Odonata, Coleoptera, and Heteroptera (OCH) Trait-Based Biomonitoring of Rivers within the Northwestern Rif of Morocco: Exploring the Responses of Traits to Prevailing Environmental Gradients
Abstract
:1. Introduction
2. Materials and Methods
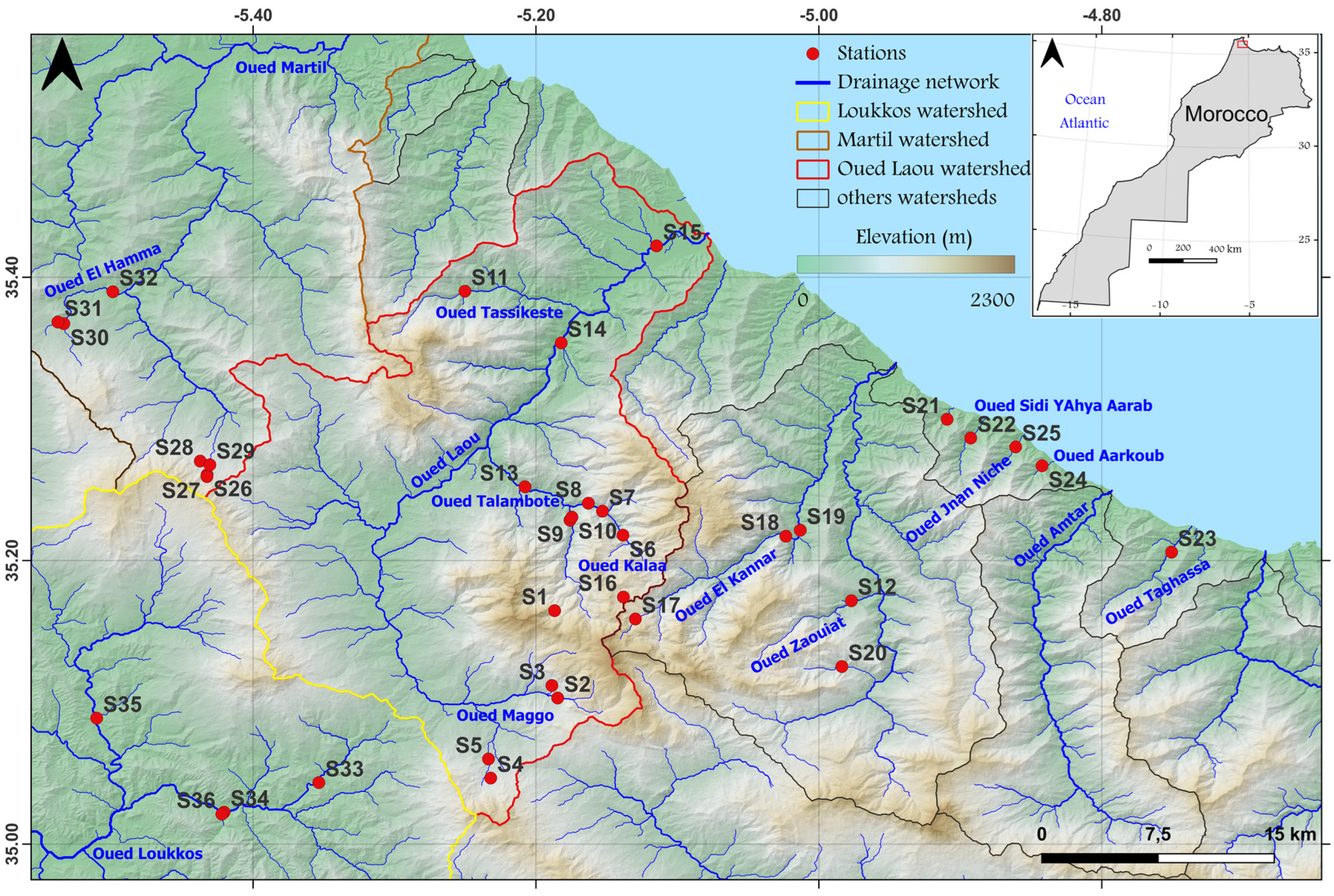
2.1. Study area Description
2.2. Evaluation of OCH Sampling and Analyses of Physico-Chemical and Hydraulic Parameters
2.2.1. Odonata, Coleoptera, and Heteroptera Group Sampling and Identification
2.2.2. Physico-Chemical and Hydraulic Parameter Measurements
2.3. Odonata, Coleoptera, and Heteroptera Trait Selection Fuzzy Coding System
2.4. Data Analysis
2.4.1. Sampling Delineation into Impact Categories
2.4.2. RLQ and Other Associated Tests—Identifying and Classifying Vulnerable and Tolerant OCH Traits
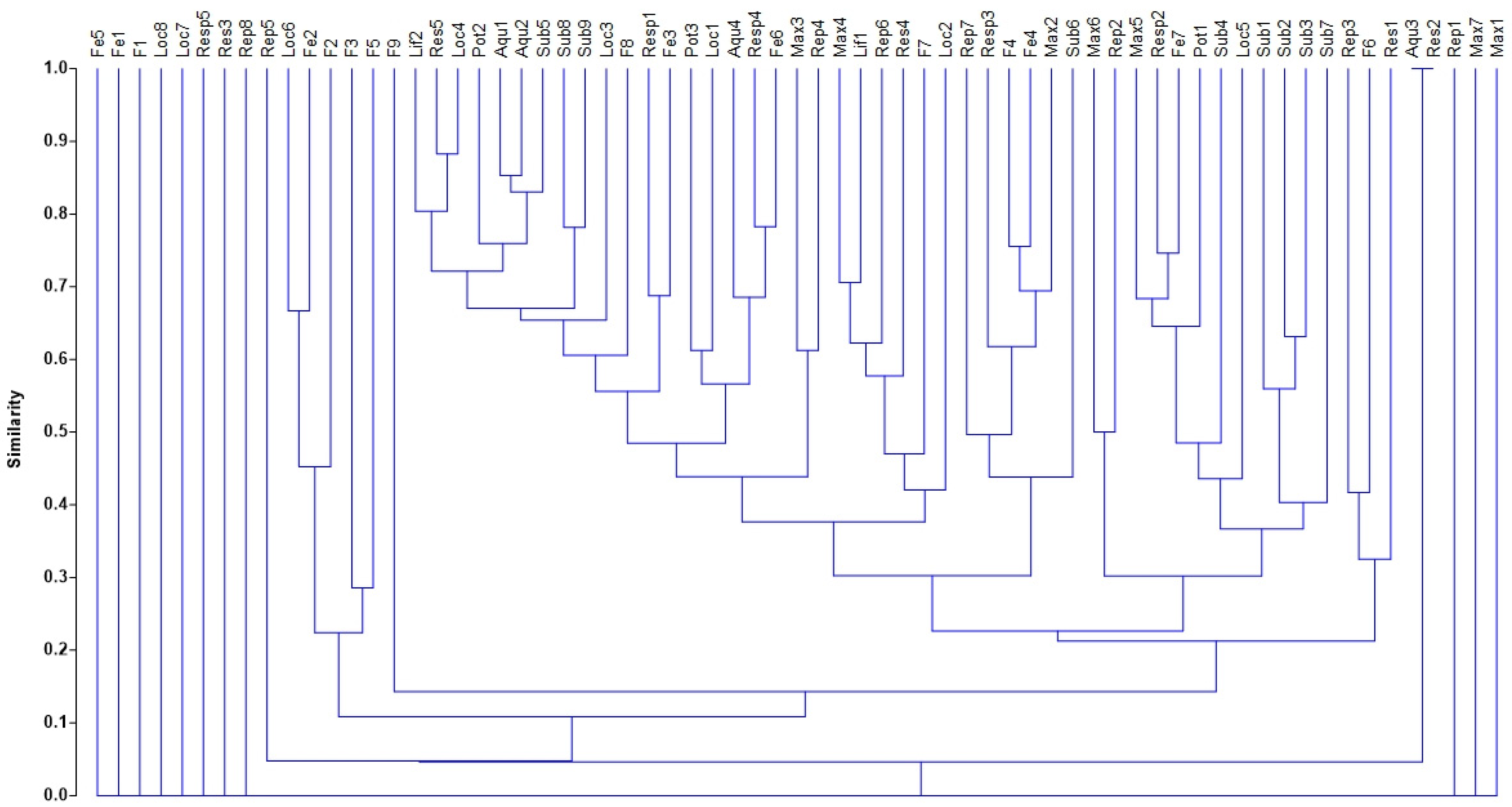
2.4.3. Inter-Trait Co-Occurrence Based on Cluster Analysis
3. Results
3.1. Site Delineation in the Study Area Catchments along an Impact Gradient in the Study Area
3.2. Odonata, Coleoptera, and Heteroptera (OCH) Taxa and Trait Distribution Patterns along Impact Gradient in the Study Area—Selection of Vulnerable and Tolerant OCH Traits
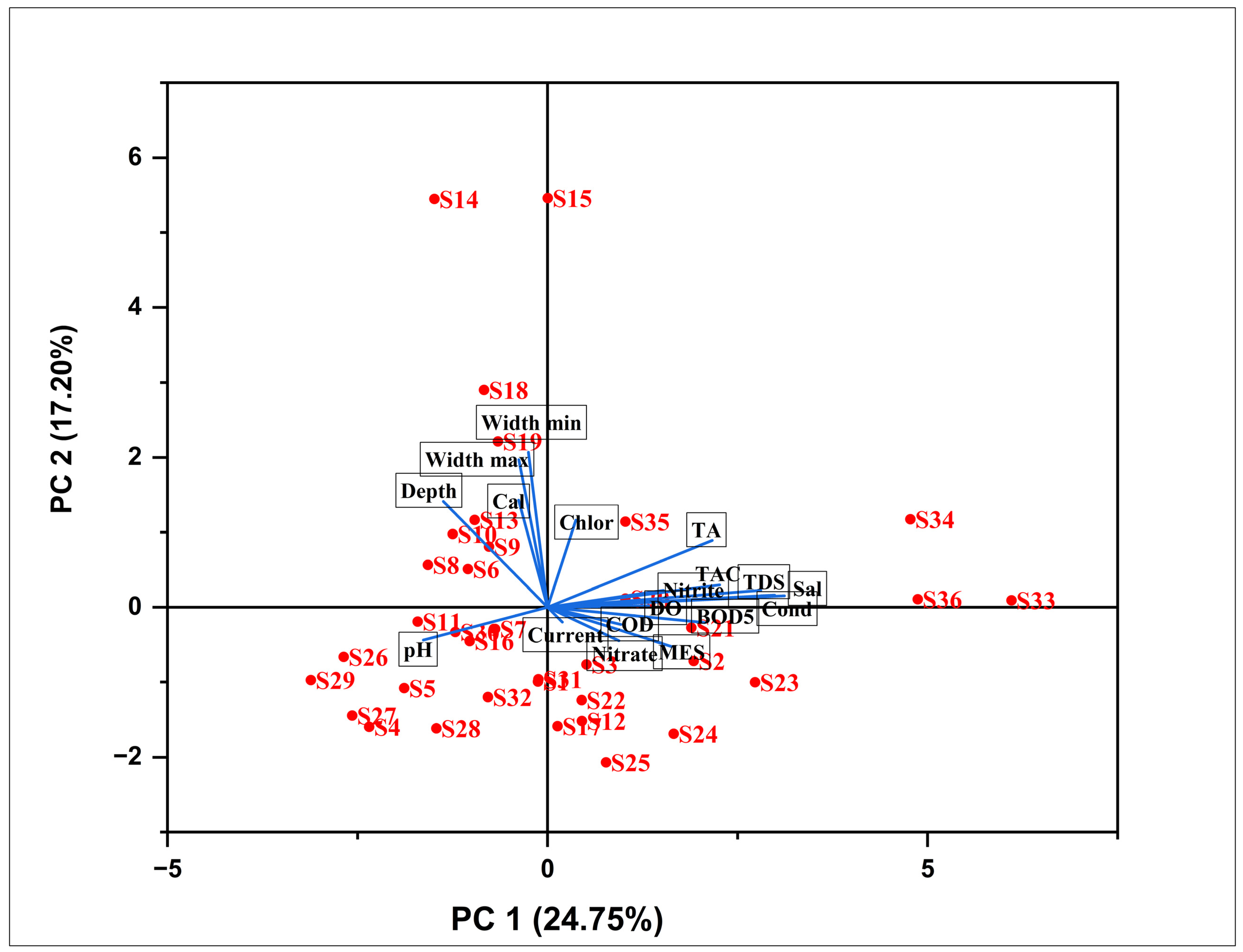
3.2.1. Relationships between Sampling Sites and OCH Taxa
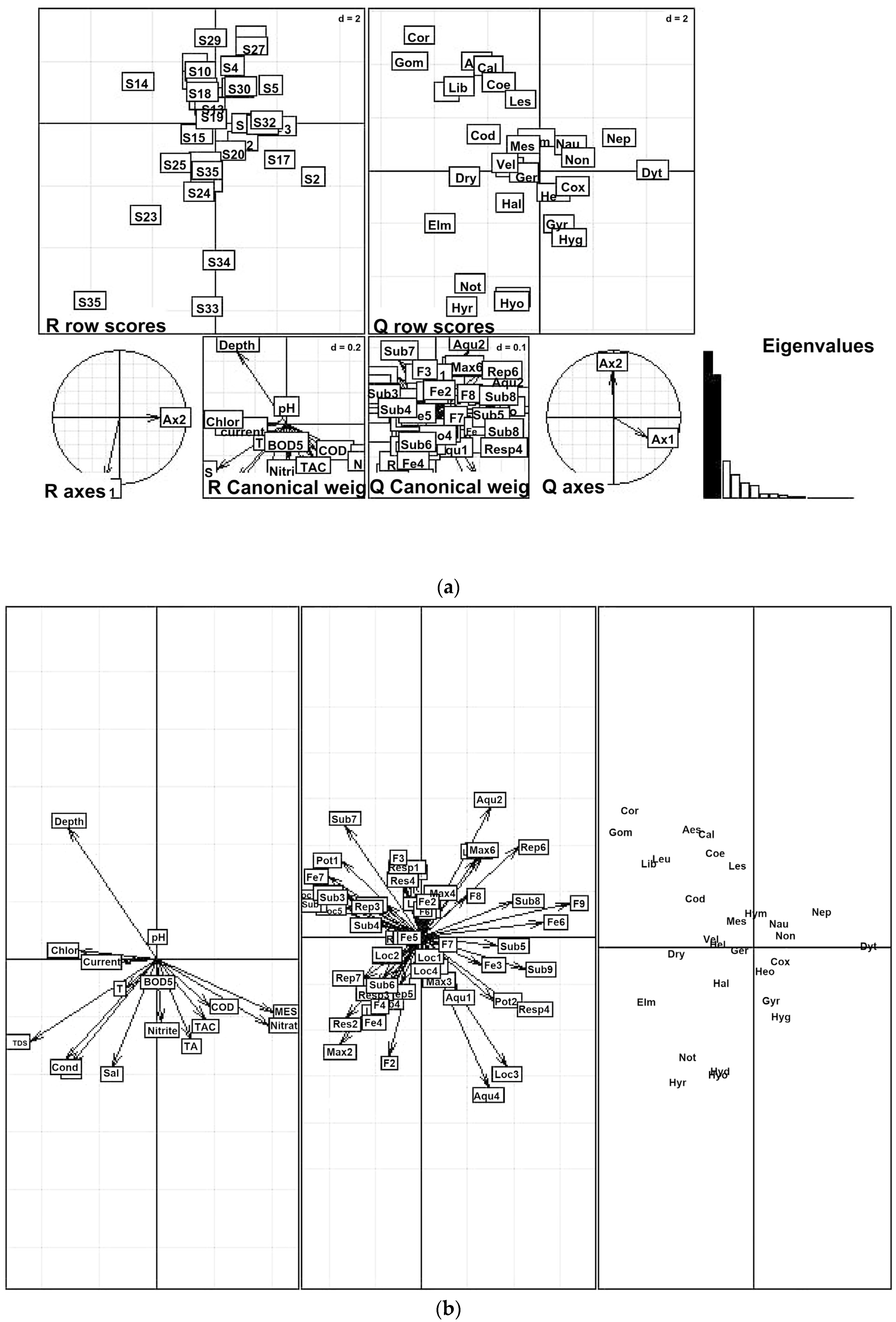
3.2.2. Relationships between Physico-Chemical Parameters and OCH Traits in the Site Impact Categories
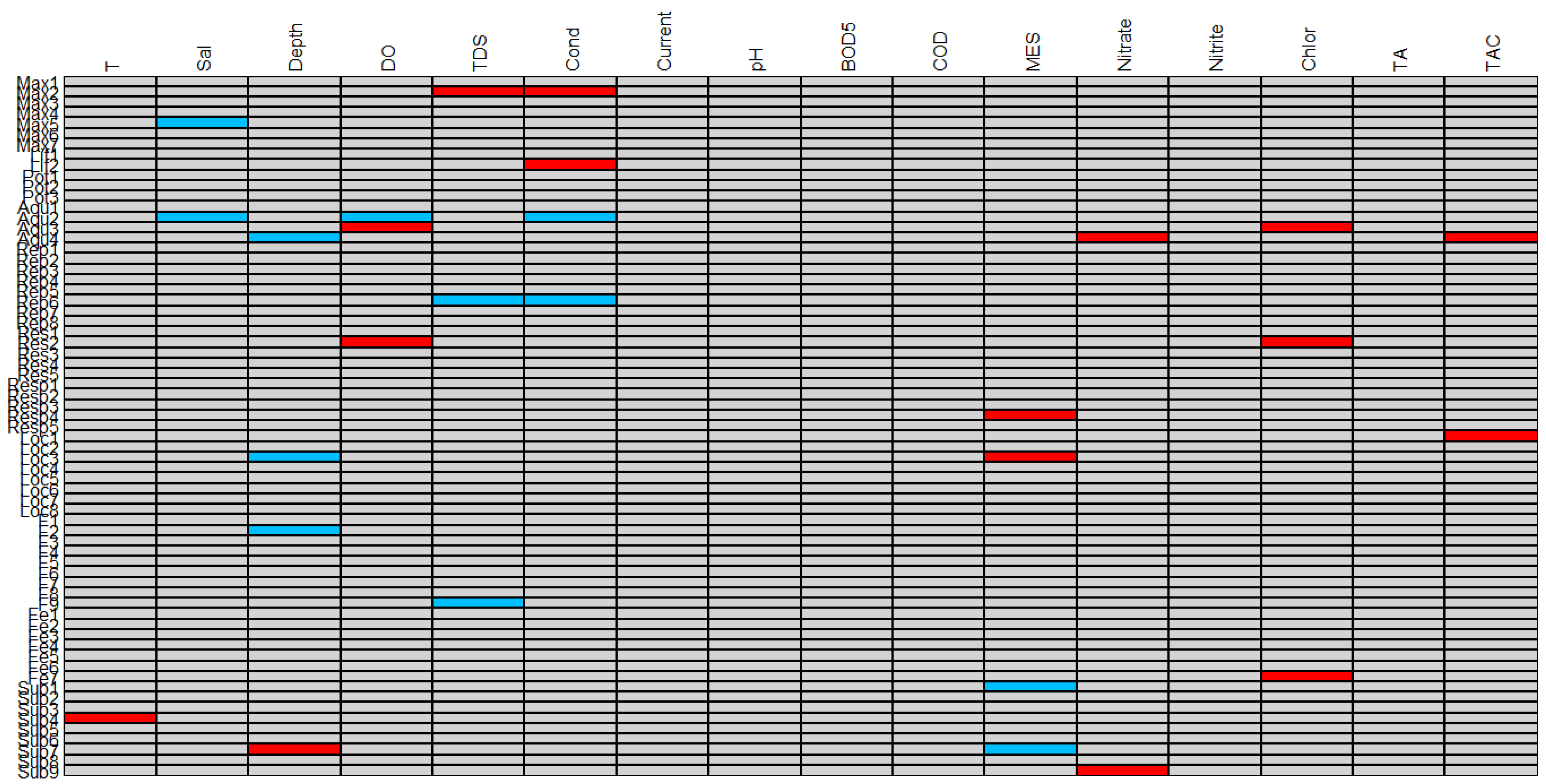
3.3. Relationships between Physico-Chemical Parameters and OCH Traits in the Site Impact Categories Based on Fourth Corner Test—Identifying Vulnerable and Tolerant Traits
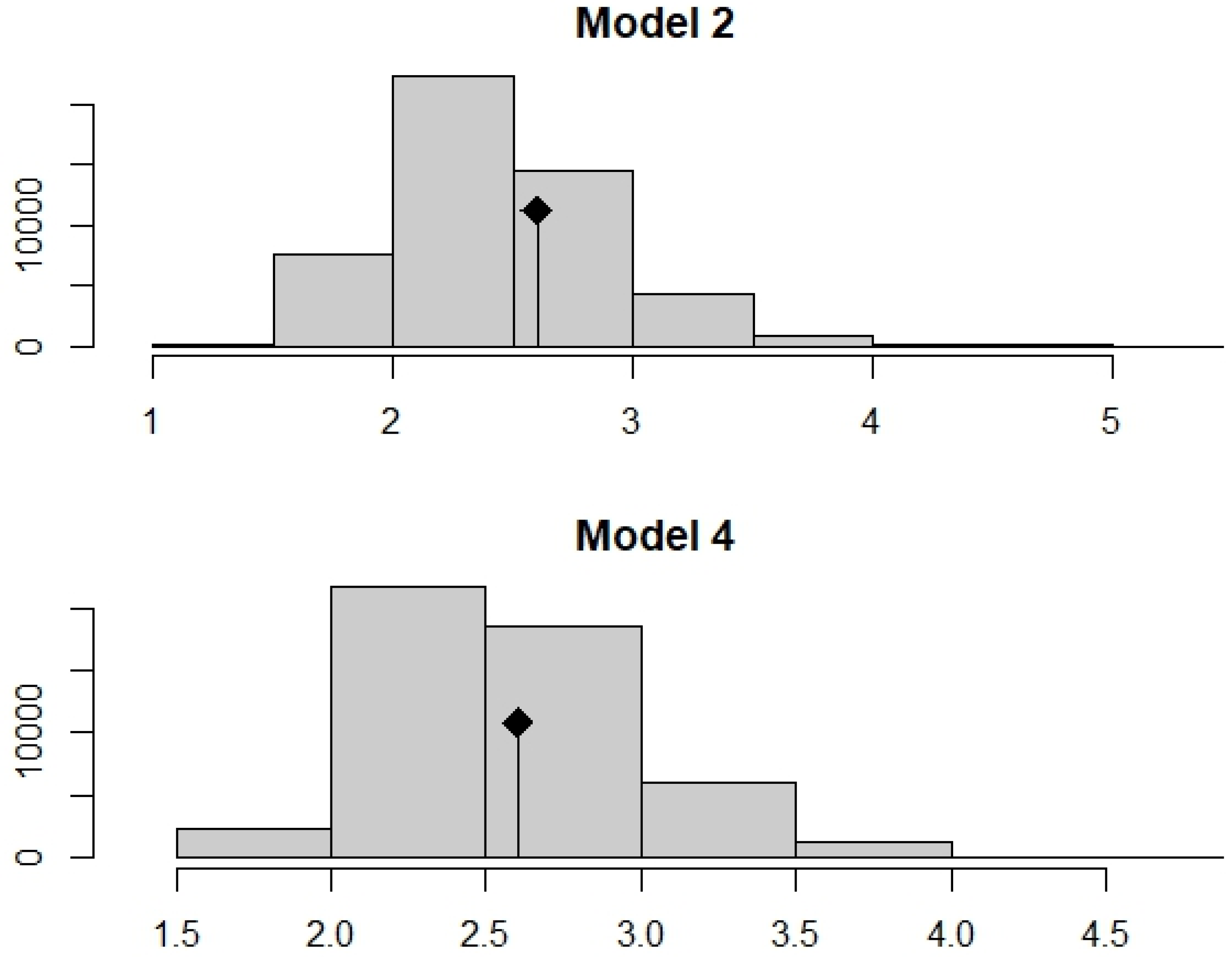
3.4. Testing the Strength of Fourth-Corner Test to Detect the Level Significance Difference between Physico-Chemical Parameters and OCH Traits
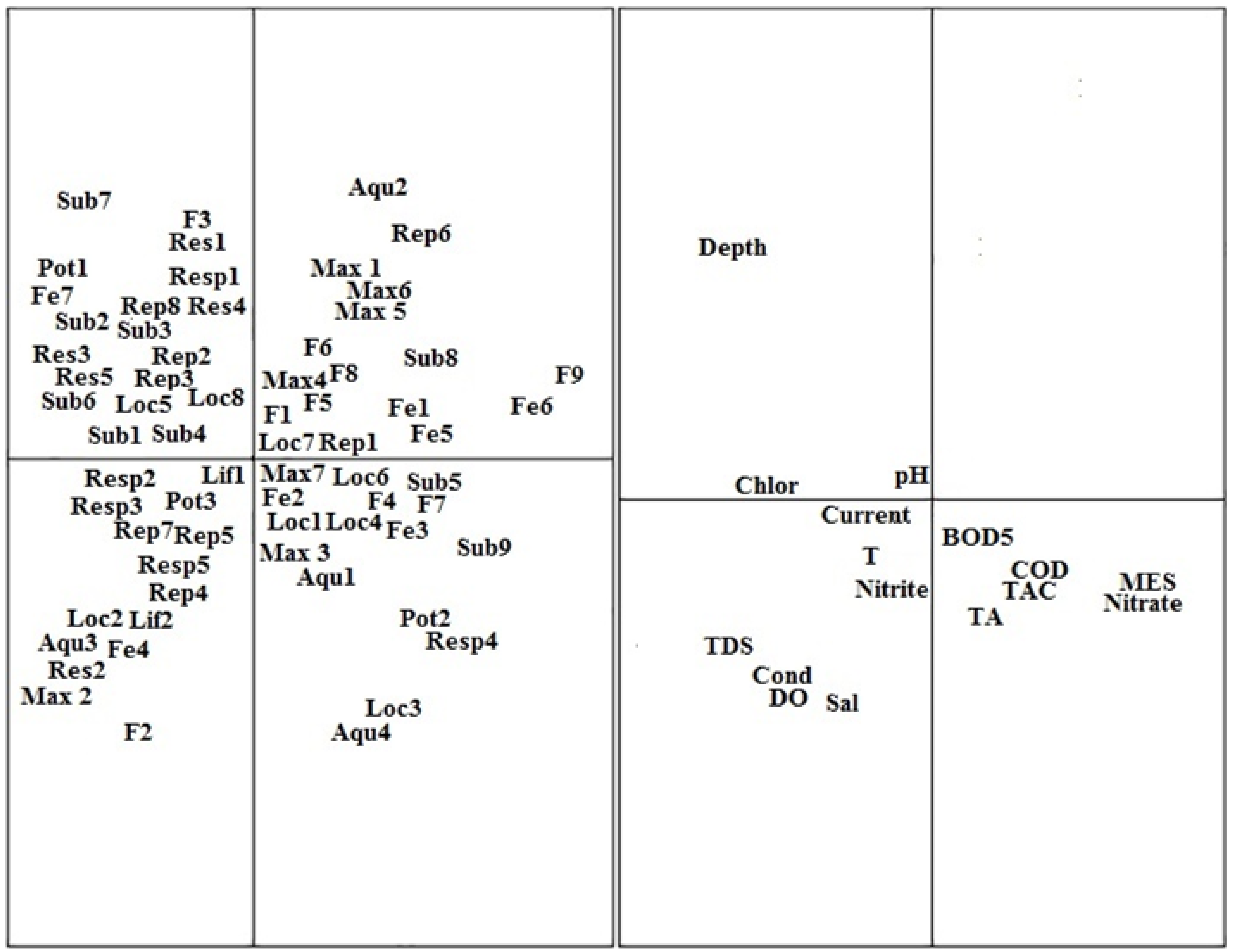
3.5. Visualization of Odonata, Coleoptera, and Heteroptera (OCH) Inter-Trait Classes and Attribute Relationships in the Sampled Sites
4. Discussion
4.1. Odonata, Coleoptera, and Heteroptera (OCH) Taxa and Trait Distribution Patterns along Impact Gradient in the Study Area—Selection of Vulnerable and Tolerant OCH Traits
4.1.1. Relationships between Sampling Sites and OCH Taxa
4.1.2. Relationship between Physico-Chemical Parameters and OCH Traits in the Site Impact Categories
4.2. Confirming the Relationship between Physico-Chemical Parameters and OCH Traits Based on Fourth Corner Test—Identifying Vulnerable and Tolerant Traits
4.3. Visualization of Odonata, Coleoptera, and Heteroptera (OCH) Inter-Trait Classes and Attribute Relationship in the Sampled Sites
4.4. Implications of the Use of OCH Traits for Riverine Ecosystem Biomonitoring in the Mediterranean Region
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dudgeon, D. Prospects for Sustaining Freshwater Biodiversity in the 21st Century: Linking Ecosystem Structure and Function. Curr. Opin. Environ. Sustain. 2010, 2, 422–430. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging Threats and Persistent Conservation Challenges for Freshwater Biodiversity. Biol. Rev. 2018, 94, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Zablocki, J.; Newsock, A.; Krolopp, A.; Tabas, P.; Salama, M. Durable Freshwater Protection: A Framework for Establishing and Maintaining Long-Term Protection for Freshwater Ecosystems and the Values They Sustain. Sustainability 2021, 13, 1950. [Google Scholar] [CrossRef]
- Franin, K.; Barić, B.; Kuštera, G. The Role of Ecological Infrastructure on Beneficial Arthropods in Vineyards. Span. J. Agric. Res. 2016, 14, e0303. [Google Scholar] [CrossRef]
- El Harche, H.; El Hassouni, S.; Fadli, M. Spatial, seasonal variation and impacts of anthropogenic factors on insect assemblages (Arthropoda: Insecta) in Northwest Morocco. Biodiversitas J. Biol. Divers. 2023, 24, 5368–5375. [Google Scholar] [CrossRef]
- Sánchez-Montoya, M.M. Estado Ecológico de Los Ríos Mediterráneos: Tipología, Condiciones de Referencia y Establecimiento de Clases. Ph.D. Thesis, Universidad de Murcia, Murcia, Spain, 2008; p. 179. [Google Scholar]
