Sex Selection Bias in Schizophrenia Antipsychotic Trials—An Update Systematic Review
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
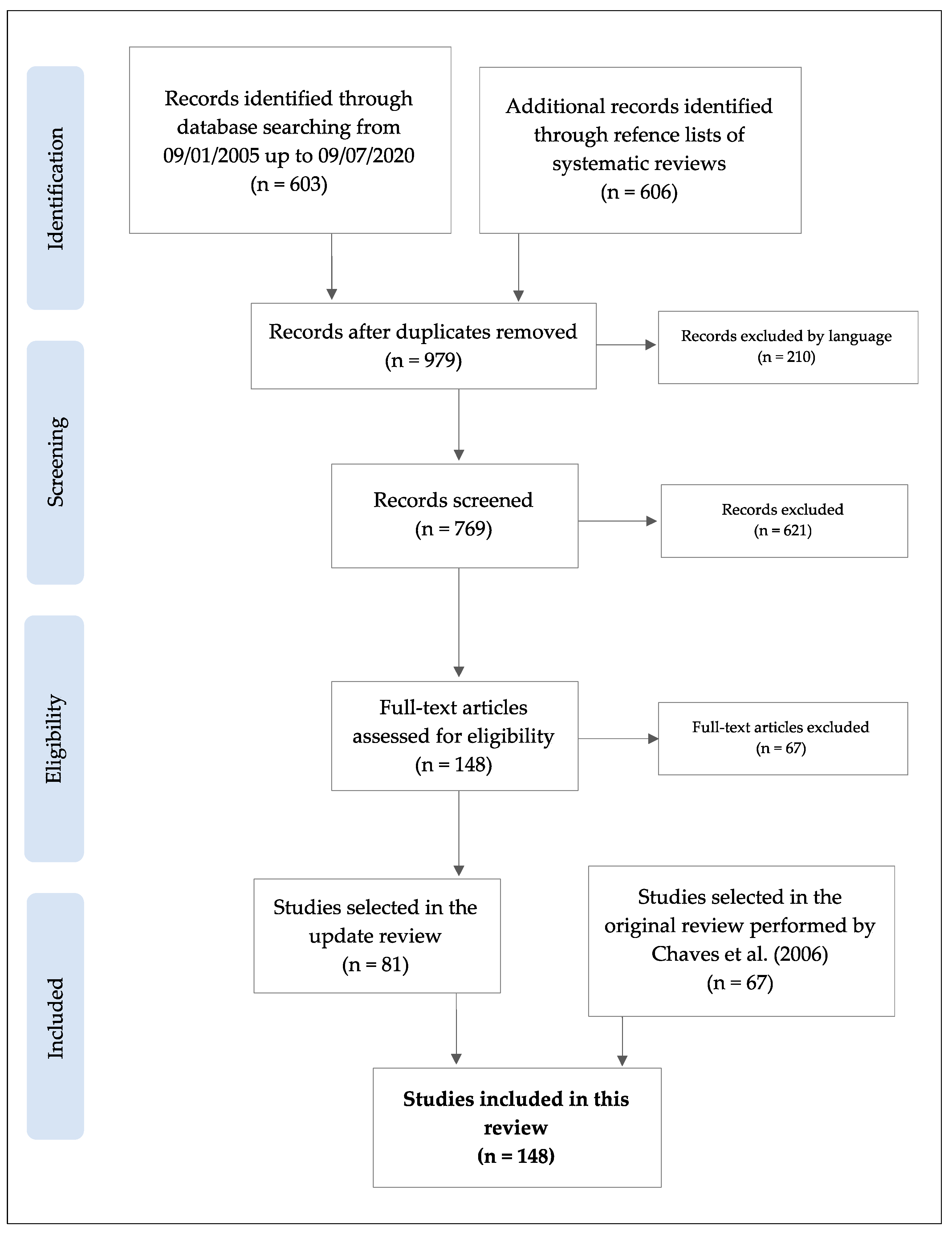
4.1. Search Strategy
4.2. Selection Criteria
4.3. Screening and Data Extraction
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lally, J.; Watkins, R.; Nash, S.; Shetty, H.; Gardner-Sood, P.; Smith, S.; Murray, R.M.; Gaughran, F. The Representativeness of Participants with Severe Mental Illness in a Psychosocial Clinical Trial. Front. Psychiatry 2018, 9, 654. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Arnold, A.P. Are Females More Variable than Males in Gene Expression? Meta-Analysis of Microarray Datasets. Biol. Sex Differ. 2015, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Tannenbaum, C.; Ellis, R.P.; Eyssel, F.; Zou, J.; Schiebinger, L. Sex and Gender Analysis Improves Science and Engineering. Nature 2019. [Google Scholar] [CrossRef] [Green Version]
- Clayton, J.A.; Collins, F.S. NIH to Balance Sex in Cell and Animal Studies. Nature 2014. [Google Scholar] [CrossRef]
- Clayton, J.A.; Tannenbaum, C. Reporting Sex, Gender, or Both in Clinical Research? JAMA J. Am. Med. Assoc. 2016, 316, 1863–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakiniaeiz, Y.; Cosgrove, K.P.; Potenza, M.N.; Mazure, C.M. Balance of the Sexes: Addressing Sex Differences in Preclinical Research. Yale J. Biol. Med. 2016, 89, 255–259. [Google Scholar]
- Saha, S.; Chant, D.; Welham, J.; McGrath, J. A Systematic Review of the Prevalence of Schizophrenia. PLoS Med. 2005, 2, 0413–0433. [Google Scholar] [CrossRef] [PubMed]
- Chaves, A.C.; Seeman, M.V. Sex Selection Bias in Schizophrenia Antipsychotic Trials. J. Clin. Psychopharmacol. 2006, 26, 489–494. [Google Scholar] [CrossRef]
- Santos-Casado, M.; García-Avello, A. Systematic Review of Gender Bias in the Clinical Trials of New Long-Acting Antipsychotic Drugs. J. Clin. Psychopharmacol. 2019, 39, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Cotton, S.M.; Lambert, M.; Schimmelmann, B.G.; Foley, D.L.; Morley, K.I.; McGorry, P.D.; Conus, P. Gender Differences in Premorbid, Entry, Treatment, and Outcome Characteristics in a Treated Epidemiological Sample of 661 Patients with First Episode Psychosis. Schizophr. Res. 2009, 114, 17–24. [Google Scholar] [CrossRef]
- Varma, V.K.; Wig, N.N.; Phookun, H.R.; Misra, A.K.; Khare, C.B.; Tripathi, B.M.; Behere, P.B.; Yoo, E.S.; Susser, E.S. First-Onset Schizophrenia in the Community: Relationship of Urbanization with Onset, Early Manifestations and Typology. Acta Psychiatr. Scand. 1997, 96, 431–438. [Google Scholar] [CrossRef]
