Serum Creatine Kinase Increases after Acute Strength Training in College Athletes with Menstrual Irregularities
Abstract
1. Introduction
2. Results
2.1. Anthropometric Characteristics of the Participants
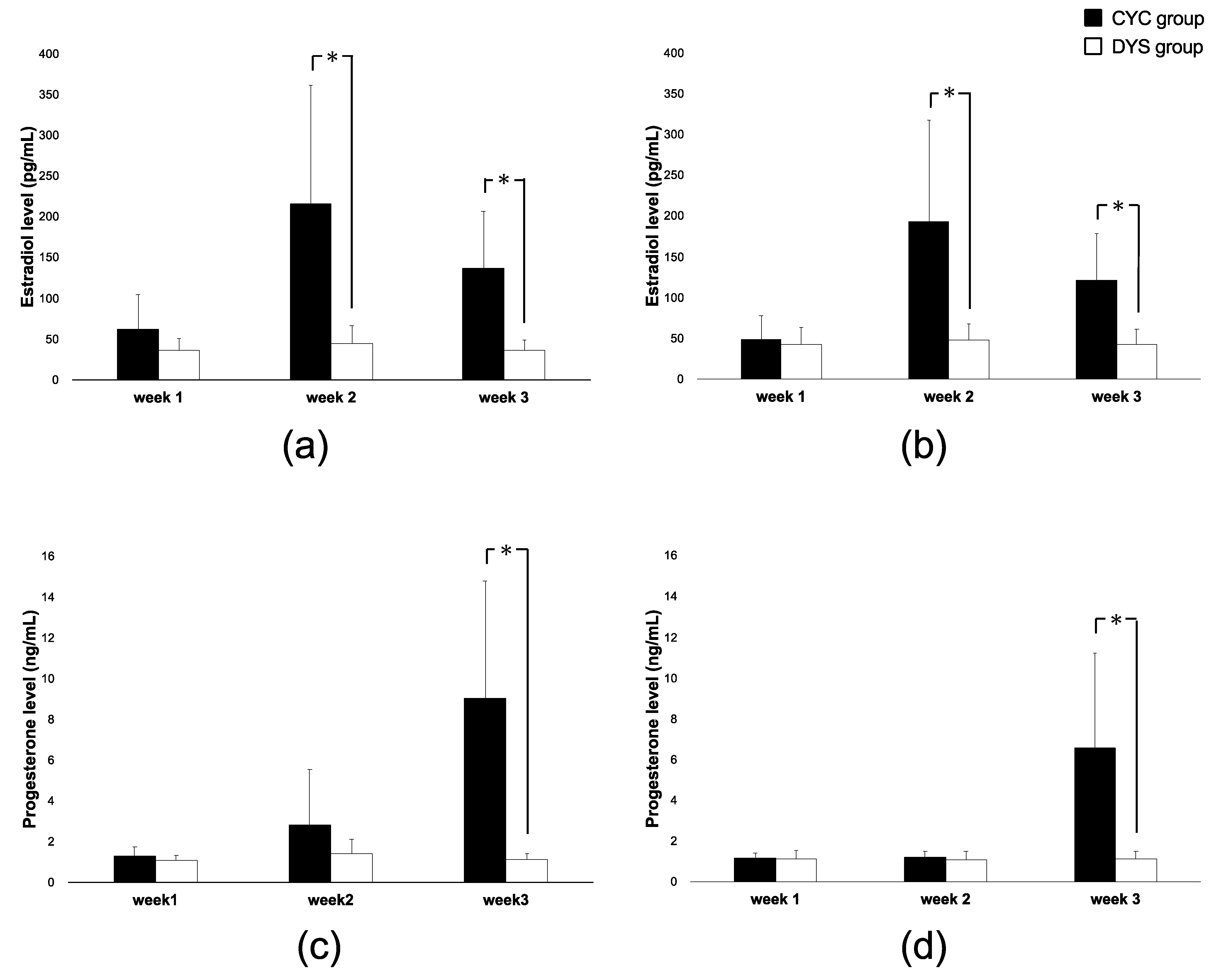
2.2. Hormone Levels of the Participants
2.3. CK Levels of the Participants
2.4. Post-Exercise Stiffness in the Participants
3. Discussion
4. Materials and Methods
4.1. Measurements
4.2. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armstrong, M.J.; Colberg, S.R.; Sigal, R.J. Moving Beyond Cardio: The Value of Resistance Training, Balance Training, and Other Forms of Exercise in the Management of Diabetes. Diabetes Spectr. 2015, 28, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Safran, M.R.; Seaber, A.V.; Garrett, W.E., Jr. Warm-up and muscular injury prevention. An update. Sports Med. 1989, 8, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T. Diagnosis, Treatment and Management of Gynecologic Disease. Acta Obstet. Gynaecol. Jpn. 2009, 61, N643–N657. [Google Scholar]
- Chidi-Ogbolu, N.; Baar, K. Effect of Estrogen on Musculoskeletal Performance and Injury Risk. Front. Physiol. 2019. [CrossRef]
- Enns, D.L.; Tiidus, P.M. The influence of estrogen on skeletal muscle: Sex matters. Sports Med. 2010, 40, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Pallavi, L.C.; De Souza, U.J.; Shivaprakash, G. Assessment of Musculoskeletal Strength and Levels of Fatigue during Different Phases of Menstrual Cycle in Young Adults. J. Clin. Diagnostic Res. 2017, 11, CC11–CC13. [Google Scholar] [CrossRef]
- Tiidus, P.M.; Holden, D.; Bombardier, E.; Zajchowski, S.; Enns, D.; Belcastro, A. Estrogen effect on post-exercise skeletal muscle neutrophil infiltration and calpain activity. Can. J. Physiol. Pharmacol. 2001, 79, 400–406. [Google Scholar] [CrossRef]
- Nose, S.; Dohi, M.; Namba, A.; Akimori, K.; Mesaki, N.; Komatsu, Y.; Akama, T.; Kawahara, T. Investigation of amenorrhea and stress fractures in elite female athletes. Jpn. J. Soc. Clin. Sports Med. 2014, 22, 67–74. [Google Scholar]
- Sawai, A.; Tochigi, Y.; Kavaliova, N.; Zaboronok, A.; Warashina, Y.; Mathis, B.J.; Mesaki, N.; Shiraki, H.; Watanabe, K. MRI reveals menstrually-related muscle edema that negatively affects athletic agility in young women. PLoS ONE 2018, 13, e0191022. [Google Scholar] [CrossRef]