- Gasith, A.; Resh, V.H. Streams in Mediterranean Climate Regions: Abiotic Influences and Biotic Responses to Predictable Seasonal Events. Annu. Rev. Ecol. Syst. 1999, 30, 51–81. [Google Scholar] [CrossRef]
- Prat, N. Water Use and Quality and Stream Flow in a Mediterranean Stream. Water Res. 2000, 34, 3876–3881. [Google Scholar] [CrossRef]
- Bonada, N.; Dolédec, S.; Statzner, B. Taxonomic and Biological Trait Differences of Stream Macroinvertebrate Communities between Mediterranean and Temperate Regions: Implications for Future Climatic Scenarios. Glob. Change Biol. 2007, 13, 1658–1671. [Google Scholar] [CrossRef]
- Bruno, D.; Belmar, O.; Sánchez-Fernández, D.; Guareschi, S.; Millán, A.; Velasco, J. Responses of Mediterranean Aquatic and Riparian Communities to Human Pressures at Different Spatial Scales. Ecol. Indic. 2014, 45, 456–464. [Google Scholar] [CrossRef]
- Guellaf, A.; Bennas, N.; El Haissoufi, M.; L’Mohdi, O.; Kettani, K. Nuevos Datos Sobre La Biodiversidad y Corología de Insectos Acuáticos (Odonata, Coleoptera y Hemiptera) de La Cuenca Del Río Martil (Noroeste de Marruecos). Graellsia 2021, 77, e149. [Google Scholar] [CrossRef]
- Bennas, N.; Sáinz-Cantero, C.E.; Alba-Tercedor, J. Preliminary data for a biogeographic study of the Baetic-Rif Mountains based on aquatic Coleoptera. Zool. Baet 1992, 3, 167–180. [Google Scholar]
- Blondel, J.; Aronson, J.; Bodiou, J.-Y.; Boeuf, G. The Mediterranean Region; OUP Oxford: New York, NY, USA, 2010; p. 392. [Google Scholar]
- Kernan, M.; Battarbee, R.W.; Moss, B.R. Climate Change Impacts on Freshwater Ecosystems; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Poff, N.L.; Ward, J.V. Physical Habitat Template of Lotic Systems: Recovery in the Context of Historical Pattern of Spatiotemporal Heterogeneity. Environ. Manage 1990, 14, 629–645. [Google Scholar] [CrossRef]
- Townsend, C.R.; Hildrew, A.G. Species Traits in Relation to a Habitat Templet for River Systems. Fresh. Biol. 1994, 31, 265–275. [Google Scholar] [CrossRef]
- Townsend, C.; Dolédec, S.; Scarsbrook, M. Species Traits in Relation to Temporal and Spatial Heterogeneity in Streams: A Test of Habitat Templet Theory. Fresh. Biol. 1997, 37, 367–387. [Google Scholar] [CrossRef]
- Statzner, B.; Bis, B.; Dolédec, S.; Usseglio-Polatera, P. Perspectives for Biomonitoring at Large Spatial Scales: A Unified Measure for the Functional Composition of Invertebrate Communities in European Running Waters. Basic Appl. Ecol. 2001, 2, 73–85. [Google Scholar] [CrossRef]
- Walsh, C.J. Biological Indicators of Stream Health Using Macroinvertebrate Assemblage Composition: A Comparison of Sensitivity to an Urban Gradient. Mar. Freshw. Res. 2006, 57, 37. [Google Scholar] [CrossRef]
- Wang, X.; Su, P.; Lin, Q.; Song, J.; Sun, H.; Cheng, D.; Wang, S.; Peng, J.; Fu, J. Distribution, Assessment and Coupling Relationship of Heavy Metals and Macroinvertebrates in Sediments of the Weihe River Basin. Sustain. Cities Soc. 2019, 50, 101665. [Google Scholar] [CrossRef]
- De Castro-Català, N.; Dolédec, S.; Kalogianni, E.; Skoulikidis, N.T.; Paunovic, M.; Vasiljević, B.; Sabater, S.; Tornés, E.; Muñoz, I. Unravelling the Effects of Multiple Stressors on Diatom and Macroinvertebrate Communities in European River Basins Using Structural and Functional Approaches. Sci. Total Environ. 2020, 742, 140543. [Google Scholar] [CrossRef]
- Mangadze, T.; Wasserman, R.J.; Froneman, P.W.; Dalu, T. Macroinvertebrate Functional Feeding Group Alterations in Response to Habitat Degradation of Headwater Austral Streams. Sci. Total Environ. 2019, 695, 133910. [Google Scholar] [CrossRef]
- Fernandes, G.W.; Arantes-Garcia, L.; Barbosa, M.; Barbosa, N.P.U.; Batista, E.K.L.; Beiroz, W.; Resende, F.M.; Abrahão, A.; Almada, E.D.; Alves, E.; et al. Biodiversity and Ecosystem Services in the Campo Rupestre: A Road Map for the Sustainability of the Hottest Brazilian Biodiversity Hotspot. Perspect. Ecol. Conserv. 2020, 18, 213–222. [Google Scholar] [CrossRef]
