From Protected Habitat to Agricultural Land: Dogs and Small Mammals Link Habitats in Northern Thailand
Abstract
:1. Introduction
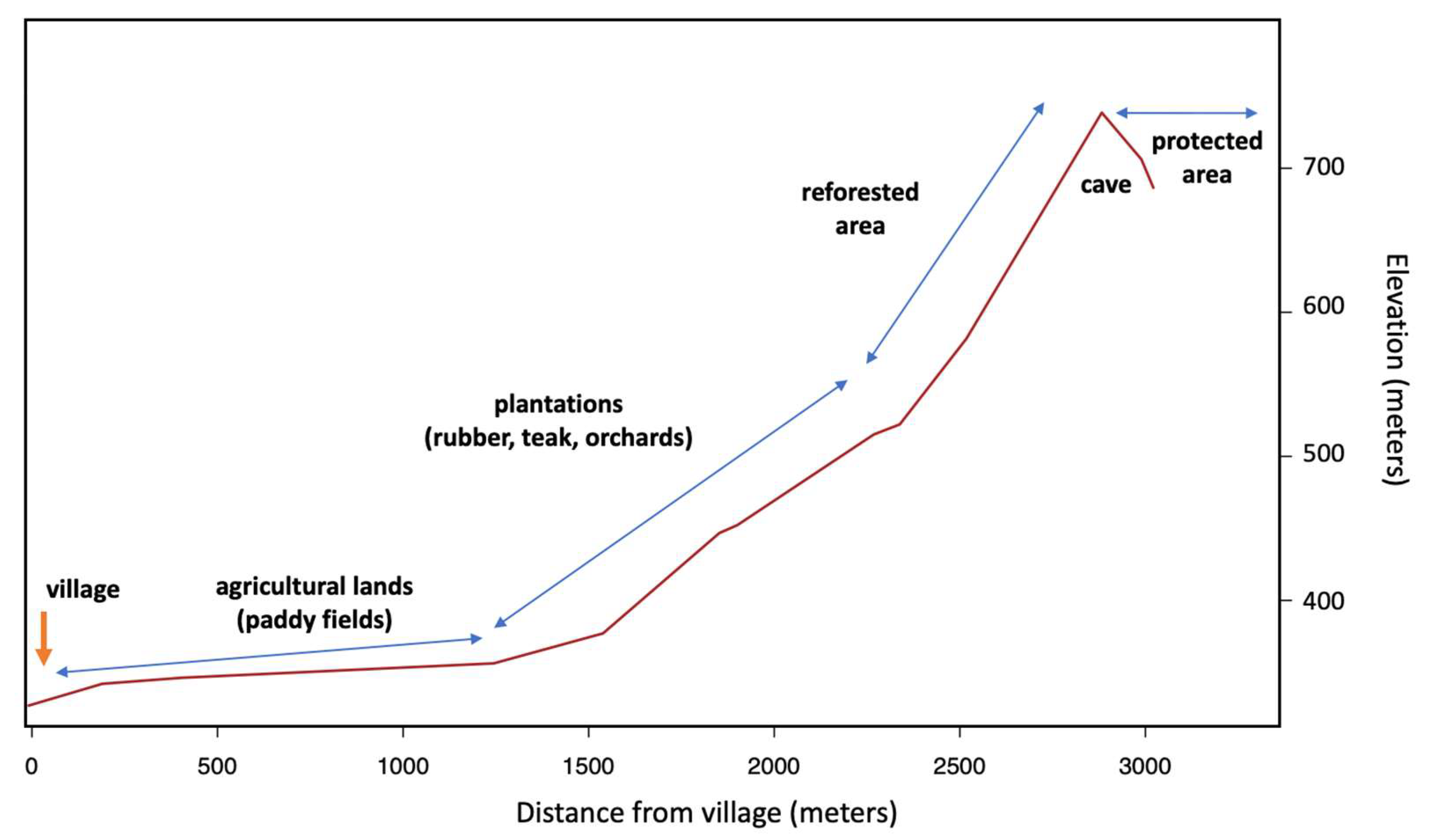
2. Materials and Methods
3. Results
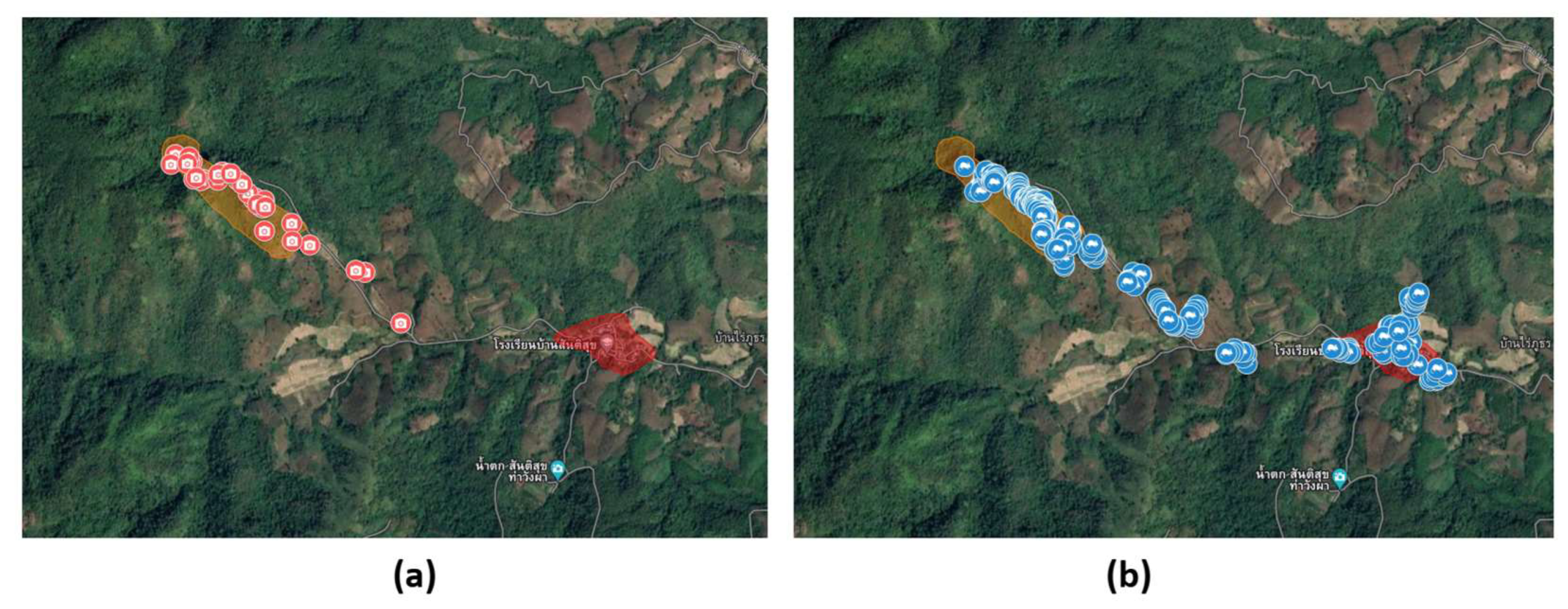
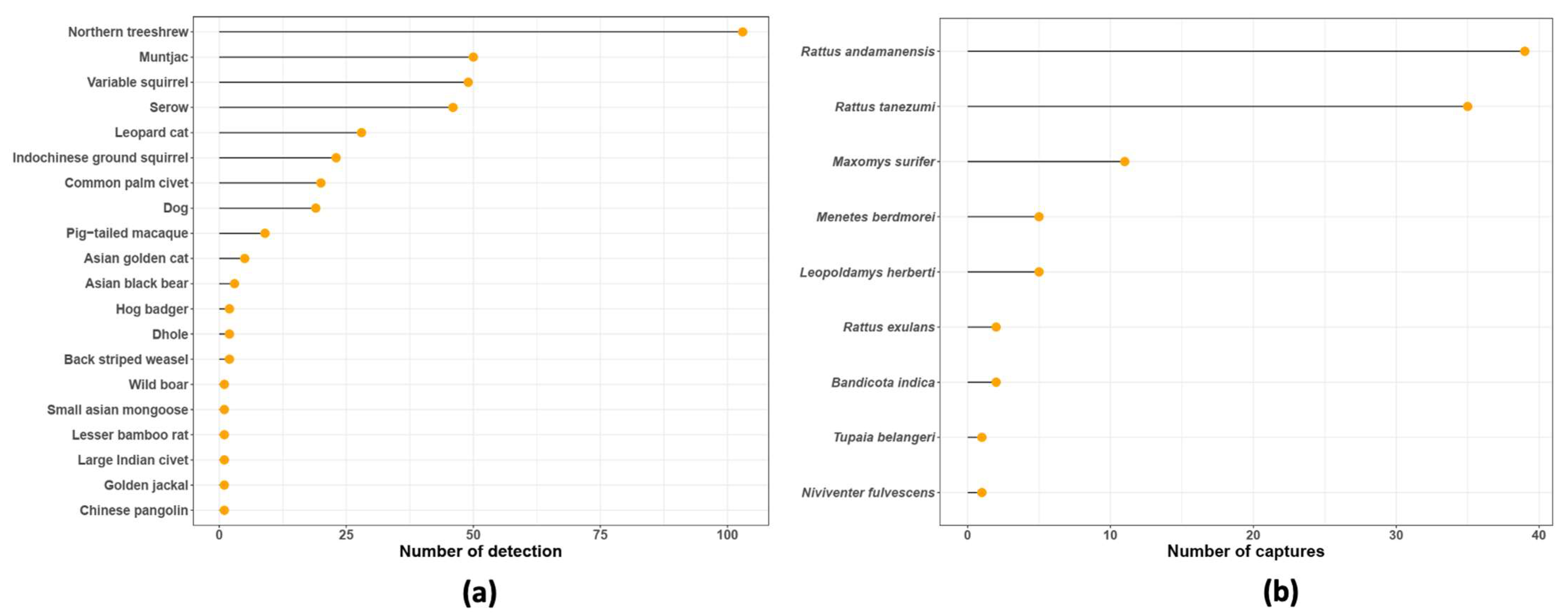
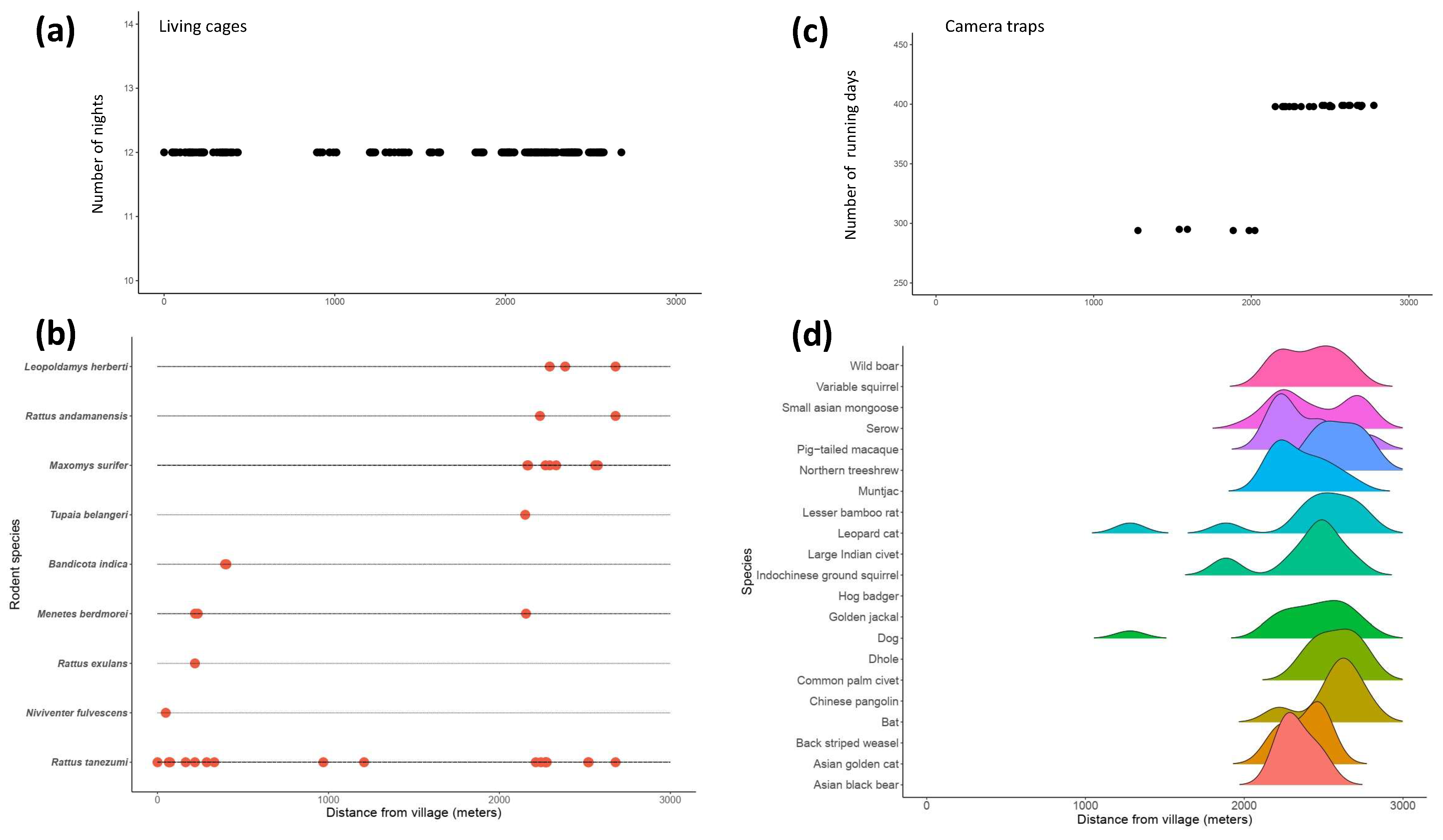
3.1. Camera Trapping
3.2. Live Trapping of Small Mammals
3.3. Species Richness
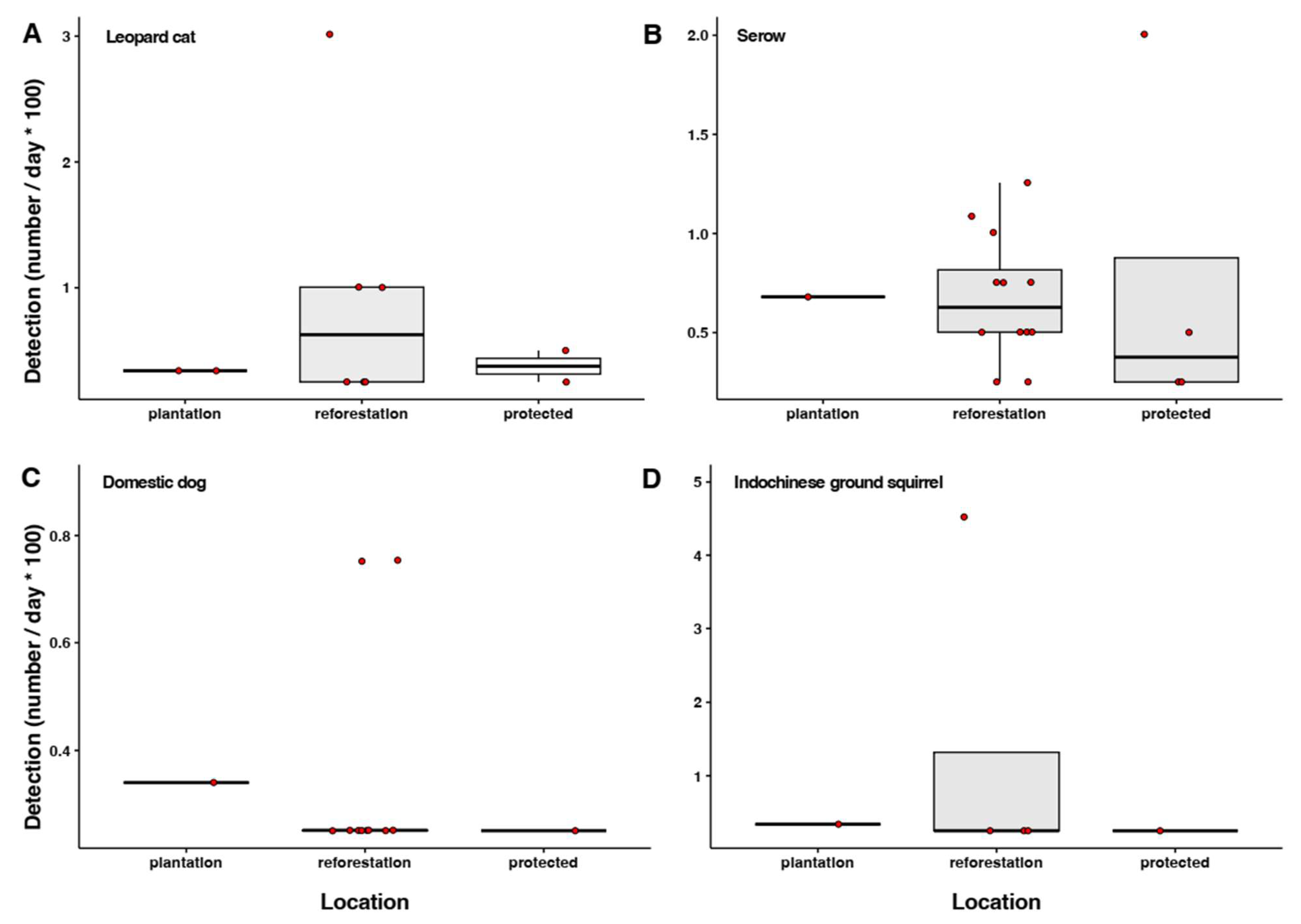
3.4. Camera Trap Detections
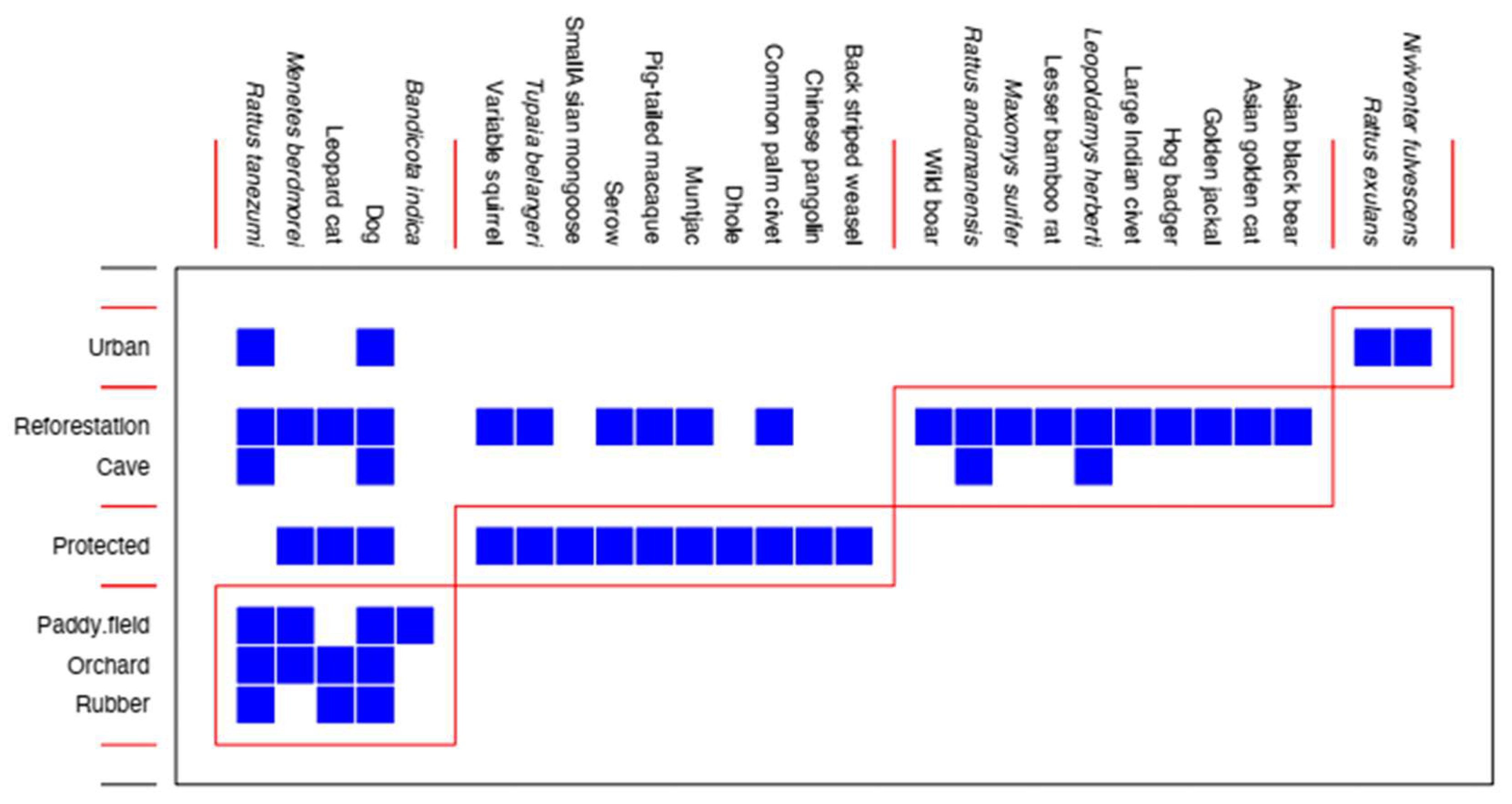
3.5. Species Occurrence in Space
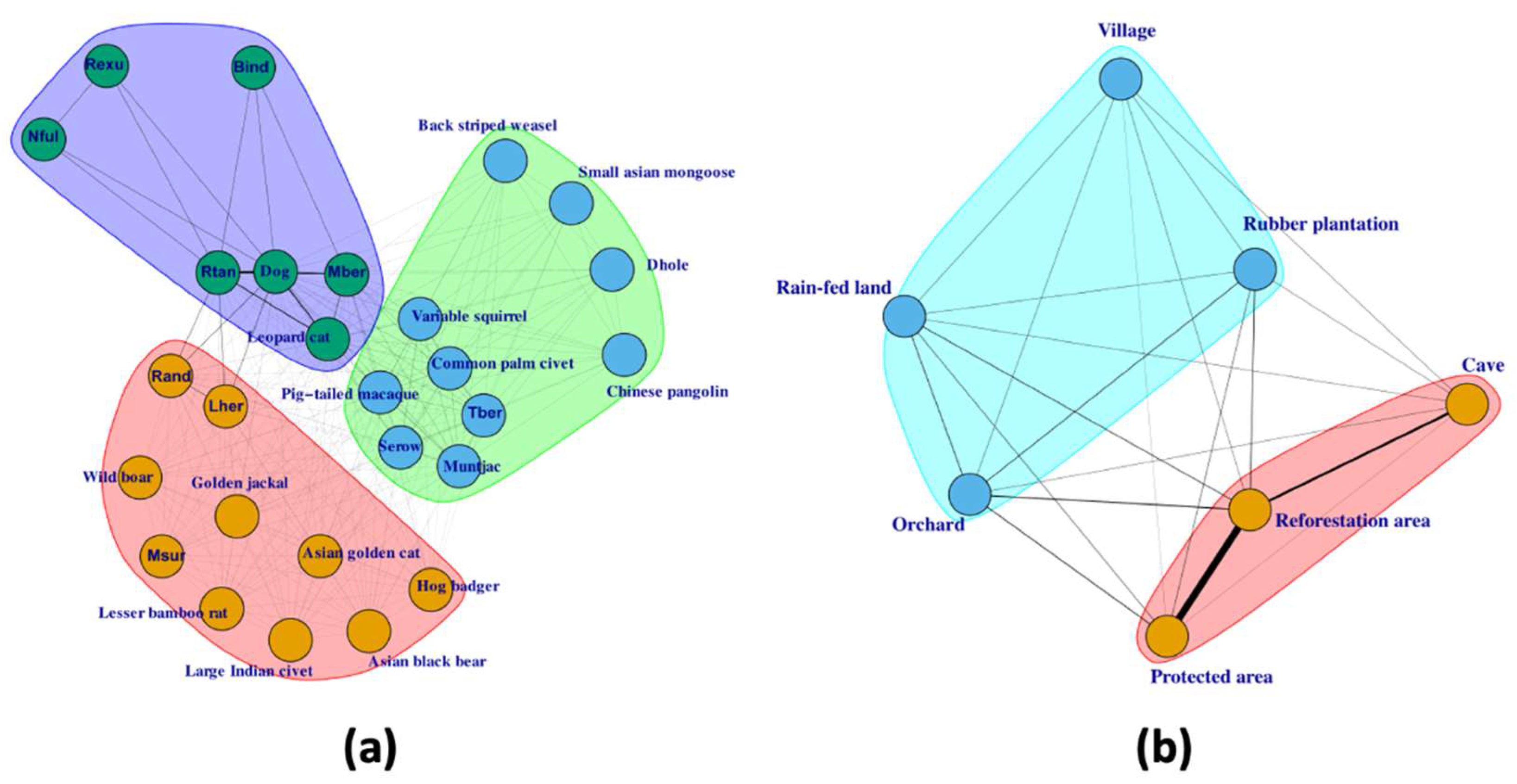
3.6. Network Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Mammal Species | Scientific Name | Number of Detections | Number of Stations |
|---|---|---|---|
| Asian black bear | Ursus thibetanus | 3 | 3 |
| Asian golden cat | Catopuma temminckii | 5 | 5 |
| Back-striped weasel | Mustela strigidorsa | 2 | 1 |
| Chinese pangolin | Manis pentadactaly | 1 | 1 |
| Common palm civet | Paradoxurus hermaphroditus | 20 | 7 |
| Dhole | Cuon alpinus | 2 | 1 |
| Domestic dog | Canis lupus domesticus | 19 | 14 |
| Golden jackal | Canis aureus | 1 | 1 |
| Greater hog badger | Arctonyx collari | 2 | 1 |
| Indochinese ground squirrel | Menetes berdmorei | 23 | 6 |
