi using denaturing gradient gel electrophoresis. Int. J. Food Microbiol. 2008, 126, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.Y.; Lee, S.H.; Jin, H.M.; Hahn, Y.; Madsen, E.L.; Jeon, C.O. Metatranscriptomic analysis of lactic acid bacterial gene expression during kimchi fermentation. Int. J. Food Microbiol. 2013, 163, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Jung, J.Y.; Lee, S.H.; Jin, H.M.; Jeon, C.O. Microbial succession and metabolite changes during fermentation of dongchimi, traditional Korean watery kimchi. Int. J. Food Microbiol. 2013, 164, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Lee, S.H.; Jung, J.Y.; Choi, E.J.; Jeon, C.O. Microbial succession and metabolite changes during long-term storage of kimchi. J. Food Sci. 2013, 78, M763–M769. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Yang, H.S.; Chang, H.C.; Kim, H.Y. Comparison of bacterial community changes in fermenting kimchi at two different temperatures using a denaturing gradient gel electrophoresis analysis. J. Microbiol. Biotechnol. 2013, 23, 76–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.C. Healthy and safe Korean traditional fermented foods: Kimchi and chongkukjang. J. Ethn. Foods 2018, 5, 161–166. [Google Scholar] [CrossRef]
- Harris, L.J. The Microbiology of vegetable fermentations. In Microbiology of Fermented Foods; Wood, B.J.B., Ed.; Blackie Academic and Professional: London, UK, 1998; pp. 45–72. [Google Scholar]
- Barrangou, R.; Yoon, S.S.; Breidt, F., Jr.; Fleming, H.P.; Klaenhammer, T.R. Identification and characterization of Leuconostoc fallax strains isolated from an industrial sauerkraut fermentation. Appl. Environ. Microbiol. 2002, 68, 2877–2884. [Google Scholar] [CrossRef] [Green Version]
- Beganovic, J.; Kos, B.; Lebos Pavunc, A.; Uroic, K.; Jokic, M.; Suskovi, J. Traditionally produced sauerkraut as source of autochthonous functional starter cultures. Microbiol. Res. 2014, 169, 623–632. [Google Scholar] [CrossRef]
- Gobbetti, M.; Corsetti, A.; Rossi, J.; La Rosa, F.; De Vincenzi, S. Identification and clustering of lactic acid bacteria and yeasts from wheat sourdoughs of central Italy. Ital. J. Food Sci. 1994, 6, 85–94. [Google Scholar]
- Garofalo, C.; Silvestri, G.; Aquilanti, L.; Clementi, F. PCR-DGGE analysis of lactic acid bacteria and yeast dynamics during the production processes of three varieties of Panettone. J. Appl. Microbiol. 2008, 105, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, A.; Lavermicocca, P.; Morea, M.; Baruzzi, F.; Tosti, N.; Gobbetti, M. Phenotypic and molecular identification and clustering of lactic acid bacteria and yeasts from wheat (species Triticum durum and Triticum aestivum) sourdoughs of southern Italy. Int. J. Food Microbiol. 2001, 64, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, C.L.; Heilig, H.; Restuccia, C.; Giudici, P.; Caggia, C. Bacterial population in traditional sourdough evaluated by molecular methods. J. Appl. Microbiol. 2005, 99, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Zotta, T.; Piraino, P.; Parente, E.; Salzano, G.; Ricciardi, A. Characterization of lactic acid bacteria isolated from sourdoughs for Cornetto, a traditional bread produced in Basilicata (Southern Italy). World J. Microbiol. Biotechnol. 2008, 24, 1785–1795. [Google Scholar] [CrossRef]
- Iacumin, L.; Cecchini, F.; Manzano, M.; Osualdini, M.; Boscolo, D.; Orlic, S.; Comi, G. Description of the microflora of sourdoughs by culture-dependent and culture independent methods. Food Microbiol. 2009, 26, 128–135. [Google Scholar] [CrossRef]
- Osimani, A.; Zannini, E.; Aquilanti, L.; Mannazzu, I.; Comitini, F.; Clementi, F. Lactic acid bacteria and yeasts from wheat sourdoughs of the Marche Region. Ital. J. Food Sci. 2009, 21, 269–286. [Google Scholar]
- Minervini, F.; Di Cagno, R.; Lattanzi, A.; De Angelis, M.; Antonielli, L.; Cardinali, G.; Cappelle, S.; Gobbetti, M. Lactic acid bacterium and yeast microbiotas of 19 sourdoughs used for traditional/typical Italian breads: Interactions between ingredients and microbial species diversity. Appl. Environ. Microbiol. 2012, 78, 1251–1264. [Google Scholar] [CrossRef] [Green Version]