- Vandewalle, M.; de Bello, F.; Berg, M.P.; Bolger, T.; Dolédec, S.; Dubs, F.; Feld, C.K.; Harrington, R.; Harrison, P.A.; Lavorel, S.; et al. Functional Traits as Indicators of Biodiversity Response to Land Use Changes across Ecosystems and Organisms. Biodivers. Conserv. 2010, 19, 2921–2947. [Google Scholar] [CrossRef]
- El Yaagoubi, S.; Edegbene, A.O.; El Alami, M.; Errochdi, S.; Harrak, R. Ephemeroptera, Plecoptera and Trichoptera (EPT) Trait-Based Biomonitoring of Rivers within the Northwestern Rif of Morocco: Implications for Determining Riverine Ecosystem Ecological Health in Africa. Aqua. Sci. 2024; (under review). [Google Scholar]
- Downing, A.L. Relative effects of species composition and richness on ecosystem properties in ponds. Ecology 2005, 86, 701–715. [Google Scholar] [CrossRef]
- Karouna-Renier, N.K.; Sparling, D.W. Relationships between Ambient Geochemistry, Watershed Land-Use and Trace Metal Concentrations in Aquatic Invertebrates Living in Stormwater Treatment Ponds. Environ. Pollut. 2001, 112, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Oertli, B.; Auderset Joye, D.; Castella, E.; Juge, R.; Lehmann, A.; Lachavanne, J. PLOCH: A Standardized Method for Sampling and Assessing the Biodiversity in Ponds. Aquatic Conserv. Mar. Freshw. Ecosyst. 2005, 15, 665–679. [Google Scholar] [CrossRef]
- Bilton, D.T.; Mcabendroth, L.; Bedford, A.; Ramsay, P.M. How Wide to Cast the Net? Cross-taxon Congruence of Species Richness, Community Similarity and Indicator Taxa in Ponds. Freshw. Biol. 2006, 51, 578–590. [Google Scholar] [CrossRef]
- Corbet, P.S. Are Odonata useful as bioindicators? Libellula 1993, 12, 91–102. [Google Scholar]
- Vilenica, M.; Rebrina, F.; Matoničkin Kepčija, R.; Šegota, V.; Rumišek, M.; Ružanović, L.; Brigić, A. Aquatic Macrophyte Vegetation Promotes Taxonomic and Functional Diversity of Odonata Assemblages in Intermittent Karst Rivers in the Mediterranean. Diversity 2022, 14, 31. [Google Scholar] [CrossRef]
- Violle, C.; Navas, M.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the Concept of Trait Be Functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Dolédec, S.; Statzner, B. Responses of Freshwater Biota to Human Disturbances: Contribution of J-NABSto Developments in Ecological Integrity Assessments. J. North Am. Benthol. Soc. 2010, 29, 286–311. [Google Scholar] [CrossRef]
- Berger, E.; Haase, P.; Schäfer, R.B.; Sundermann, A. Towards Stressor-Specific Macroinvertebrate Indices: Which Traits and Taxonomic Groups Are Associated with Vulnerable and Tolerant Taxa? Sci. Total. Environ. 2018, 619–620, 144–154. [Google Scholar] [CrossRef]
- Firmiano, K.R.; Castro, D.M.P.; Linares, M.S.; Callisto, M. Functional Responses of Aquatic Invertebrates to Anthropogenic Stressors in Riparian Zones of Neotropical Savanna Streams. Sci. Total. Environ. 2021, 753, 141865. [Google Scholar] [CrossRef]
- Bady, P.; Dolédec, S.; Fesl, C.; Gayraud, S.; Bacchi, M.; Schöll, F. Use of Invertebrate Traits for the Biomonitoring of European Large Rivers: The Effects of Sampling Effort on Genus Richness and Functional Diversity. Fresh. Biol. 2004, 50, 159–173. [Google Scholar] [CrossRef]
- Charvet, S.; Statzner, B.; Usseglio-Polatera, P.; Dumont, B. Traits of Benthic Macroinvertebrates in Semi-natural French Streams: An Initial Application to Biomonitoring in Europe. Freshw. Biol. 2000, 43, 277–296. [Google Scholar] [CrossRef]
- Dolédec, S.; Statzner, B.; Bournard, M. Species Traits for Future Biomonitoring across Ecoregions: Patterns along a Human-impacted River. Freshw. Biol. 1999, 42, 737–758. [Google Scholar] [CrossRef]
- Dolédec, S.; Chessel, D.; Gimaret-Carpentier, C. Niche Separation in Community Analysis: A New Method. Ecology 2000, 81, 2914. [Google Scholar] [CrossRef]