| Large Indian civet | Viverra zibetha | 1 | 1 |
| Leopard cat | Prionailurus bengalensis | 28 | 10 |
| Lesser bamboo rat | Cannomys badius | 1 | 1 |
| Red muntjac | Muntiacus muntjak | 50 | 12 |
| Northern treeshrew | Tupaia belangeri | 103 | 9 |
| Northern pig-tailed macaque | Macaca leonina | 9 | 7 |
| Indochinese serow | Capricornis milneedwardsi | 46 | 17 |
| Small Asian mongoose | Herpestes javanicus | 1 | 1 |
| Variable squirrel | Callosciurus finalaysonii | 49 | 14 |
| Wild boar | Sus scrofa | 1 | 1 |
| Mammal Species | Centrality | Habitat | Centrality |
|---|---|---|---|
| Domestic dog | 1.000 | Reforestation area | 1.000 |
| Rattus tanezumi | 0.962 | Protected area | 0.935 |
| Leopard cat | 0.653 | Cave | 0.359 |
| Menetes berdmorei | 0.592 | Orchards | 0.289 |
| Leopoldamys herberti | 0.286 | Rice field | 0.206 |
| Rattus andamanensis | 0.286 | Rubber plantation | 0.206 |
| Bandicota indica | 0.240 | ||
| Niviventer mekongis | 0.204 | ||
| Rattus exulans | 0.204 | ||
| Common palm civet | 0.156 | ||
| Red muntjac | 0156 | ||
| Northern pig-tailed macaque | 0.156 | ||
| Indochinese serow | 0.156 | ||
| Northern treeshrew | 0.156 | ||
| Variable squirrel | 0.156 | ||
| Back-striped weasel | 0.080 | ||
| Chinese pangolin | 0.080 | ||