- Kirkbride, J.B.; Errazuriz, A.; Croudace, T.J.; Morgan, C.; Jackson, D.; Boydell, J.; Murray, R.M.; Jones, P.B. Incidence of Schizophrenia and Other Psychoses in England, 1950–2009: A Systematic Review and Meta-Analyses. PLoS ONE 2012, 7, e31660. [Google Scholar] [CrossRef]
- Cersosimo, M.G.; Benarroch, E.E. Estrogen Actions in the Nervous System: Complexity and Clinical Implications. Neurology 2015. [Google Scholar] [CrossRef]
- Køster, A.; Lajer, M.; Lindhardt, A.; Rosenbaum, B. Gender Differences in First Episode Psychosis. Soc. Psychiatry Psychiatr. Epidemiol. 2008, 43, 940–946. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, S.; Usall, J.; Cobo, J.; Labad, X.; Kulkarni, J. Gender Differences in Schizophrenia and First-Episode Psychosis: A Comprehensive Literature Review. Schizophr. Res. Treat. 2012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeman, M.V. Does Gender Influence Outcome in Schizophrenia? Psychiatr. Q. 2019, 90, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.B.; DeLisi, L.E. Issues Related to Sex Differences in Antipsychotic Treatment. Curr. Opin. Psychiatry 2016, 29, 211–217. [Google Scholar] [CrossRef]
- Seeman, M.V. Are There Gender Differences in the Response to Antipsychotic Drugs? Neuropharmacology 2019. [Google Scholar] [CrossRef]
- Gillies, G.E.; Virdee, K.; McArthur, S.; Dalley, J.W. Sex-Dependent Diversity in Ventral Tegmental Dopaminergic Neurons and Developmental Programing: A Molecular, Cellular and Behavioral Analysis. Neuroscience 2014, 282, 69–85. [Google Scholar] [CrossRef] [Green Version]
- Eugene, A.R.; Masiak, J. A Pharmacodynamic Modelling and Simulation Study Identifying Gender Differences of Daily Olanzapine Dose and Dopamine D2-Receptor Occupancy. Nord. J. Psychiatry 2017, 71, 417–424. [Google Scholar] [CrossRef]
- Seeman, M.V. Men and Women Respond Differently to Antipsychotic Drugs. Neuropharmacology 2020, 163, 107631. [Google Scholar] [CrossRef]
- Jones, I.; Chandra, P.S.; Dazzan, P.; Howard, L.M. Bipolar Disorder, Affective Psychosis, and Schizophrenia in Pregnancy and the Post-Partum Period. Lancet 2014, 384, 1789–1799. [Google Scholar] [CrossRef]
- Grigoriadis, S.; Seeman, M.V. The Role of Estrogen in Schizophrenia: Implications for Schizophrenia Practice Guidelines for Women. Can. J. Psychiatry 2002, 47, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Soldin, O.P.; Chung, S.H.; Mattison, D.R. Sex Differences in Drug Disposition. J. Biomed. Biotechnol. 2011, 2011, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Abel, K.M.; Drake, R.; Goldstein, J.M. Sex Differences in Schizophrenia. Int. Rev. Psychiatry 2010, 22, 417–428. [Google Scholar] [CrossRef]