- Gayraud, S.; Statzner, B.; Bady, P.; Haybachp, A.; Schöll, F.; Usseglio-Polatera, P.; Bacchi, M. Invertebrate Traits for the Biomonitoring of Large European Rivers: An Initial Assessment of Alternative Metrics. Freshw. Biol. 2003, 48, 2045–2064. [Google Scholar] [CrossRef]
- Menezes, S.; Baird, D.J.; Soares, A.M.V.M. Beyond Taxonomy: A Review of Macroinvertebrate Trait-based Community Descriptors as Tools for Freshwater Biomonitoring. J. Appl. Ecol. 2010, 47, 711–719. [Google Scholar] [CrossRef]
- Usseglio-Polatera, P.; Bournaud, M.; Richoux, P.; Tachet, H. Biological and Ecological Traits of Benthic Freshwater Macroinvertebrates: Relationships and Definition of Groups with Similar Traits. Freshw. Biol. 2000, 43, 175–205. [Google Scholar] [CrossRef]
- Chessman, B.C.; Royal, M.J. Bioassessment without Reference Sites: Use of Environmental Filters to Predict Natural Assemblages of River Macroinvertebrates. J. North Am. Benthol. Soc. 2004, 23, 599–615. [Google Scholar] [CrossRef]
- Dolédec, S.; Phillips, N.; Scarsbrook, M.; Riley, R.H.; Townsend, C.R. Comparison of Structural and Functional Approaches to Determining Landuse Effects on Grassland Stream Invertebrate Communities. J. North Am. Benthol. Soc. 2006, 25, 44–60. [Google Scholar] [CrossRef]
- Merritt, R.W.; Cummins, K.W.; Berg, M.B.; Novak, J.A.; Higgins, M.J.; Wessell, K.J.; Lessard, J.L. Development and Application of a Macroinvertebrate Functional-Group Approach in the Bioassessment of Remnant River Oxbows in Southwest Florida. J. North Am. Benthol. Soc. 2002, 21, 290–310. [Google Scholar] [CrossRef]
- Vieira, N.K.M.; Clements, W.H.; Guevara, L.S.; Jacobs, B.F. Resistance and Resilience of Stream Insect Communities to Repeated Hydrologic Disturbances after a Wildfire. Freshw. Biol. 2004, 49, 1243–1259. [Google Scholar] [CrossRef]
- Sotomayor, G.; Hampel, H.; Vázquez, R.F.; Forio, M.A.E.; Goethals, P.L.M. Implications of Macroinvertebrate Taxonomic Resolution for Freshwater Assessments Using Functional Traits: The Paute River Basin (Ecuador) Case. Divers. Distrib. 2021, 28, 1735–1747. [Google Scholar] [CrossRef]
- Akamagwuna, F.C.; Ntloko, P.; Edegbene, A.O.; Odume, O.N. Are Ephemeroptera, Plecoptera and Trichoptera Traits Reliable Indicators of Semi-Urban Pollution in the Tsitsa River, Eastern Cape Province of South Africa? Environ. Monit. Assess. 2021, 193, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Benzina, I.; Si Bachir, A.; Santoul, F.; Céréghino, R. Macroinvertebrate Functional Trait Responses to Environmental Gradients and Anthropogenic Disturbance in Arid-Land Streams of North Africa. J. Arid Environ. 2021, 195, 104626. [Google Scholar] [CrossRef]
- Edegbene, A.O.; Arimoro, F.O.; Odume, O.N. Developing and Applying a Macroinvertebrate-based Multimetric Index for Urban Rivers in the Niger Delta, Nigeria. Ecol. Evol. 2019, 9, 12869–12885. [Google Scholar] [CrossRef] [PubMed]
- Edegbene, A.O.; Arimoro, F.O.; Odume, O.N. How Does Urban Pollution Influence Macroinvertebrate Traits in Forested Riverine Systems? Water 2020, 12, 3111. [Google Scholar] [CrossRef]
- Edegbene, A.O.; Arimoro, F.O.; Odume, O.N. Exploring the Distribution Patterns of Macroinvertebrate Signature Traits and Ecological Preferences and Their Responses to Urban and Agricultural Pollution in Selected Rivers in the Niger Delta Ecoregion, Nigeria. Aquat. Ecol. 2020, 54, 553–573. [Google Scholar] [CrossRef]
- Edegbene, A.O.; Arimoro, F.O.; Odume, O.N.; Ogidiaka, E.; Keke, U.N. Can Macroinvertebrate Traits Be Explored and Applied in Biomonitoring Riverine Systems Draining Forested Catchments? Front. Water 2021, 3, 607556. [Google Scholar] [CrossRef]
- Edegbene, A.O.; Odume, O.N.; Arimoro, F.O.; Keke, U.N. Identifying and Classifying Macroinvertebrate Indicator Signature Traits and Ecological Preferences along Urban Pollution Gradient in the Niger Delta. Environ. Pollut. 2021, 281, 117076. [Google Scholar] [CrossRef] [PubMed]