| Dhole | 0.080 | ||
| Small Asian mongoose | 0.080 | ||
| Asian black bear | 0.078 | ||
| Golden jackal | 0.078 | ||
| Maxomys surifer | 0.078 | ||
| Wild boar | 0.078 | ||
| Asian golden cat | 0.078 | ||
| Greater hog badger | 0.078 | ||
| Large Indian civet | 0.078 | ||
| Lesser bamboo rat | 0.078 |
| Species | Males (n) | Females (n) |
|---|---|---|
| Bandicota indica | 0 | 2 |
| Leopoldamys herberti | 1 | 4 |
| Maxomys surifer | 3 | 4 |
| Menetes berdmorei | 3 | 2 |
| Niviventer mekongis | 0 | 2 |
| Rattus andamanensis | 14 | 6 |
| Rattus exulans | 0 | 2 |
| Rattus tanezumi | 10 | 17 |
| Tupaia belangeri | 0 | 1 |
References
- Dirzo, R.; Young, H.S.; Galetti, M.; Ceballos, G.; Isaac, N.J.B.; Collen, B. Defaunation in the Anthropocene. Science 2014, 345, 401–406. [Google Scholar] [CrossRef]
- Butti, M.; Pacca, L.; Santos, P.; Alonso, A.C.; Buss, G.; Ludwig, G.; Jerusalinsky, L.; Martins, A.B. Habitat loss estimation for assessing terrestrial mammalian species extinction risk: An open data framework. PeerJ 2022, 10, e14289. [Google Scholar] [CrossRef]
- Yue, S.; Brodie, J.F.; Zipkin, E.F.; Bernard, H. Oil palm plantations fail to support mammal diversity. Ecol. Appl. 2015, 25, 2285–2292. [Google Scholar] [CrossRef]