- Bell, D.R.; Blackburn, J.T.; Norcorss, M.F.; Ondrak, K.S.; Hudson, J.D.; Hackney, A.C.; Padua, D.A. Estrogen and muscle stiffness have a negative relationship in females. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, J.T.; Bell, D.R.; Norcross, M.F.; Hudson, J.D.; Kimsey, M.H. Sex comparison of hamstring structural and material properties. Clin. Biomech. 2009, 24, 65–70. [Google Scholar] [CrossRef]
- Doize, F.; Laporte, R.; Deroth, L. Effects of exercise on skeletal muscle and serum enzyme activities in pigs. Vet. Res. Commun. 1989, 13, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-kinase- and exercise-related muscle damage implications for muscle performance and recovery. J. Nutr. Metab. 2012. [Google Scholar] [CrossRef] [PubMed]
- Lorimer, A.V.; Hume, P.A. Stiffness as a Risk Factor for Achilles Tendon Injury in Running Athletes. Sports Med. 2016, 46, 1921–1938. [Google Scholar] [CrossRef] [PubMed]
- Dragin, N.; Nancy, P.; Villegas, J.; Roussin, R.; Le Panse, R.; Berrih-Aknin, S. Balance between Estrogens and Proinflammatory Cytokines Regulates Chemokine Production Involved in Thymic Germinal Center Formation. Sci. Rep. 2017, 7, 7970. [Google Scholar] [CrossRef]
- Shivers, K.Y.; Amador, N.; Abrams, L.; Hunter, D.; Jenab, S.; Quiñones-Jenab, V. Estrogen alters baseline and inflammatory-induced cytokine levels independent from hypothalamic-pituitary-adrenal axis activity. Cytokine 2015, 72, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Gaida, J.E.; Alfredson, H.; Forsgren, S.; Cook, J.L. A pilot study on biomarkers for tendinopathy: Lower levels of serum TNF-α and other cytokines in females but not males with Achilles tendinopathy. BMC Sports Sci. Med. Rehabil. 2016, 8, 5. [Google Scholar] [CrossRef]
- Harkey, M.S.; Luc, B.A.; Golightly, Y.M.; Thomas, A.C.; Driban, J.B.; Hackney, A.C.; Pietrosimone, B. Osteoarthritis-related biomarkers following anterior cruciate ligament injury and reconstruction: A systematic review. Osteoarthr. Cartil. 2015, 23, 1–12. [Google Scholar] [CrossRef]
- Lattermann, C.; Conley, C.E.; Johnson, D.L.; Reinke, E.K.; Huston, L.J.; Huebner, J.L.; Chou, C.H.; Kraus, V.B.; Spindler, K.P.; Jacobs, C.A. Select Biomarkers on the Day of Anterior Cruciate Ligament Reconstruction Predict Poor Patient-Reported Outcomes at 2-Year Follow-Up: A Pilot Study. BioMed. Res. Int. 2018, 2018, 9387809. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.N.; Li, Y.P.; Liu, C.L.; Zhang, Z.J. Assessing the elastic properties of skeletal muscle and tendon using shearwave ultrasound elastography and MyotonPRO. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Zinder, S.M.; Padua, D.A. Reliability, Validity, and Precision of a Handheld Myometer for Assessing in Vivo Muscle Stiffness. J. Sport Rehabil. 2011, 20, jsr.2010-0051. [Google Scholar] [CrossRef]