- Reale, A.; Tremonte, P.; Succi, M.; Sorrentino, E.; Coppola, R. Exploration of lactic acid bacteria ecosystem of sourdoughs from the Molise region. Ann. Microbiol. 2005, 55, 17–22. [Google Scholar]
- Ricciardi, A.; Parente, E.; Piraino, P.; Paraggio, M.; Romano, P. Phenotypic characterization of lactic acid bacteria from sourdoughs for Altamura bread produced in Apulia (Southern Italy). Int. J. Food Microbiol. 2005, 98, 63–72. [Google Scholar] [CrossRef]
- Reale, A.; Di Renzo, T.; Succi, M.; Tremonte, P.; Coppola, R.; Sorrentino, E. Identification of lactobacilli isolated in traditional ripe wheat sourdoughs by using molecular methods. World J. Microbiol. Biotechnol. 2011, 27, 237–244. [Google Scholar] [CrossRef]
- Yagmur, G.; Tanguler, H.; Leventdurur, S.; Elmaci, E.U.; Francesca, N.; Settanni, L.; Moschetti, G.; Erten, H. Identification of predominant lactic acid bacteria and yeasts of Turkish sourdoughs and selection of starter cultures for liquid sourdough production using different flours and dough yields. Pol. J. Food Nutr. Sci. 2016, 66, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Arena, M.P.; Russo, P.; Spano, G.; Capozzi, V. Exploration of the microbial biodiversity associated with north Apulian sourdoughs and the effect of the increasing number of inoculated lactic acid bacteria strains on the biocontrol against fungal spoilage. Fermentation 2019, 5, 97. [Google Scholar] [CrossRef] [Green Version]
- Sevgili, A.; Erkmen, O.; Koçaslan, S. Identification of lactic acid bacteria and yeasts from traditional sourdoughs and sourdough production by enrichment. Czech J. Food Sci. 2021, 39, 312–318. [Google Scholar] [CrossRef]
- Valmorri, S.; Settanni, L.; Suzzi, G.; Gardini, F.; Vernocchi, P.; Corsetti, A. Application of a novel polyphasic approach to study the lactobacilli composition of sourdoughs from the Abruzzo region (central Italy). Lett. Appl. Microbiol. 2006, 43, 343–349. [Google Scholar] [CrossRef]
- Palomba, S.; Blaiotta, G.; Ventorino, V.; Saccone, A.; Pepe, O. Microbial characterization of sourdough for sweet baked products in the Campania region (southern Italy) by a polyphasic approach. Ann. Microbiol. 2011, 61, 307–314. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, W.; Sun, Z.; Bao, Q.; Wang, F.; Yu, J.; Chen, W.; Zhang, H. Diversity of lactic acid bacteria and yeasts in traditional sourdoughs collected from western region in Inner Mongolia of China. Food Control 2011, 22, 767–774. [Google Scholar] [CrossRef]
- Palla, M.; Cristani, C.; Giovannetti, M.; Agnolucci, M. Identification and characterization of lactic acid bacteria and yeasts of PDO Tuscan bread sourdough by culture dependent and independent methods. Int. J. Food Microbiol. 2017, 250, 19–26. [Google Scholar] [CrossRef]
- Reale, A.; Di Renzo, T.; Boscaino, F.; Nazzaro, F.; Fratianni, F.; Aponte, M. Lactic acid bacteria biota and aroma profile of Italian traditional sourdoughs from the Irpinian area in Italy. Front. Microbiol. 2019, 10, 1621. [Google Scholar] [CrossRef] [Green Version]
- Scheirlinck, I.; Van der Meulen, R.; Van Schoor, A.; Vancanneyt, M.; De Vuyst, L.; Vandamme, P.; Huys, G. Influence of geographical origin and flour type on diversity of lactic acid bacteria in traditional Belgian sourdoughs. Appl. Environ. Microbiol. 2007, 73, 6262–6269. [Google Scholar] [CrossRef] [Green Version]
- Scheirlinck, I.; Van der Meulen, R.; Van Schoor, A.; Vancanneyt, M.; De Vuyst, L.; Vandamme, P.; Huys, G. Taxonomic structure and stability of the bacterial community in Belgian sourdough ecosystems as assessed by culture and population fingerprinting. Appl. Environ. Microbiol. 2008, 74, 2414–2423. [Google Scholar] [CrossRef] [Green Version]
- Lhomme, E.; Lattanzi, A.; Xavier, D.; Minervini, F.; De Angelis, M.; Lacaze, G.; Onno, B.; Gobbetti, M. Lactic acid bacterium and yeast microbiotas of sixteen French traditional sourdoughs. Int. J. Food Microbiol. 2015, 215, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Pino, A.; Russo, N.; Solieri, L.; Sola, L.; Caggia, C.; Randazzo, C.L. Microbial consortia involved in traditional Sicilian sourdough: Characterization of lactic acid bacteria and yeast populations. Microorganisms 2022, 10, 283. [Google Scholar] [CrossRef]