- Atkinson, J.; Brudvig, L.A.; Mallen-Cooper, M.; Nakagawa, S.; Moles, A.T.; Bonser, S.P. Terrestrial ecosystem restoration increases biodiversity and reduces its variability, but not to reference levels: A global meta-analysis. Ecol. Lett. 2022, 25, 1725–1737. [Google Scholar] [CrossRef]
- Morris, R.J. Anthropogenic impacts on tropical forest biodiversity: A network structure and ecosystem functioning perspective. Philos. Trans. R. Soc. Lond. B 2010, 365, 3709–3718. [Google Scholar] [CrossRef] [PubMed]
- Koerner, S.E.; Poulsen, J.R.; Blanchard, E.J.; Okouyi, J.; Clark, C.J. Vertebrate community composition and diversity declines along a defaunation gradient radiating from rural villages in Gabon. J. Appl. Ecol. 2017, 54, 805–814. [Google Scholar] [CrossRef]
- McFarlane, R.; Sleigh, A.; McMichael, T. Synanthropy of wild mammals as a determinant of emerging infectious diseases in the Asian-Australasian region. EcoHealth 2012, 9, 24–35. [Google Scholar] [CrossRef]
- Ecke, F.; Han, B.A.; Hörnfeldt, B.; Khalil, H.; Magnusson, M.; Singh, N.J.; Ostfeld, R.S. Population fluctuations and synanthropy explain transmission risk in rodent-borne zoonoses. Nat. Commun. 2022, 13, 7532. [Google Scholar] [CrossRef]