- Errochdi, S.; El Alami, M.; Bennas, N.; Belqat, B.; Ater, M.; Fdil, F. Étude de La Qualité Physicochimique et Microbiologique de Deux Réseaux Hydrographiques Nord Marocains: Laou et Tahaddart. Méditerranée 2012, 118, 41–51. [Google Scholar] [CrossRef]
- El Yaagoubi, S.; El Alami, M.; Harrak, R.; Azmizem, A.; Ikssi, M.; Aoulad Mansour, M.R. Assessment of Functional Feeding Groups (FFG) Structure of Aquatic Insects in North- Western Rif—Morocco. Biodivers. Data J. 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Kettani, K.; Ebejer, M.J.; Ackland, D.M.; Bächli, G.; Barraclough, D.; Barták, M.; Carles-Tolrá, M.; Černý, M.; Cerretti, P.; Chandler, P.; et al. Catalogue of the Diptera (Insecta) of Morocco—An Annotated Checklist, with Distributions and a Bibliography. ZooKeys 2022, 1094, 1–466. [Google Scholar] [CrossRef] [PubMed]
- Tachet, H.; Richoux, P.; Bournaud, M.; Usseglio-Polatera, P. Invertébrés D’eau Douce: Systématique, Biologie, Écologie, 3rd ed.; CNRS: Paris, France, 2010. [Google Scholar]
- Rodier, J.; Legube, B.; Merlet, N. L’analyse de L’eau, 10th ed.; Dunod: Paris, France, 2016. [Google Scholar]
- Bis, B.; Usseglio-Polatera, P. Species Traits Analysis. European Commission, STAR (Standardisation of River Classifications), Deliverable N2. A Project under 5th Framework Programme Energy, Environment and Sustainable Development Key Action 1: Sustainable Management and Quality of Water Contract No: EVK1-CT 2001-00089; University of Lodz: Lodz, Poland; University of Metz: Metz, France, 2004; p. 148. [Google Scholar]
- Chevenet, F.; Dolédec, S.; Chessel, D. A Fuzzy Coding Approach for the Analysis of Long-term Ecological Data. Freshw. Biol. 1994, 31, 295–309. [Google Scholar] [CrossRef]
- Edegbene, A.O. Developing Macroinvertebrate Trait-and Taxonomically-Based Approaches for Biomonitoring Wadeable Riverine Systems in the Niger Delta, Nigeria. Ph.D. Thesis, Rhodes University, Grahamstown, South Africa, 2020. [Google Scholar]
- Dolédec, S.; Chessel, D.; ter Braak, C.J.F.; Champely, S. Matching Species Traits to Environmental Variables: A New Three-Table Ordination Method. Environ. Ecol. Stat. 1996, 3, 143–166. [Google Scholar] [CrossRef]
- Dray, S.; Dufour, A.-B. Theade4Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22. [Google Scholar] [CrossRef]
- Edegbene, A.O.; Elakhame, L.A.; Arimoro, F.O.; Osimen, E.C.; Edegbene Ovie, T.T.; Akumabor, E.C.; Ubanatu, N.C.; Njuguna, C.W.; Sankoh, A.A.; Akamagwuna, F.C. How Do the Traits of Macroinvertebrates in the River Chanchaga Respond to Illegal Gold Mining Activities in North Central Nigeria. Front. Ecol. Evol. 2023, 11. [Google Scholar] [CrossRef]
- Lamouroux, N.; Dolédec, S.; Gayraud, S. Biological Traits of Stream Macroinvertebrate Communities: Effects of Microhabitat, Reach, and Basin Filters. J. North Am. Benthol. Soc. 2004, 23, 449–466. [Google Scholar] [CrossRef]
- Tierno de Figueroa, J.M.; López-Rodríguez, M.J.; Fenoglio, S.; Sánchez-Castillo, P.; Fochetti, R. Freshwater Biodiversity in the Rivers 844 of the Mediterranean Basin. Hydrobiologia 2012, 719, 137–186. [Google Scholar] [CrossRef]
- Villalobos-Jiménez, G.; Dunn, A.M.; Hassall, C. Dragonflies and Damselflies (Odonata) in Urban Ecosystems: A Review. Eur. J. Entomol. 2016, 113, 217–232. [Google Scholar] [CrossRef]
- Jäch, M.A.; Balke, M. Global Diversity of Water Beetles (Coleoptera) in Freshwater. Hydrobiologia 2007, 595, 419–442. [Google Scholar] [CrossRef]
- Pérez-Bilbao, A.; Calapez, A.R.; Feio, M.J. Aquatic Coleoptera Distribution Patterns and Their Environmental Drivers in Central Portugal, Iberian Peninsula. Limnologica 2014, 46, 45–57. [Google Scholar] [CrossRef]
- Liess, M.; Schäfer, R.B.; Schriever, C.A. The Footprint of Pesticide Stress in Communities—Species Traits Reveal Community Effects of Toxicants. Sci. Total Environ. 2008, 406, 484–490. [Google Scholar] [CrossRef]
- Pallottini, M.; Cappelletti, D.; Fabrizi, A.; Gaino, E.; Goretti, E.; Selvaggi, R.; Céréghino, R. Macroinvertebrate Functional Trait Responses to Chemical Pollution in Agricultural–Industrial Landscapes. River Res. Appl. 2016, 33, 505–513. [Google Scholar] [CrossRef]