- Ferchichi, M.; Valcheva, R.; Oheix, N.; Kabadjova, P.; Prevost, H.; Onno, B.; Dousset, X. Rapid investigation of French sourdough microbiota by restriction fragment length polymorphism of the 16S-23S rRNA gene intergenic spacer region. World J. Microbiol. Biotechnol. 2008, 24, 2425–2434. [Google Scholar] [CrossRef]
- Fu, W.; Rao, H.; Tian, Y.; Xue, W. Bacterial composition in sourdoughs from different regions in China and the microbial potential to reduce wheat allergens. LWT-Food Sci. Technol. 2020, 117, 108669. [Google Scholar] [CrossRef]
- De Vuyst, L.; Schrijvers, V.; Paramithiotis, S.; Hoste, B.; Vancanneyt, M.; Swings, J.; Kalantzopoulos, G.; Tsakalidou, E.; Messens, W. The biodiversity of Lactic Acid Bacteria in Greek traditional wheat sourdough is reflected in both composition and metabolite formation. Appl. Environ. Microbiol. 2002, 68, 6059–6069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paramithiotis, S.; Tsiasiotou, S.; Drosinos, E.H. Comparative study of spontaneously fermented sourdoughs originating from two regions of Greece: Peloponnesus and Thessaly. Eur. Food Res. Technol. 2010, 231, 883–890. [Google Scholar] [CrossRef]
- Syrokou, M.K.; Themeli, C.; Paramithiotis, S.; Mataragas, M.; Bosnea, L.; Argyri, A.; Chorianopoulos, N.G.; Skandamis, P.N.; Drosinos, E.H. Microbial ecology of Greek wheat sourdoughs identified by culture-dependent and culture-independent approach. Foods 2020, 9, 1603. [Google Scholar] [CrossRef]
- Lattanzi, A.; Minervini, F.; Di Cagno, R.; Diviccaro, A.; Antonielli, L.; Cardinali, G.; Cappelle, S.; De Angelis, M.; Gobbetti, M. The lactic acid bacteria and yeast microbiota of eighteen sourdoughs used for the manufacture of traditional Italian sweet leavened baked goods. Int. J. Food Microbiol. 2013, 15, 71–79. [Google Scholar] [CrossRef]
- Michel, E.; Monfort, C.; Deffrasnes, M.; Guezenec, S.; Lhomme, E.; Barret, M.; Sicard, D.; Dousset, X.; Onno, B. Characterization of relative abundance of lactic acid bacteria species in French organic sourdough by cultural, qPCR and MiSeq high-throughput sequencing methods. Int. J. Food Microbiol. 2016, 239, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Nionelli, L.; Curri, N.; Curiel, J.A.; Di Cagno, R.; Pontonio, E.; Cavoski, I.; Gobbetti, M.; Rizzello, C.G. Exploitation of Albanian wheat cultivars: Characterization of the flours and lactic acid bacteria microbiota, and selection of starters for sourdough fermentation. Food Microbiol. 2014, 44, 96–107. [Google Scholar] [CrossRef]
- Comasio, A.; Verce, M.; Van Kerrebroeck, S.; De Vuyst, L. Diverse microbial composition of sourdoughs from different origins. Front. Microbiol. 2020, 11, 1212. [Google Scholar] [CrossRef]
- Kitahara, M.; Sakata, S.; Benno, Y. Biodiversity of Lactobacillus sanfranciscensis strains isolated from five sourdoughs. Lett. Appl. Microbiol. 2005, 40, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Fraberger, V.; Unger, C.; Kummer, C.; Domig, K.J. Insights into microbial diversity of traditional Austrian sourdough. LWT-Food Sci. Technol. 2020, 127, 109358. [Google Scholar] [CrossRef]
- Petkova, M.; Stefanova, P.; Gotcheva, V.; Angelov, A. Isolation and characterisation of lactic acid bacteria and yeasts from typical Bulgarian sourdoughs. Microorganisms 2021, 9, 1346. [Google Scholar] [CrossRef] [PubMed]
- Boreczek, J.; Litwinek, D.; Zylinska-Urban, J.; Izak, D.; Buksa, K.; Gawor, J.; Gromadka, R.; Bardowski, J.K.; Kowalczyk, M. Bacterial community dynamics in spontaneous sourdoughs made from wheat, spelt, and rye wholemeal flour. MicrobiologyOpen 2020, 9, e1009. [Google Scholar] [CrossRef]
- Ganchev, I.; Koleva, Z.; Kizheva, Y.; Moncheva, P.; Hristova, P. Lactic acid bacteria from spontaneously fermented rye sourdough. Bulg. J. Agric. Sci. 2014, 20, 69–73. [Google Scholar]
- Rosenquist, H.; Hansen, A. The microbial stability of two bakery sourdoughs made from conventionally and organically grown rye. Food Microbiol. 2000, 17, 241–250. [Google Scholar] [CrossRef]