- Bordes, F.; Caron, A.; Blasdell, K.; de Garine Wichatitsky, M.; Morand, S. Forecasting potential emergence of zoonotic diseases in South-East Asia: Network analysis identifies key rodent hosts. J. Appl. Ecol. 2017, 5, 691–700. [Google Scholar] [CrossRef]
- Thinphovong, C.; Nordstrom-Schuler, E.; Soisook, P.; Kritiyakan, A.; Chakngean, R.; Saggapitakwong, S. A protocol and a data-based prediction to investigate virus spillover at the wildlife interface in human-dominated and protected habitats in Thailand: The Spillover Interface project. PLoS ONE.
- Francis, C.M. Field Guide to the Mammals of South-East Asia; Bloomsbury: London, UK, 2019. [Google Scholar]
- Niedballa, J.; Sollmann, R.; Courtiol, A.; Wilting, A. camtrapR: An R package for efficient camera trap data management. Methods Ecol. Evol. 2016, 7, 1457–1462. [Google Scholar] [CrossRef]
- R Development Core Team. The R Project for Statistical Computing, R Version 4.2.0. 2022. Available online: https://www.r-project.org (accessed on 5 January 2023).
- Morand, S.; Blasdell, K.; Bordes, F.; Buchy, P.; Carcy, B.; Chaisiri, K.; Chaval, Y.; Claude, J.; Cosson, J.-F.; Desquesnes, M.; et al. Changing landscapes of Southeast Asia and rodent-borne diseases: Decreased diversity but increased transmission risks. Ecol. Appl. 2019, 29, e01886. [Google Scholar] [CrossRef]