- Pruyn, E.C.; Watsford, M.L.; Murphy, A.J. Validity and reliability of three methods of stiffness assessment. J. Sport Health Sci. 2016, 5, 476–483. [Google Scholar] [CrossRef]
- Tani, M.; Sakuma, A. Applicability Evaluation of Young’s Modulus Measurement using Equivalent Indentation Strain in Spherical Indentation Testing for Soft Materials. Trans. Jpn. Soc. Mech. Eng. A 2010, 76, 102–108. [Google Scholar] [CrossRef][Green Version]
- Tani, M.; Sakuma, A.; Shinomiya, M. Evaluation of Thickness and Young’s Modulus of Soft Materials by using Spherical Indentation Testing. Trans. Jpn. Soc. Mech. Eng. A 2009, 75, 901–908. [Google Scholar] [CrossRef]
- Kubo, K.; Miyamoto, M.; Tanaka, S.; Maki, A.; Tsunoda, N.; Kanehisa, H. Muscle and tendon properties during menstrual cycle. Int. J. Sports Med. 2009, 30, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.A.; Baltgalvis, K.A.; Greising, S.M. Mechanisms behind estrogen’s beneficial effect on muscle strength in females. Exerc. Sport Sci. Rev. 2010, 38, 61–67. [Google Scholar] [CrossRef]
- Zhao, Z.; Han, F.; Yang, S.; Wu, J.; Zhan, W. Oxamate-mediated inhibition of lactate dehydrogenase induces protective autophagy in gastric cancer cells: Involvement of the Akt–mTOR signaling pathway. Cancer Lett. 2015, 358, 17–26. [Google Scholar] [CrossRef]
- Copeland, J.L.; Consitt, L.A.; Tremblay, M.S. Hormonal responses to endurance and resistance exercise in females aged 19-69 years. J. Gerontol. A Biol. Sci. Med. Sci. 2002, 57, B158–B165. [Google Scholar] [CrossRef]
- McTiernan, A.; Tworoger, S.S.; Ulrich, C.M.; Yasui, Y.; Irwin, M.L.; Rajan, K.B.; Sorensen, B.; Rudolph, R.E.; Bowen, D.; Stanczyk, F.Z.; et al. Effect of exercise on serum estrogens in postmenopausal women: A 12-month randomized clinical trial. Cancer Res. 2004, 64, 2923–2928. [Google Scholar] [CrossRef]
- De Crée, C. Sex steroid metabolism and menstrual irregularities in the exercising female. A review. Sports Med. 1998, 25, 369–406. [Google Scholar] [CrossRef]
- Hansen, M.; Skovgaard, D.; Reitelseder, S.; Holm, L.; Langbjerg, H.; Kjaer, M. Effects of Estrogen Replacement and Lower Androgen Status on Skeletal Muscle Collagen and Myofibrillar Protein Synthesis in Postmenopausal Women. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1005–1013. [Google Scholar] [CrossRef]
- Hansen, M. Female hormones: Do they influence muscle and tendon protein metabolism? Proc. Nutr. Soc. 2018, 77, 32–41. [Google Scholar] [CrossRef]