- Mueller, M.R.A.; Wolfrum, G.; Stolz, P.; Ehrmann, M.A.; Vogel, R.F. Monitoring the growth of Lactobacillus species during a rye flour fermentation. Food Microbiol. 2001, 18, 217–227. [Google Scholar] [CrossRef]
- Rocha, J.M.; Malcata, F.X. On the microbiological profile of traditional Portuguese sourdough. J. Food Prot. 1999, 62, 1416–1429. [Google Scholar] [CrossRef]
- Ispirli, H.; Demirbaş, F.; Yüzer, M.O.; Dertli, E. Identification of lactic acid bacteria from spontaneous rye sourdough and determination of their functional characteristics. Food Biotechnol. 2018, 32, 222–235. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Lactic acid bacteria isolation from spontaneous sourdough and their characterization including antimicrobial and antifungal proper-ties evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meroth, C.B.; Walter, J.; Hertel, C.; Brandt, M.J.; Hammes, W.P. Monitoring the bacterial population dynamics in sourdough fermentation processes by using PCR denaturing gradient gel electrophoresis. Appl. Environ. Microbiol. 2003, 69, 475–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viiard, E.; Mihhalevski, A.; Rühka, T.; Paalme, T.; Sarand, I. Evaluation of the microbial community in industrial rye sourdough upon continuous back-slopping propagation revealed Lactobacillus helveticus as the dominant species. J. Appl. Microbiol. 2013, 114, 404–442. [Google Scholar] [CrossRef] [PubMed]
- Albano, H.; Henriques, I.; Correia, A.; Hogg, T.; Teixeira, P. Characterization of microbial population of ‘Alheira’ (a traditional Portuguese fermented sausage) by PCR-DGGE and traditional cultural microbiological methods. J. Appl. Microbiol. 2008, 105, 2187–2194. [Google Scholar] [CrossRef]
- Ammor, S.; Rachman, C.; Chaillou, S.; Prévost, H.; Dousset, X.; Zagorec, M.; Dufour, E.; Chevallier, I. Phenotypic and genotypic identification of lactic acid bacteria isolated from a small-scale facility producing traditional dry sausages. Food Microbiol. 2005, 22, 373–382. [Google Scholar] [CrossRef]
- Aquilanti, L.; Santarelli, S.; Silvestri, G.; Osimani, A.; Petruzzelli, A.; Clementi, F. The microbial ecology of a typical Italian salami during its natural fermentation. Int. J. Food Microbiol. 2007, 120, 136–145. [Google Scholar] [CrossRef]
- Aymerich, T.; Martin, B.; Garriga, M.; Vidal-Carou, M.C.; Bover-Cid, S.; Hugas, M. Safety properties and molecular strain typing of lactic acid bacteria from slightly fermented sausages. J. Appl. Microbiol. 2006, 100, 40–49. [Google Scholar] [CrossRef]
- Benito, M.J.; Martin, A.; Aranda, E.; Perez-Nevado, F.; Ruiz-Moyano, S.; Cordoba, M.G. Characterization and selection of autochthonous lactic acid bacteria isolated from traditional Iberian dry-fermented Salchichon and Chorizo sausages. J. Food Sci. 2007, 72, M193–M201. [Google Scholar] [CrossRef]
- Bonomo, M.G.; Ricciardi, A.; Zotta, T.; Parente, E.; Salzano, G. Molecular and technological characterization of lactic acid bacteria from traditional fermented sausages of Basilicata region (Southern Italy). Meat Sci. 2008, 80, 1238–1248. [Google Scholar] [CrossRef]
- Comi, G.; Urso, R.; Iacumin, L.; Rantsiou, K.; Cattaneo, P.; Cantoni, C.; Cocolin, L. Characterisation of naturally fermented sausages produced in the North East of Italy. Meat Sci. 2005, 69, 381–392. [Google Scholar] [CrossRef]
- Coppola, R.; Giagnacovo, B.; Iorizzo, M.; Grazia, L. Characterization of lactobacilli involved in the ripening of soppressata molisana, a typical southern Italy fermented sausage. Food Microbiol. 1998, 15, 47–353. [Google Scholar] [CrossRef]
- Di Cagno, R.; Chaves Lopez, C.; Tofalo, R.; Gallo, G.; De Angelis, M.; Paparella, A.; Hammes, W.; Gobbetti, M. Comparison of the compositional, microbiological, biochemical and volatile profile characteristics of three Italian PDO fermented sausages. Meat Sci. 2008, 79, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Drosinos, E.H.; Mataragas, M.; Xiraphi, N.; Moschonas, G.; Gaitis, F.; Metaxopoulos, J. Characterization of the microbial flora from a traditional Greek fermented sausage. Meat Sci. 2005, 69, 307–317. [Google Scholar] [CrossRef]