- Karaouzas, I.; Gritzalis, K.C. Local and Regional Factors Determining Aquatic and Semi-Aquatic Bug (Heteroptera) Assemblages in Rivers and Streams of Greece. Hydrobiologia 2006, 573, 199–212. [Google Scholar] [CrossRef]
- Polhemus, J.T.; Polhemus, D.A. Global Diversity of True Bugs (Heteroptera; Insecta) in Freshwater. Hydrobiologia 2007, 595, 379–391. [Google Scholar] [CrossRef]
- Carbonell, J.A.; Abellán, P.; Arribás, P.; Elder, J.F.; Millan, A. The Genus Aphelocheirus Westwood, 1833 (Hemiptera: Aphelocheiridae) in the Iberian Peninsula. Zootaxa 2011, 2771, 1. [Google Scholar] [CrossRef]
- Mathers, K.L.; Rice, S.P.; Wood, P.J. Temporal Effects of Enhanced Fine Sediment Loading on Macroinvertebrate Community Structure and Functional Traits. Sci. Total Environ. 2017, 599–600, 513–522. [Google Scholar] [CrossRef]
- Murphy, J.F.; Jones, J.I.; Arnold, A.; Duerdoth, C.P.; Pretty, J.L.; Naden, P.S.; Sear, D.A.; Collins, A.L. Can Macroinvertebrate Biological Traits Indicate Fine-grained Sediment Conditions in Streams? River Res. Appl. 2017, 33, 1606–1617. [Google Scholar] [CrossRef]
- Wilkes, M.A.; Mckenzie, M.; Murphy, J.F.; Chadd, R.P. Assessing the Mechanistic Basis for Fine Sediment Biomonitoring: Inconsistencies among the Literature, Traits and Indices. River Res. Appl. 2017, 33, 1618–1629. [Google Scholar] [CrossRef]
- Chapman, L.J.; Schneider, K.R.; Apodaca, C.; Chapman, C.A. Respiratory Ecology of Macroinvertebrates in a Swamp–River System of East Africa1. Biotropica 2004, 36, 572. [Google Scholar] [CrossRef]
- Tomanova, S.; Moya, N.; Oberdorff, T. Using Macroinvertebrate Biological Traits for Assessing Biotic Integrity of Neotropical Streams. River Res. Appl. 2008, 24, 1230–1239. [Google Scholar] [CrossRef]
- Ding, N.; Yang, W.; Zhou, Y.; González-Bergonzoni, I.; Zhang, J.; Chen, K.; Vidal, N.; Jeppesen, E.; Liu, Z.; Wang, B. Different Responses of Functional Traits and Diversity of Stream Macroinvertebrates to Environmental and Spatial Factors in the Xishuangbanna Watershed of the Upper Mekong River Basin, China. Sci. Total Environ. 2017, 574, 288–299. [Google Scholar] [CrossRef]
- Desrosiers, M.; Usseglio-Polatera, P.; Archaimbault, V.; Larras, F.; Méthot, G.; Pinel-Alloul, B. Assessing Anthropogenic Pressure in the St. Lawrence River Using Traits of Benthic Macroinvertebrates. Sci. Total Environ. 2019, 649, 233–246. [Google Scholar] [CrossRef]
- Minshall, G.W. Stream Ecosystem Theory: A Global Perspective. J. North Am. Benthol. Soc. 1988, 7, 263–288. [Google Scholar] [CrossRef]
- Carvalho, A.L.; Nessimian, J.L. Odonata Do Estado Do Rio de Janeiro, Brasil: Hábitats e Hábitos Das Larvas. Oecol. Bras. 1998, 5, 3–28. [Google Scholar] [CrossRef]
- Fidelis, A.; Overbeck, G.; Pillar, V.D.; Pfadenhauer, J. Effects of Disturbance on Population Biology of the Rosette Species Eryngium Horridum Malme in Grasslands in Southern Brazil. Plant Ecol. 2007, 195, 55–67. [Google Scholar] [CrossRef]
- Pimentel, D.R.; Couceiro, S.R.M.; Salcedo, A.K.M. Diet of Phylloicus (Trichoptera: Calamoceratidae) Caddisfly Larvae in Forest Streams of Western Pará, Central Brazilian Amazonia. Acta Limnol. Bras. 2020, 32. [Google Scholar] [CrossRef]
- Statzner, B.; Bêche, L.A. Can Biological Invertebrate Traits Resolve Effects of Multiple Stressors on Running Water Ecosystems? Freshw. Biol. 2010, 55, 80–119. [Google Scholar] [CrossRef]
- Williams, D.D. Some Relationships between Stream Benthos and Substrate Heterogeneity. Limnol. Oceanogr. 1980, 25, 166–172. [Google Scholar] [CrossRef]
- Statzner, B. Growth and Reynolds Number of Lotic Macroinvertebrates: A Problem for Adaptation of Shape to Drag. Oikos 1988, 51, 84. [Google Scholar] [CrossRef]
- Vogel, S. Life in Moving Fluids; Princeton University Press: Princeton, NJ, USA, 1996. [Google Scholar]
- Gayraud, S.; Philippe, M. Does Subsurface Interstitial Space Influence General Features and Morphological Traits of the Benthic Macroinvertebrate Community in Streams? Fundam. Appl. Limnol. 2001, 151, 667–686. [Google Scholar] [CrossRef]