- Mahuzier, C.; Morand, S.; Chaisiri, K.; De Rouw, A.; Soulileuth, B.; Thinphovong, C.; Tran, A.; Valentin, C. Random Forest Land Cover Classifications of Sentinel Satellite Images in 2019, Saen Thong, Thailand; DataSuds: Paris, France, 2022. [Google Scholar] [CrossRef]
- VanDerWal, J.; Falconi, L.; Januchowski, S.; Shoo, L.; Storlie, C. SDMTools: Species Distribution Modelling Tools: Tools for Processing Data Associated with Species Distribution Modelling Exercises; R Package Version 1.1-221.2 2014. Available online: https://cran.r-project.org/src/contrib/Archive/SDMTools/ (accessed on 14 October 2023).
- Walther, B.A.; Morand, S. Comparative performance of species richness estimation methods. Parasitology 1998, 116, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; OHara, R.B.; Solymos, P.; Stevens, M.H.H.; et al. Vegan: Community Ecology Package. R Package Version 2.5–3. 2018. Available online: https://CRAN.R-project.org/package=vegan (accessed on 14 October 2023).
- Kindt, R. Package for Community Ecology and Suitability Analysis. R Package Version 2.12–3. 2018. Available online: https://cran.r-project.org/web/packages/BiodiversityR/BiodiversityR.pdf (accessed on 14 October 2023).