- Drosinos, E.H.; Paramithiotis, S.; Kolovos, G.; Tsikouras, I.; Metaxopoulos, I. Phenotypic and technological diversity of lactic acid bacteria and staphylococci isolated from traditionally fermented sausages in Southern Greece. Food Microbiol. 2007, 24, 260–270. [Google Scholar] [CrossRef]
- Federici, S.; Ciarrocchi, F.; Campana, R.; Ciandrini, E.; Blasi, G.; Baffone, W. Identification and functional traits of lactic acid bacteria isolated from Ciauscolo salami produced in Central Italy. Meat Sci. 2014, 98, 575–584. [Google Scholar] [CrossRef]
- Fonseca, S.; Cachaldora, A.; Gómez, M.; Franco, I.; Carballo, J. Monitoring the bacterial population dynamics during the ripening of Galician chorizo, a traditional dry fermented Spanish sausage. Food Microbiol. 2013, 33, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Fontana, C.; Cocconcelli, P.S.; Vignolo, G. Monitoring the bacterial population dynamics during fermentation of artisanal Argentinean sausages. Int. J. Food Microbiol. 2005, 103, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Garcia Fontan, M.C.; Lorenzo, J.M.; Parada, A.; Franco, I.; Carballo, J. Microbiological characteristics of “androlla”, a Spanish traditional pork sausage. Food Microbiol. 2007, 24, 52–58. [Google Scholar] [CrossRef]
- Garcia Fontan, M.C.; Lorenzo, J.M.; Martinez, S.; Franco, I.; Carballo, J. Microbiological characteristics of Botillo, a Spanish traditional pork sausage. LWT-Food Sci. Technol. 2007, 40, 610–1622. [Google Scholar] [CrossRef]
- Greppi, A.; Ferrocino, I.; La Storia, A.; Rantsiou, K.; Ercolini, D.; Cocolin, L. Monitoring of the microbiota of fermented sausages by culture independent rRNA-based approaches. Int. J. Food Microbiol. 2015, 212, 67–75. [Google Scholar] [CrossRef]
- Martin, B.; Garriga, M.; Hugas, M.; Bover-Cid, S.; Veciana-Nogués, M.T.; Aymerich, T. Molecular, technological and safety characterization of Gram-positive catalase-positive cocci from slightly fermented sausages. Int. J. Food Microbiol. 2006, 107, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Papamanoli, E.; Tzanetakis, N.; Litopoulou-Tzanetaki, E.; Kotzekidou, P. Characterization of lactic acid bacteria isolated from a Greek dry-fermented sausage in respect of their technological and probiotic properties. Meat Sci. 2003, 65, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Parente, E.; Grieco, S.; Crudele, M.A. Phenotypic diversity of lactic acid bacteria isolated from fermented sausages produced in Basilicata (Southern Italy). J. Appl. Microbiol. 2001, 90, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Pini, F.; Aquilani, C.; Giovannetti, L.; Viti, C.; Pugliese, C. Characterization of the microbial community composition in Italian Cinta Senese sausages dry-fermented with natural extracts as alternatives to sodium nitrite. Food Microbiol. 2020, 89, 103417. [Google Scholar] [CrossRef] [PubMed]
- Pisacane, V.; Callegari, M.L.; Puglisi, E.; Dallolio, G.; Rebecchi, A. Microbial analyses of traditional Italian salami reveal microorganisms transfer from the natural casing to the meat matrix. Int. J. Food Microbiol. 2015, 207, 57–65. [Google Scholar] [CrossRef]
- Polka, J.; Rebecchi, A.; Pisacane, V.; Morelli, L. Bacterial diversity in typical Italian salami at different ripening stages as revealed by high-throughput sequencing of 16s rRNA amplicons. Food Microbiol. 2015, 46, 342–356. [Google Scholar] [CrossRef]
- Prado, N.; Sampayo, M.; González, P.; Lombó, F.; Díaz, J. Physicochemical, sensory and microbiological characterization of Asturian Chorizo, a traditional fermented sausage manufactured in Northern Spain. Meat Sci. 2019, 156, 118–124. [Google Scholar] [CrossRef]
- Rantsiou, K.; Drosinos, E.H.; Gialitaki, M.; Metaxopoulos, I.; Comi, G.; Cocolin, L. Use of molecular tools to characterize Lactobacillus spp. isolated from Greek traditional fermented sausages. Int. J. Food Microbiol. 2006, 112, 215–222. [Google Scholar] [CrossRef]
- Rantsiou, K.; Drosinos, E.H.; Gialitaki, M.; Urso, R.; Krommer, J.; Gasparik-Reichardt, J.; Toth, S.; Metaxopoulos, I.; Comi, G.; Cocolin, L. Molecular characterization of Lactobacillus species isolated from naturally fermented sausages produced in Greece, Hungary and Italy. Food Microbiol. 2005, 22, 19–28. [Google Scholar] [CrossRef]