- Feio, M.J.; Dolédec, S. Integration of Invertebrate Traits into Predictive Models for Indirect Assessment of Stream Functional Integrity: A Case Study in Portugal. Ecol. Indic. 2012, 15, 236–247. [Google Scholar] [CrossRef]
- Kuzmanovic, M.; Dolédec, S.; de Castro-Catala, N.; Ginebreda, A.; Sabater, S.; Muñoz, I.; Barceló, D. Environmental Stressors as a Driver of the Trait Composition of Benthic Macroinvertebrate Assemblages in Polluted Iberian Rivers. Environ. Res. 2017, 156, 485–493. [Google Scholar] [CrossRef]
- Wood, B.M.; Bain, M.B. Morphology and Microhabitat Use in Stream Fish. Can. J. Fish. Aquat. Sci. 1995, 52, 1487–1498. [Google Scholar] [CrossRef]
- Lamouroux, N.; Poff, N.L.; Angermeier, P.L. Intercontinental convergence of stream fish community traits along geomorphic and hydraulic gradients. Ecology 2002, 83, 1792–1807. [Google Scholar] [CrossRef]
- Bêche, L.A.; Mcelravy, E.P.; Resh, V.H. Long-term Seasonal Variation in the Biological Traits of Benthic-macroinvertebrates in Two Mediterranean-climate Streams in California, U.S.A. Freshw. Biol. 2005, 51, 56–75. [Google Scholar] [CrossRef]
- Lytle, D.A.; Poff, N.L. Adaptation to Natural Flow Regimes. Trends Ecol. Evol. 2004, 19, 94–100. [Google Scholar] [CrossRef]
- Shiffer, C.N.; White, H.B. Dragonfly and Damselfly Colonization and Recolonization of a Large, Semi-Permanent Pennsylvania Pond. Northeast. Nat. 2014, 21, 630–651. [Google Scholar] [CrossRef]
- Brito, J.G.; Roque, F.O.; Martins, R.T.; Nessimian, J.L.; Oliveira, V.C.; Hughes, R.M.; de Paula, F.R.; Ferraz, S.F.B.; Hamada, N. Small Forest Losses Degrade Stream Macroinvertebrate Assemblages in the Eastern Brazilian Amazon. Biol. Conserv. 2020, 241, 108263. [Google Scholar] [CrossRef]
- Davies, S.P.; Jackson, S.K. The biological condition gradient: A descriptive model for interpreting change in aquatic ecosystems. Ecol. Appl. 2006, 16, 1251–1266. [Google Scholar] [CrossRef]
- Stevčić, Č.; Pérez-Miguel, M.; Drake, P.; Tovar-Sánchez, A.; Cuesta, J.A. Macroinvertebrate Communities on Rocky Shores: Impact Due to Human Visitors. Estuar. Coast. Shelf Sci. 2018, 211, 127–136. [Google Scholar] [CrossRef]
- Hilsenhoff, W.L. Rapid Field Assessment of Organic Pollution with a Family-Level Biotic Index. J. North Am. Benthol. Soc. 1988, 7, 65–68. [Google Scholar] [CrossRef]
- Sánchez-Fernández, D.; Abellán, P.; Mellado, A.; Velasco, J.; Millán, A. Are Water Beetles Good Indicators of Biodiversity in Mediterranean Aquatic Ecosystems? The Case of the Segura River Basin (SE Spain). Biodivers. Conserv. 2006, 15, 4507–4520. [Google Scholar] [CrossRef]
- Nieser, N.; Baena, M.; Martínez-Aviles, J.; Millán, A. Claves Para la Identificación de los Heterópteros Acuáticos (Nepomorpha y Gerromorpha) de la Península Ibérica. Con Notas Sobre las Especies de las Islas Azores, Baleares, Canarias y Madeira; Asociación Española de Limnología: Madrid, Spain, 1994; p. 112. [Google Scholar]
- Velasco, J.; Millán, A. Insect dispersal in a drying desert stream: Effects of temperature and water loss. Southwest. Nat. 1998, 43, 80–87. [Google Scholar]
- Mellado, A. The Ecology of Stream Macroinvertebrate Assemblages from the Segura River Basin (SE Spain). In Environmental Factors, Spatiotemporal Variability, Indicator Taxa, Diversity Trends, Biological-Ecological Traits, and Applications for Bioassessment; University of Murcia: Spain, Murcia, 2005; p. 200. [Google Scholar]
- Picazo, F.; Moreno, J.L.; Millán, A. The Contribution of Standing Waters to Aquatic Biodiversity: The Case of Water Beetles in Southeastern Iberia. Aquat. Ecol. 2009, 44, 205–216. [Google Scholar] [CrossRef]
- Hauer, F.R.; Lamberti, G.A. Methods in Stream Ecology; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Yadamsuren, O.; Morse, J.C.; Hayford, B.; Gelhaus, J.K.; Adler, P.H. Macroinvertebrate Community Responses to Land Use: A Trait-Based Approach for Freshwater Biomonitoring in Mongolia. Hydrobiologia 2020, 847, 1887–1902. [Google Scholar] [CrossRef]