- Van Spall, H.G.C.; Toren, A.; Kiss, A.; Fowler, R.A. Eligibility Criteria of Randomized Controlled Trials Published in High-Impact General Medical Journals: A Systematic Sampling Review. J. Am. Med. Assoc. 2007, 297, 1233–1240. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Ethical Considerations for Including Women as Research Participants; American College of Obstetricians and Gynecologists: Washington, DC, USA, 2016; Volume 127. [Google Scholar]
- González-Rodríguez, A.; Seeman, M.V. The Association between Hormones and Antipsychotic Use: A Focus on Postpartum and Menopausal Women. Ther. Adv. Psychopharmacol. 2019, 9, 204512531985997. [Google Scholar] [CrossRef] [Green Version]
- Robinson, D.; Woerner, M.G.; Pollack, S.; Lerner, G. Subject Selection Biases in Clinical Trials: Data from a Multicenter Schizophrenia Treatment Study. J. Clin. Psychopharmacol. 1996, 16, 170–176. [Google Scholar] [CrossRef]
- Lange, B.; Mueller, J.K.; Leweke, F.M.; Bumb, J.M. How Gender Affects the Pharmacotherapeutic Approach to Treating Psychosis—A Systematic Review. Expert Opin. Pharmacother. 2017, 18, 351–362. [Google Scholar] [CrossRef]
- Goldstein, J.M.; Cohen, L.S.; Horton, N.J.; Lee, H.; Andersen, S.; Tohen, M.; Crawford, A.M.K.; Tollefson, G. Sex Differences in Clinical Response to Olanzapine Compared with Haloperidol. Psychiatry Res. 2002, 110, 27–37. [Google Scholar] [CrossRef]
- González-Rodríguez, A.; Catalán, R.; Penadés, R.; Ruiz Cortés, V.; Torra, M.; Seeman, M.V.; Bernardo, M. Antipsychotic Response Worsens With Postmenopausal Duration in Women With Schizophrenia. J. Clin. Psychopharmacol. 2016, 36, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Riecher-Rössler, A.; Häfner, H. Schizophrenia and Oestrogens—Is There an Association? Eur. Arch. Psychiatry Clin. Neurosci. 1993, 242, 323–328. [Google Scholar] [CrossRef]
- Seeman, M. Neuroleptic Prescription for Men and Women. J. Soc. Pharmacol. 1989, 3, 219–236. [Google Scholar]
- Olten, B.; Bloch, M.H. Meta Regression: Relationship between Antipsychotic Receptor Binding Profiles and Side-Effects. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 84, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, J.W. Antipsychotic Medications: Metabolic and Cardiovascular Risk. J. Clin. Psychiatry 2007, 68 (Suppl. 4), 8–13. [Google Scholar]
- Li, L.; Wang, Z. Ovarian aging and osteoporosis. In Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; Volume 1086, pp. 199–215. [Google Scholar]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer Immunotherapy Efficacy and Patients’ Sex: A Systematic Review and Meta-Analysis. Lancet Oncol. 2018. [Google Scholar] [CrossRef]