- Rebecchi, A.; Crivori, S.; Sarra, P.G.; Cocconcelli, P.S. Physiological and molecular techniques for the study of bacterial community development in sausage fermentation. J. Appl. Microbiol. 1998, 84, 1043–1049. [Google Scholar] [CrossRef]
- Samelis, J.; Maurogenakis, F.; Metaxopoulos, J. Characterisation of lactic acid bacteria isolated from naturally fermented Greek dry salami. Int. J. Food Microbiol. 1994, 23, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Samelis, J.; Metaxopoulos, J.; Vlassi, M.; Pappa, A. Stability and safety of traditional Greek salami—A microbiological ecology study. Int. J. Food Microbiol. 1998, 44, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, G.; Santarelli, S.; Aquilanti, L.; Beccaceci, A.; Osimani, A.; Tonucci, F.; Clementi, F. Investigation of the microbial ecology of Ciauscolo, a traditional Italian salami, by culture–dependent techniques and PCR-DGGE. Meat Sci. 2007, 77, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Urso, R.; Comi, G.; Cocolin, L. Ecology of lactic acid bacteria in Italian fermented sausages: Isolation, identification and molecular characterization. Syst. Appl. Microbiol. 2006, 29, 671–680. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Drosinos, E.H. The sourdough micro-ecosystem: An update. In Fermented Foods, Part II: Technological Interventions; Ray, R.C., Montet, D., Eds.; CRC Science: Cleveland, OH, USA, 2017; pp. 263–283. [Google Scholar]
- Shao, L.; Chen, S.; Wang, H.; Zhang, J.; Xu, X.; Wang, H. Advances in understanding the predominance, phenotypes, and mechanisms of bacteria related to meat spoilage. Trends Food Sci. Technol. 2021, 118, 822–832. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Skandamis, P.N.; Nychas, G.J.E. Insights into fresh meat spoilage. In Safety of Meat and Processed Meat; Toldra, F., Ed.; Springer: New York, NY, USA, 2009; pp. 55–82. [Google Scholar]
- Lorenzo, J.M.; Munekata, P.E.; Dominguez, R.; Pateiro, M.; Saraiva, J.A.; Franco, D. Main groups of microorganisms of relevance for food safety and stability: General aspects and overall description. In Innovative Technologies for Food Preservation. Inactivation of Spoilage and Pathogenic Microorganisms; Barba, G.J., Sant’Ana, A.S., Orlien, V., Koubaa, M., Eds.; Academic Press: Amsterdam, The Netherlands, 2018; pp. 53–107. [Google Scholar]
- Borch, E.; Kant-Muermans, M.-L.; Blixt, Y. Bacterial spoilage of meat and cured meat products. Int. J. Food Microbiol. 1996, 33, 103–120. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Drosinos, E.H. Foodborne pathogens of fermented meat products In Fermented Meat Products: Health Aspects; Zdolec, N., Ed.; CRC Science: Cleveland, OH, USA, 2016; pp. 196–227. [Google Scholar]
- Moschopoulou, E.; Moatsou, G.; Syrokou, M.K.; Paramithiotis, S.; Drosinos, E.H. Food quality changes during shelf life. In Food Quality and Shelf Life; Galanakis, C.M., Ed.; Academic Press: London, UK, 2019; pp. 1–32. [Google Scholar]
- Erkmen, O.; Bozoglu, T.F. Spoilage of milk and milk products. In Food Microbiology: Principles into Practice; Erkmen, O., Bozoglu, T.F., Eds.; J. Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 307–336. [Google Scholar]
- Leff, J.W.; Fierer, N. Bacterial communities associated with the surfaces of fresh fruits and vegetables. PLoS ONE 2013, 8, e59310. [Google Scholar] [CrossRef] [Green Version]
- Paramithiotis, S.; Drosinos, E.H.; Skandamis, P. Microbial ecology of fruits and fruit-based products. In Quantitative Microbiology in Food Processing—Modeling the Microbial Ecology; de Souza Sant’Ana, A., Ed.; J. Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 358–381. [Google Scholar]
- Michl, K.; Berg, G.; Cernava, T. The microbiome of cereal plants: The current state of knowledge and the potential for future applications. Environ. Microbiome 2023, 18, 28. [Google Scholar] [CrossRef]