- Ge, Y.; Liu, Z.; García-Girón, J.; Chen, X.; Yan, Y.; Li, Z.; Xie, Z. Human-Induced Loss of Functional and Phylogenetic Diversity Is Mediated by Concomitant Deterministic Processes in Subtropical Aquatic Insect Communities. Ecol. Indic. 2022, 136, 108600. [Google Scholar] [CrossRef]
| Site | Axis 1 | Inter-Site Distance | Percentage Inter-Site Distance | Impact Categorization |
|---|---|---|---|---|
| S1 | 0.1141 | 0.2274 | 50.1759 | MIS |
| S2 | 0.1192 | 0.2223 | 65.09517 | MIS |
| S3 | 0.1085 | 0.233 | 68.2284 | MIS |
| S4 | 0.07525 | 0.26625 | 77.96486 | SIS |
| S5 | 0.07832 | 0.26318 | 77.06589 | SIS |
| S6 | 0.1184 | 0.2231 | 65.32943 | MIS |
| S7 | 0.1357 | 0.2058 | 60.26354 | MIS |
| S8 | 0.1258 | 0.2157 | 63.16252 | MIS |
| S9 | 0.1074 | 0.2341 | 68.55051 | MIS |
| S10 | 0.1321 | 0.2094 | 61.31772 | MIS |
| S11 | 0.1318 | 0.2097 | 61.40556 | MIS |
| S12 | 0.1458 | 0.1957 | 57.306 | MIS |
| S13 | 0.1358 | 0.2057 | 60.23426 | MIS |
| S14 | 0.2067 | 0.1348 | 39.47291 | HIS |
| S15 | 0.1689 | 0.1726 | 50.54173 | MIS |
| S16 | 0.1318 | 0.2097 | 61.40556 | MIS |
| S17 | 0.1406 | 0.2009 | 58.8287 | MIS |
| S18 | 0.1443 | 0.1972 | 57.74524 | MIS |
| S19 | 0.1489 | 0.1926 | 56.39824 | MIS |
| S20 | 0.1777 | 0.1638 | 47.96486 | HIS |
| S21 | 0.2261 | 0.1154 | 33.79209 | HIS |
| S22 | 0.2108 | 0.1307 | 38.27233 | HIS |
| S23 | 0.3415 | 0 | 0 | HIS |
| S24 | 0.237 | 0.1045 | 30.60029 | HIS |
| S25 | 0.2008 | 0.1407 | 41.20059 | HIS |
| S26 | 0.03733 | 0.30417 | 89.06881 | SIS |
| S27 | 0.03674 | 0.30476 | 89.24158 | SIS |
| S28 | 0.07779 | 0.26371 | 77.22108 | SIS |
| S29 | 0.05678 | 0.28472 | 83.37335 | SIS |
| S30 | 0.09439 | 0.24711 | 72.36018 | SIS |
| S31 | 0.1147 | 0.2268 | 66.41288 | MIS |
| S32 | 0.1031 | 0.2384 | 69.80966 | MIS |
| S33 | 0.3218 | 0.0197 | 5.768668 | HIS |
| S34 | 0.2033 | 0.1382 | 40.46852 | HIS |
| S35 | 0.1653 | 0.1762 | 51.5959 | MIS |
| S36 | 0.326 | 0.0155 | 4.538799 | HIS |
| Cluster | Clustered Trait Attributes |
|---|---|
| 1 | Loc6, Fe2, F2, F3, F5 |
| 2 | Lif2, Res5, Loc4 |
| 3 | Pot2, Aqu1, Aqu2, Sub5 |
| 4 | Sub8, Sub9 |
| 5 | Resp1, Fe3 |
| 6 | Pot3, Loc1 |
| 7 | Aqu4, Rep4, Fe6 |
| 8 | Max3, Rep4 |
| 9 | Max4, Lif1, Rep6, Res4, F7, Loc2 |
| 10 | Rep7, Resp3, F4, Fe4, Max2, Sub6 |
| 11 | Max6, Rep2 |
| 12 | Max5, Resp2, Fe7, Pot1, Sub4, Loc5 |
| 13 | Sub1, Sub2, Sub3 |
| 14 | Rep3, F6, Res1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Yaagoubi, S.; Edegbene, A.O.; El Haissoufi, M.; Harrak, R.; El Alami, M. Odonata, Coleoptera, and Heteroptera (OCH) Trait-Based Biomonitoring of Rivers within the Northwestern Rif of Morocco: Exploring the Responses of Traits to Prevailing Environmental Gradients. Ecologies 2024, 5, 132-154. https://doi.org/10.3390/ecologies5010009
El Yaagoubi S, Edegbene AO, El Haissoufi M, Harrak R, El Alami M. Odonata, Coleoptera, and Heteroptera (OCH) Trait-Based Biomonitoring of Rivers within the Northwestern Rif of Morocco: Exploring the Responses of Traits to Prevailing Environmental Gradients. Ecologies. 2024; 5(1):132-154. https://doi.org/10.3390/ecologies5010009
Chicago/Turabian StyleEl Yaagoubi, Sara, Augustine Ovie Edegbene, Mohamed El Haissoufi, Rihab Harrak, and Majida El Alami. 2024. "Odonata, Coleoptera, and Heteroptera (OCH) Trait-Based Biomonitoring of Rivers within the Northwestern Rif of Morocco: Exploring the Responses of Traits to Prevailing Environmental Gradients" Ecologies 5, no. 1: 132-154. https://doi.org/10.3390/ecologies5010009
APA StyleEl Yaagoubi, S., Edegbene, A. O., El Haissoufi, M., Harrak, R., & El Alami, M. (2024). Odonata, Coleoptera, and Heteroptera (OCH) Trait-Based Biomonitoring of Rivers within the Northwestern Rif of Morocco: Exploring the Responses of Traits to Prevailing Environmental Gradients. Ecologies, 5(1), 132-154. https://doi.org/10.3390/ecologies5010009