- Dormann, C.F.; Fruend, J.; Bluethgen, N.; Gruber, B. Indices, graphs and null models: Analyzing bipartite ecological networks. Open Ecol. J. 2009, 2, 7–24. [Google Scholar] [CrossRef]
- Dormann, C.F.; Gruber, B.; Fruend, J. Introducing the bipartite Package: Analysing Ecological Networks. R News 2008, 8, 8–11. [Google Scholar]
- Opsahl, T. Structure and Evolution of Weighted Networks; University of London (Queen Mary College): London, UK, 2009; pp. 104–122. [Google Scholar]
- Csardi, G.; Nepusz, T. The igraph software package for complex network research. Int. J. Complex Syst. 2006, 1695, 1–9. [Google Scholar]
- Blondel, V.D.; Guillaume, J.L.; Lambiotte, R.; Lefebvre, É. Fast unfolding of community hierarchies in large networks. J. Stat. Mech. 2008, 10, 10008. [Google Scholar] [CrossRef]
- Dormann, C.F.; Strau, R. Detecting modules in quantitative bipartite networks: The QuaBiMo algorithm. arXiv 2013, arXiv:1304.3218. [Google Scholar]
- Magioli, M.; Paschoaletto, K.M.; de Barros Ferraz, M.; Chiarello, A.G.; Galetti, M.; Freire Setz, E.Z.; Paglia, A.P.; Abrego, N.; Ribeiro, M.C.; Ovaskainen, O. Land-use changes lead to functional loss of terrestrial mammals in a Neotropical rainforest. Perspect. Ecol. Cons. 2021, 19, 161–170. [Google Scholar] [CrossRef]
- Galetti, M.; dos Santos Pires, A.; Santin Brancalion, P.H.; Fernandez, F.A.S. Reversing defaunation by trophic rewilding in empty forests. Biotropica 2017, 49, 5–8. [Google Scholar] [CrossRef]
- Hornok, S.; Földvári, G.; Rigó, K.; Meli, M.L.; Gönczi, E.; Répási, A.; Farkas, R.; Papp, I.; Kontschán, J.; Hofmann-Lehmann, R. Synanthropic rodents and their ectoparasites as carriers of a novel haemoplasma and vector-borne, zoonotic pathogens indoors. Parasites Vectors 2015, 8, 27. [Google Scholar] [CrossRef]
- Nawtaisong, P.; Robinson, M.T.; Khammavong, K.; Milavong, P.; Rachlin, A.; Dittrich, S.; Dubot-Pérès, A.; Vongsouvath, M.; Horwood, P.F.; Dussart, P.; et al. Zoonotic Pathogens in Wildlife Traded in Markets for Human Consumption, Laos. Emerg. Infect. Dis. 2022, 28, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Doherty, T.S.; Dickman, C.R.; Glen, A.S.; Newsome, T.M.; Nimmo, D.G.; Ritchie, E.G.; Vanak, A.T.; Wirsing, A.J. The global impacts of domestic dogs on threatened vertebrates. Biol. Cons. 2017, 210, 56–59. [Google Scholar] [CrossRef]
- Morand, S.; McIntyre, K.M.; Baylis, M. Domesticated animals and human infectious diseases of zoonotic origins: Domestication time matters. Infect. Genet. Evol. 2014, 24, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Wells, K.; Morand, S.; Wardeh, M.; Baylis, M. Distinct spread of DNA and RNA viruses among mammals amid prominent role of domestic species. Glob. Ecol. Biogeogr. 2020, 29, 470–481. [Google Scholar] [CrossRef]
- Kays, R.; Arbogast, B.S.; Baker-Whatton, M.; Beirne, C.; Boone, H.M.; Bowler, M.; Burneo, S.F.; Cove, M.V.; Ding, P.; Espinosa, S.; et al. An empirical evaluation of camera trap study design: How many, how long and when? Methods Ecol. Evol. 2020, 11, 700–713. [Google Scholar] [CrossRef]
- Derhé, M.A.; Murphy, H.T.; Preece, N.D.; Lawes, M.J.; Menéndez, R. Recovery of mammal diversity in tropical forests: A functional approach to measuring restoration. Restor. Ecol. 2018, 26, 778–786. [Google Scholar] [CrossRef]
- Beirne, C.; Sun, C.; Tattersall, E.R.; Burgar, J.M.; Fisher, J.T.; Burton, A.C. Multispecies modelling reveals potential for habitat restoration to re-establish boreal vertebrate community dynamics. J. Appl. Ecol. 2021, 58, 2821–2832. [Google Scholar] [CrossRef]
- Prist, P.R.; Prado, A.; Tambosi, L.R.; Umetsu, F.; de Arruda Bueno, A.; Pardini, R.; Metzger, J.P. Moving to healthier landscapes: Forest restoration decreases the abundance of Hantavirus reservoir rodents in tropical forests. Sci. Total Environ. 2021, 752, 141967. [Google Scholar] [CrossRef]