- Erkmen, O.; Bozoglu, T.F. Spoilage of vegetables and fruits. In Food Microbiology: Principles into Practice; Erkmen, O., Bozoglu, T.F., Eds.; J. Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 337–363. [Google Scholar]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, 6406. [Google Scholar]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Champomier Vergès, M.-C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Tilocca, B.; Soggiu, A.; Iavarone, F.; Greco, V.; Putignani, L.; Ristori, M.V.; Macari, G.; Spina, A.A.; Morittu, V.M.; Ceniti, C.; et al. The functional characteristics of goat cheese microbiota from the one-health perspective. Int. J. Mol. Sci. 2022, 23, 14131. [Google Scholar] [CrossRef]
- Nelli, A.; Venardou, B.; Skoufos, I.; Voidarou, C.; Lagkouvardos, I.; Tzora, A. An insight into goat cheese: The tales of artisanal and industrial Gidotyri microbiota. Microorganisms 2023, 11, 123. [Google Scholar] [CrossRef]
- Tsigkrimani, M.; Bakogianni, M.; Paramithiotis, S.; Bosnea, L.; Pappa, E.; Drosinos, E.H.; Skandamis, P.Ν.; Mataragas, M. Microbial ecology of artisanal Feta and Kefalograviera cheeses. Part I: Bacterial community and its functional characteristics with focus on lactic acid bacteria as determined by culture-dependent methods and phenotype microarrays. Microorganisms 2022, 10, 161. [Google Scholar] [CrossRef]
- Gram, L.; Huss, H.H. Microbiological spoilage of fish and fish products. Int. J. Food Microbiol. 1996, 33, 121–137. [Google Scholar] [CrossRef]
- Tiedje, J.M.; Bruns, M.A.; Casadevall, A.; Criddle, C.S.; Eloe-Fadrosh, E.; Karl, D.M.; Nguyen, N.K.; Zhouh, J. Microbes and climate change: A research prospectus for the future. mBio 2022, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhou, X.; Hale, L.; Yuan, M.; Ning, D.; Feng, J.; Shi, Z.; Li, Z.; Feng, B.; Gao, Q.; et al. Climate warming accelerates temporal scaling of grassland soil microbial biodiversity. Nat. Ecol. Evol. 2019, 3, 612–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brubacher, D.; Allen, M.; Déry, S.J.; Parkes, M.W.; Chhetri, B.; Mak, S.; Sobie, S.; Takaro, T.K. Associations of five food- and water-borne diseases with ecological zone, land use and aquifer type in a changing climate. Sci. Total Environ. 2020, 728, 138808. [Google Scholar] [CrossRef] [PubMed]
- Lhomme, E.; Urien, C.; Legrand, J.; Dousset, X.; Onno, B.; Sicard, D. Sourdough microbial community dynamics: An analysis during French organic bread-making processes. Food Microbiol. 2016, 53, 41–50. [Google Scholar] [CrossRef]
- Schultze, S.R.; Sabbatini, P. Implications of a climate-changed atmosphere on cool-climate viticulture. J. Appl. Meteorol. Climatol. 2019, 58, 1141–1153. [Google Scholar] [CrossRef]
- Hewer, M.J.; Gough, W.A. Climate change impact assessment on grape growth and wine production in the Okanagan valley (Canada). Clim. Risk Manag. 2021, 33, 100343. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Destrac-Irvine, A. Modified grape composition under climate change conditions requires adaptations in the vineyard. Oeno One 2017, 51, 147–154. [Google Scholar] [CrossRef]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.-T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A Review of the potential climate change impacts and adaptation options for European viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Duchenne-Moutien, R.A.; Neetoo, H. Climate change and emerging food safety issues: A review. J. Food Prot. 2021, 84, 1884–1897. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Kang, J.P.; Huo, Y.; Hoang, V.A.; Yang, D.U.; Yang, D.C.; Kang, S.C. Bombilactobacillus apium sp. nov., isolated from the gut of honeybee (Apis cerana). Arch. Microbiol. 2021, 203, 2193–2198. [Google Scholar] [CrossRef]
- Panziera, D.; Requier, F.; Chantawannakul, P.; Pirk, C.W.W.; Blacquière, T. The diversity decline in wild and managed honey bee populations urges for an integrated conservation approach. Front. Ecol. Evol. 2022, 10, 767950. [Google Scholar] [CrossRef]
- Semeniuk, V.; Cresswell, I.D. A proposed revision of diversity measures. Diversity 2013, 5, 613–626. [Google Scholar] [CrossRef] [Green Version]
- Cibario, A.; Avramova, M.; Dimopoulou, M.; Magani, M.; Miot-Sertier, C.; Mas, A.; Portillo, M.C.; Ballestra, P.; Albertin, W.; Masneuf-Pomarede, I.; et al. Brettanomyces bruxellensis wine isolates show high geographical dispersal and long persistence in cellars. PLoS ONE 2019, 14, e0222749. [Google Scholar]