- Labonté, B.; Engmann, O.; Purushothaman, I.; Menard, C.; Wang, J.; Tan, C.; Scarpa, J.R.; Moy, G.; Loh, Y.H.E.; Cahill, M.; et al. Sex-Specific Transcriptional Signatures in Human Depression. Nat. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Pinnow, E.; Sharma, P.; Parekh, A.; Gevorkian, N.; Uhl, K. Increasing Participation of Women in Early Phase Clinical Trials Approved by the FDA. Women’s Health Issues 2009. [Google Scholar] [CrossRef]
- Benjeaa, Y.; Geysels, Y. Gender Bias in the Clinical Evaluation of Effectiveness in Therapies. Appl. Clin. Trials 2020, 29, 30–33. [Google Scholar]
- Beery, A.K.; Zucker, I. Sex Bias in Neuroscience and Biomedical Research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef] [Green Version]
- Taipale, H.; Puranen, A.; Mittendorfer-Rutz, E.; Tiihonen, J.; Tanskanen, A.; Cervenka, S.; Lähteenvuo, M. Antipsychotic Use among Persons with Schizophrenia in Sweden and Finland, Trends and Differences. Nord. J. Psychiatry 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Neasham, A.; Lambrinudi, C.; Khan, A. A Quantitative Analysis of Antipsychotic Prescribing Trends for the Treatment of Schizophrenia in England and Wales. JRSM Open 2018, 9, 205427041875857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazici, E.; Cilli, A.S.; Yazici, A.B.; Baysan, H.; Ince, M.; Bosgelmez, S.; Bilgic, S.; Aslan, B.; Erol, A. Antipsychotic Use Pattern in Schizophrenia Outpatients: Correlates of Polypharmacy. Clin. Pract. Epidemiol. Ment. Heal. 2017, 13, 92–103. [Google Scholar] [CrossRef] [Green Version]
- Huhn, M.; Nikolakopoulou, A.; Schneider-Thoma, J.; Krause, M.; Samara, M.; Peter, N.; Arndt, T.; Bäckers, L.; Rothe, P.; Cipriani, A.; et al. Comparative Efficacy and Tolerability of 32 Oral Antipsychotics for the Acute Treatment of Adults with Multi-Episode Schizophrenia: A Systematic Review and Network Meta-Analysis. Lancet 2019, 394, 939–951. [Google Scholar] [CrossRef] [Green Version]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IBM Corp. IBM SPSS Statistics for Macintosh; Version 26.0; IBM Corp: Armonk, NY, USA, 2019. [Google Scholar]
| N | Mean (SD) | Median | Range | |
|---|---|---|---|---|
| Total sample | 43,961 | 297 (277.5) | 248.5 | 50–1995 |
| Men | 28,956 | 195.7 (183.8) | 154.5 | 0–1295 |
| Women | 15,005 | 101.4 (102.9) | 78.5 | 0–700 |
| Proportion of women | - | 34.1% (0.1) | 34.4% | 0–100% |
| Duration of trial (days) | - | 146.5 (196) | 56 | 1–1095 |
| Age (years) † | - | 36.7 (7) | 37 | 21.5–72 |
| Number of Trials N (%) | Total Sample N (%) | Proportion of Women Mean (SD) | Sig. | Post hoc Comparisons † | |