- Doma, K.; Nicholls, A.; Gahreman, D.; Damas, F.; Libardi, C.A.; Sinclair, W. The Effect of a Resistance Training Session on Physiological and Thermoregulatory Measures of Sub-maximal Running Performance in the Heat in Heat-Acclimatized Men. Sports Med. Open 2019, 5, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fukaya, K.; Kawamori, A.; Osada, Y.; Kitazawa, M.; Ishiguro, M. The forecasting of menstruation based on a state-space modeling of basal body temperature time series. Stat. Med. 2017, 36, 3361–3379. [Google Scholar] [CrossRef] [PubMed]
- Vanheest, J.L.; Rodgers, C.D.; Mahoney, C.E.; De Souza, M.J. Ovarian suppression impairs sport performance in junior elite female swimmers. Med. Sci. Sports Exerc. 2014, 46, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.R.; Fragala, M.S.; Volek, J.S.; Denegar, C.R.; Anderson, J.M.; Comstock, B.A.; Dunn-Lewis, C.; Hooper, D.R.; Szivak, T.K.; Luk, H.Y.; et al. Sex differences in creatine kinase after acute heavy resistance exercise on circulating granulocyte estradiol receptors. Eur. J. Appl. Physiol. 2012, 112, 3335–3340. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, B.M.; Dantas, E.; de Salles, B.F.; Miranda, H.; Koch, A.J.; Willardson, J.M.; Simão, R. Creatine kinase and lactate dehydrogenase responses after upper-body resistance exercise with different rest intervals. J. Strength Cond. Res. 2010, 24, 1657–1662. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawai, A.; Mitsuhashi, R.; Zaboronok, A.; Warashina, Y.; Mathis, B.J. Serum Creatine Kinase Increases after Acute Strength Training in College Athletes with Menstrual Irregularities. Women 2021, 1, 71-79. https://doi.org/10.3390/women1020007
Sawai A, Mitsuhashi R, Zaboronok A, Warashina Y, Mathis BJ. Serum Creatine Kinase Increases after Acute Strength Training in College Athletes with Menstrual Irregularities. Women. 2021; 1(2):71-79. https://doi.org/10.3390/women1020007
Chicago/Turabian StyleSawai, Akemi, Risa Mitsuhashi, Alexander Zaboronok, Yuki Warashina, and Bryan J. Mathis. 2021. "Serum Creatine Kinase Increases after Acute Strength Training in College Athletes with Menstrual Irregularities" Women 1, no. 2: 71-79. https://doi.org/10.3390/women1020007
APA StyleSawai, A., Mitsuhashi, R., Zaboronok, A., Warashina, Y., & Mathis, B. J. (2021). Serum Creatine Kinase Increases after Acute Strength Training in College Athletes with Menstrual Irregularities. Women, 1(2), 71-79. https://doi.org/10.3390/women1020007