- Peixoto, R.S.; Voolstra, C.R.; Sweet, M.; Duarte, C.M.; Carvalho, S.; Villela, H.; Lunshof, J.E.; Gram, L.; Woodhams, D.C.; Walter, J.; et al. Harnessing the microbiome to prevent global biodiversity loss. Nat. Microbiol. 2022, 7, 1726–1735. [Google Scholar] [CrossRef]

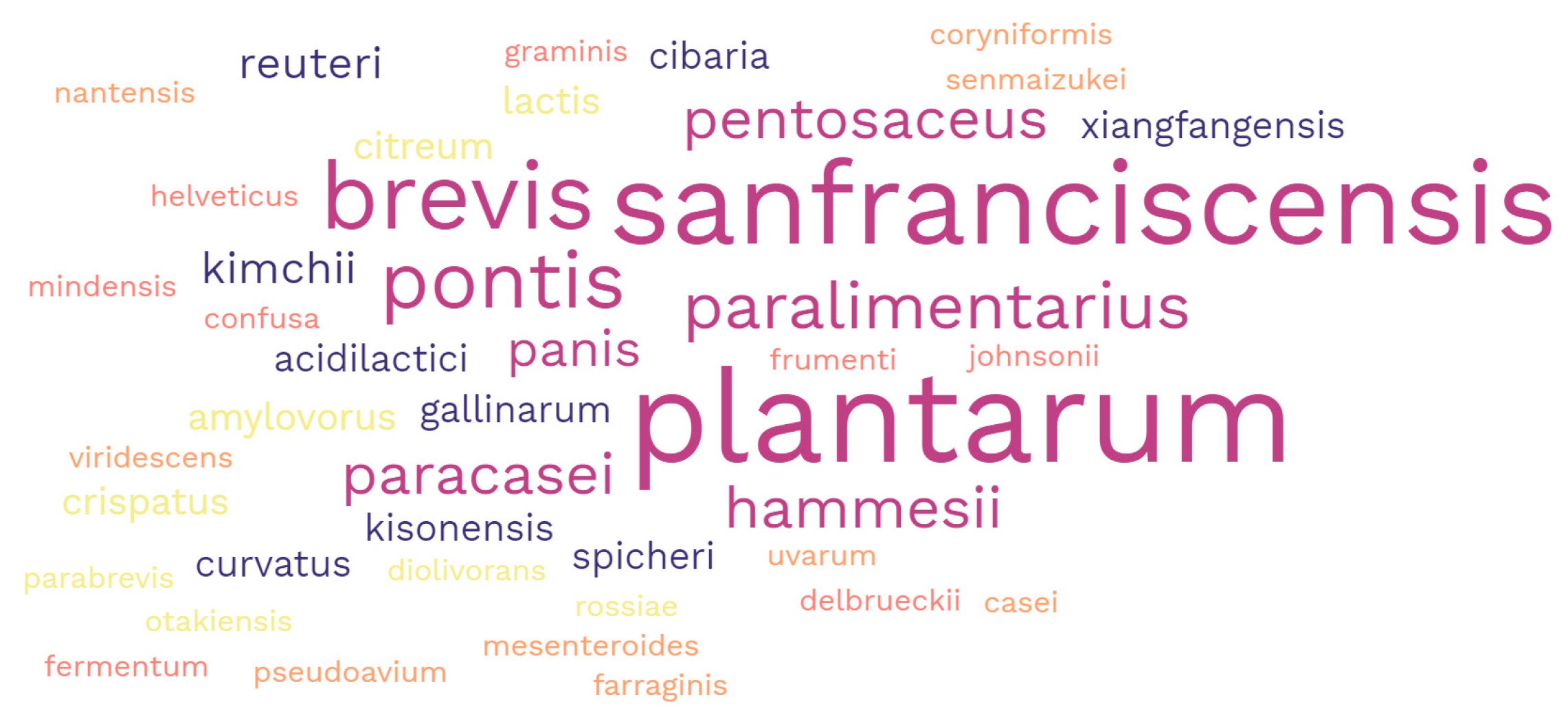
| Product | Lactic Acid Bacteria | References |
|---|---|---|
| Kimchi | Lc. gelidum, Ln. carnosum, Ln. citreum, Ln. gasicomitatum, Ln. gelidum, Ln. holzapfelii, Ln. inhae, Ln. kimchii, Ln. lactis, Ln. mesenteroides, Lp. pentosus, Lp. plantarum, Lt. curvatus, Lt. sakei, Lv. brevis, Lv. parabrevis, Lv. spicheri, W. cibaria, W. confusa, W. kandleri, W. koreensis, and W. soli | [15,16,17,18,19,20,21,22] |
| Sauerkraut | E. faecalis, Lc. lactis subsp. lactis, Ln. fallax, Ln. mesenteroides, Lp. plantarum, Lt. curvatus, Lt. sakei, Lv. brevis, P. pentosaceus, and W. confusa | [23,24,25] |
| Wheat sourdoughs | C. alimentarius, C. crustorum, C. farciminis, C. heilongjiangensis, C. kimchii, C. mindensis, C. nantensis, C. paralimentarius, E. durans, E. faecalis, E. faecium, E. hirae, Fr. fructivorans, Fr. sanfranciscensis, Fu. rossiae, La. casei, La. paracasei, La. rhamnosus, Lb. acetotolerans, Lb. acidophilus, Lb. delbrueckii, Lb. gallinarum, Lb. guizhouensis, Lb. helveticus, Lc. lactis, Le. buchneri, Le. diolivorans, Le. farraginis, Le. hilgardii, Le. kisonensis, Le. parabuchneri, Lm. fermentum, Lm. frumenti, Lm. panis, Lm. pontis, Ln. citreum, Ln. mesenteroides, Ln. pseudomesenteroides, Lo. coryniformis, Lp. paraplantarum, Lp. pentosus, Lp. plantarum, Lp. xiangfangensis, Lt. curvatus, Lt. graminis, Lt. sakei, Lt. sunkii, Lv. brevis, Lv. hammesii, Lv. koreensis, Lv. namurensis, Lv. parabrevis, Lv. senmaizukei, Lv. spicheri, Lv. zymae, P. acidilactici, P. argentinicus, P. inopinatus, P. parvulus, P. pentosaceus, Pa. vaccinostercus; Sc. harbinensis, Sc. perolens, W. cibaria, W. confusa, W. paramesenteroides, and W. viridescens | [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61] |
| Rye sourdoughs | C. kimchii, C. paralimentarius, C. mindensis, C. nantensis, E. pseudoavium, Fr. sanfranciscensis, Fu. rossiae, La. casei, La. paracasei, Lb. acidophilus, Lb. amylovorus, Lb. crispatus, Lb. delbrueckii, Lb. gallinarum, Lb. helveticus, Lb. johnsonii, Lc. lactis, Le. diolivorans, Le. farraginis, Le. kisonensis, Le. otakiensis, Lm. fermentum, Lm. frumenti, Lm. panis, Lm. pontis, Lm. reuteri, Ln. citreum, Ln. mesenteroides, Lo. coryniformis, Lp. plantarum, Lp. xiangfangensis, Lq. uvarum, Lt. curvatus, Lt. graminis, Lv. brevis, Lv. hammesii, Lv. parabrevis, Lv. senmaizukei, Lv. spicheri, P. acidilactici, P. pentosaceus, W. cibaria, W. confusa, and W. viridescens | [45,46,47,57,58,59,60,61,62,63,64,65,66,67,68,69] |
| Fermented meat products | C. alimentarius, C. farciminis, C. futsai, C. paralimentarius, C. versmoldensis, Ca. divergens, E. faecalis, E. faecium, E. gallinarum, E. pseudoavium, La. casei, La. paracasei, La. rhamnosus, La. zeae, Lb. acidophilus, Lb. helveticus, Lb. johnsonii, Lc. garveae, Lc. lactis, Le. buchneri, Li. salivarius, Lm. antri, Lm. fermentum, Lm. frumenti, Lm. oris, Lm. panis, Lm. reuteri, Lm. vaginalis, Ln. carnosum, Ln. citreum, Ln. gelidum, Ln. lactis, Ln. mesenteroides, Ln. pseudomesenteroides, Lo. coryniformis, Lp. paraplantarum, Lp. pentosus, Lp. plantarum, Lt. curvatus, Lt. graminis, Lt. sakei, Lv. brevis, P. acidilactici, P. pentosaceus, W. hellenica, W. minor, W. paramesenteroides, and W. viridescens | [70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paramithiotis, S.; Dimopoulou, M. Preserving Microbial Biodiversity: The Case of Food-Associated Microorganisms. Ecologies 2023, 4, 521-534. https://doi.org/10.3390/ecologies4030034
Paramithiotis S, Dimopoulou M. Preserving Microbial Biodiversity: The Case of Food-Associated Microorganisms. Ecologies. 2023; 4(3):521-534. https://doi.org/10.3390/ecologies4030034
Chicago/Turabian StyleParamithiotis, Spiros, and Maria Dimopoulou. 2023. "Preserving Microbial Biodiversity: The Case of Food-Associated Microorganisms" Ecologies 4, no. 3: 521-534. https://doi.org/10.3390/ecologies4030034