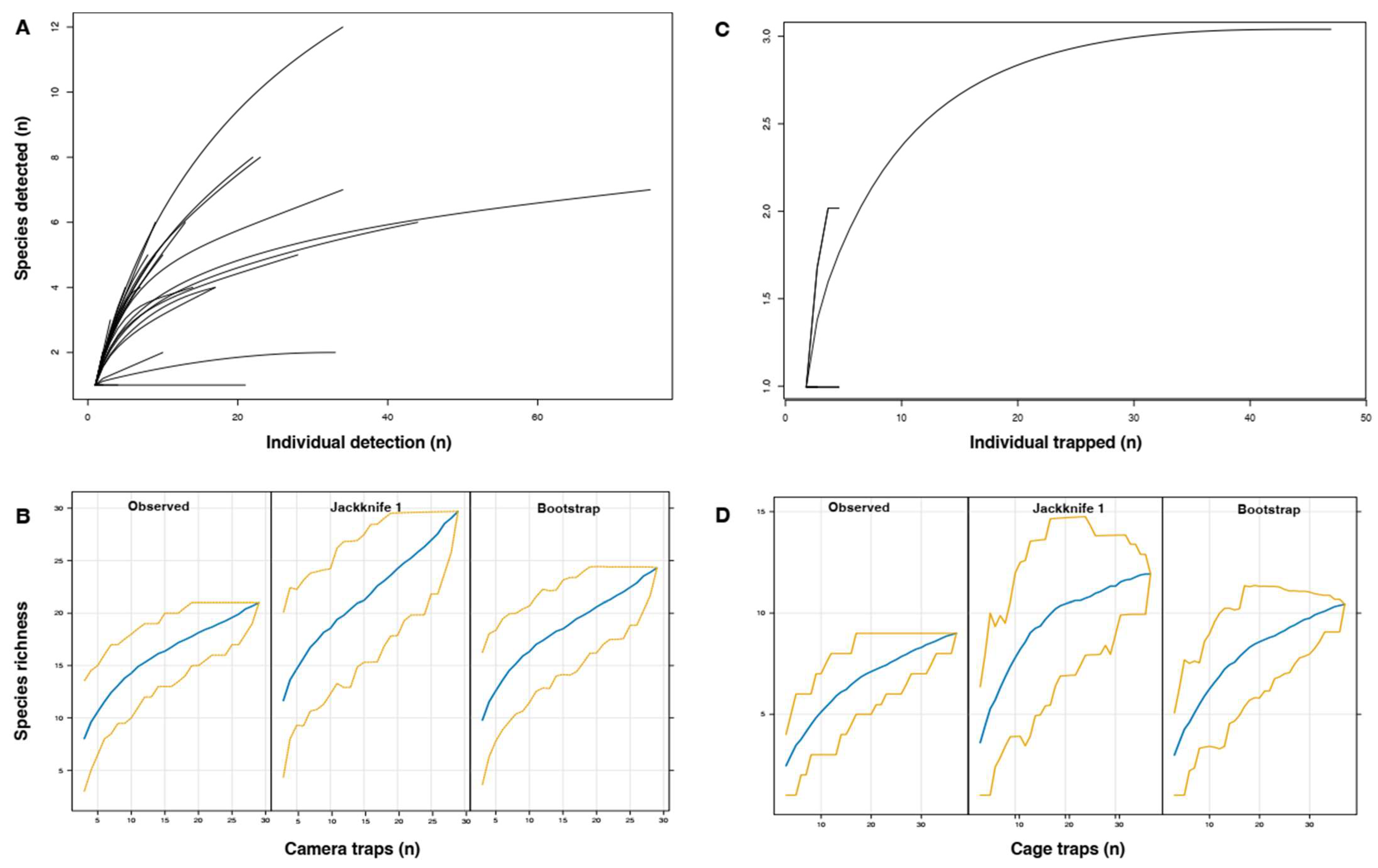
| Habitat | Cameras (n) | Set-Up to Retrieval Dates | Number of Days (by Camera) | Observed Species (n) | Jackknife 1 ±SE | Bootstrap ±SE |
|---|---|---|---|---|---|---|
| Reforestation, protected area | 25 | 19 November 2021 22 December 2022 | 398 | 21 | 29.7 ± 4.0 | 24.3 ± 1.9 |
| Plantation and orchard | 7 | 3 March 2022 22 December 2022 | 284 | 4 | 6.0 ± 1.2 | 5.0 ± 0.7 |
| All | 32 | - | - | 21 | 29.7 ± 4.0 | 24.3 ± 1.9 |
| Species | Urban | Paddy Field | Orchard | Rubber | Reforestation | Cave | Protected |
|---|---|---|---|---|---|---|---|
| Asian black bear | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Asian golden cat | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Back-striped weasel | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Bandicota indica | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Chinese pangolin | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Common palm civet | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Dhole | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Domestic dog | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Golden jackal | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Greater hog badger | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Large Indian civet | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Leopard cat | 0 | 0 | 1 | 1 | 1 | 0 | 1 |
| Leopoldamys herberti | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Lesser bamboo rat | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Maxomys surifer | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Menetes berdmorei | 0 | 1 | 1 | 0 | 1 | 0 | 1 |
| Red muntjac | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Niviventer mekongis | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Northern pig-tailed macaque | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Rattus andamanensis | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| Rattus exulans | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Rattus tanezumi | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| Indochinese serow | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Small Asian mongoose | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Tupaia belangeri | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Variable squirrel | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Wild boar | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thinphovong, C.; Kritiyakan, A.; Chakngean, R.; Paladsing, Y.; Makaew, P.; Labadie, M.; Mahuzier, C.; Phimpraphai, W.; Morand, S.; Chaisiri, K. From Protected Habitat to Agricultural Land: Dogs and Small Mammals Link Habitats in Northern Thailand. Ecologies 2023, 4, 671-685. https://doi.org/10.3390/ecologies4040044
Thinphovong C, Kritiyakan A, Chakngean R, Paladsing Y, Makaew P, Labadie M, Mahuzier C, Phimpraphai W, Morand S, Chaisiri K. From Protected Habitat to Agricultural Land: Dogs and Small Mammals Link Habitats in Northern Thailand. Ecologies. 2023; 4(4):671-685. https://doi.org/10.3390/ecologies4040044
Chicago/Turabian StyleThinphovong, Chuanphot, Anamika Kritiyakan, Ronnakrit Chakngean, Yossapong Paladsing, Phurin Makaew, Morgane Labadie, Christophe Mahuzier, Waraphon Phimpraphai, Serge Morand, and Kittipong Chaisiri. 2023. "From Protected Habitat to Agricultural Land: Dogs and Small Mammals Link Habitats in Northern Thailand" Ecologies 4, no. 4: 671-685. https://doi.org/10.3390/ecologies4040044
APA StyleThinphovong, C., Kritiyakan, A., Chakngean, R., Paladsing, Y., Makaew, P., Labadie, M., Mahuzier, C., Phimpraphai, W., Morand, S., & Chaisiri, K. (2023). From Protected Habitat to Agricultural Land: Dogs and Small Mammals Link Habitats in Northern Thailand. Ecologies, 4(4), 671-685. https://doi.org/10.3390/ecologies4040044