|---|---|---|---|---|---|
| Decade of publication | |||||
| ≤2000 | 28 (18.9%) | 9188 (20.9%) | 28.7% (0.11) | p = 0.03 * | ≤2000 vs. 2001–2010: p = 0.02 ≤2000 vs. ≥2011: p = 0.02 |
| 2001–2010 | 73 (49.3%) | 23,034 (52.4%) | 36% (0.11) | ||
| ≥2011 | 47 (31.8%) | 11,739 (26.7%) | 36.4% (0.17) | ||
| Funding | |||||
| Pharmaceutical | 109 (73.6%) | 35,015 (79.7%) | 33.6% (0.12) | p = 0.01 * | Pharm. vs. Non-pharm.: p < 0.01 |
| Non-pharmaceutical | 25 (16.9%) | 6605 (15.0%) | 42.4% (0.17) | ||
| Not reported | 14 (9.5%) | 2341 (5.3%) | 30.2% (0.14) | ||
| Sample size | |||||
| 50–100 | 31 (20.9%) | 2313 (5.3%) | 38% (0.19) | p = 0.27 | |
| 101–200 | 35 (23.6%) | 5092 (11.6%) | 35.3% (0.14) | ||
| 201–500 | 65 (43.9%) | 21,796 (49.6%) | 32.5% (0.10) | ||
| >501 | 17 (11.5%) | 14,760 (33.6%) | 36.4% (0.08) | ||
| Location | |||||
| Asia | 28 (21.4%) | 5116 (11.6%) | 44.3% (0.18) | p = 0.01 * | N. America vs. Asia: p < 0.01 N. America vs. Europe: p < 0.01 N. America vs. Other: p < 0.01 N. America vs. Multiple: p < 0.01 Asia vs. Multiple: p < 0.01 |
| Europe | 23 (17.6%) | 3820 (8.7%) | 38.6% (0.10) | ||
| Other | 4 (3.1%) | 427 (1.0%) | 45% (0.20) | ||
| North America | 34 (26.0%) | 9854 (22.4%) | 24.9% (0.07) | ||
| Multiple continents | 42 (32.1%) | 20,937 (47.6%) | 33.7% (0.09) | ||
| Number of study centers | |||||
| Single center | 17 (11.5%) | 1630 (3.7%) | 38.9% (0.21) | p = 0.15 | |
| Multicenter | 123 (83.1%) | 40,684 (92.5%) | 33.9% (0.12) | ||
| Not reported | 8 (5.4%) | 1647 (3.7%) | 39.6% (0.15) | ||
| Inclusion criteria for women | |||||
| Yes | 85 (57.4%) | 26,174 (59.5%) | 32.2% (0.13) | p = 0.02 * | Yes vs. Not specified: p = 0.02 |
| Not specified | 63 (42.6%) | 17,787 (40.5%) | 38.2% (0.14) | ||
| Symptom presentation | |||||
| Stable | 22 (14.9%) | 5557 (12.6%) | 38.8% (0.09) | p = 0.21 | |
| Acute | 98 (66.2%) | 29,516 (67.1%) | 33.5% (0.14) | ||
| Both | 18 (12.2%) | 6889 (15.7%) | 36.6% (0.16) | ||
| Not reported | 10 (6.8%) | 1999 (4.5%) | 34.5% (0.15) | ||
| Setting | |||||
| Inpatient | 63 (42.3%) | 15,194 (34.6%) | 31.1% (0.15) | p = 0.02 * | Inpatient vs. Outpatient: p = 0.04 Inpatient vs. Both: p = 0.02 |
| Outpatient | 26 (17.4%) | 7566 (17.2%) | 36.5% (0.11) | ||
| Both | 37 (24.8%) | 15,282 (34.8%) | 38% (0.13) | ||
| Not reported | 23 (15.4%) | 5919 (13.5%) | 36.4% (0.12) | ||
| Number of psychotic episodes | |||||
| First episode | 16 (10.8%) | 2922 (6.6%) | 32.7% (0.13) | p = 0.33 | |
| Multiple episode | 56 (37.8%) | 17,294 (39.3%) | 36.4% (0.16) | ||
| Both | 38 (25.7%) | 12,786 (29.1%) | 32.3% (0.11) | ||
| Not reported | 38 (25.7%) | 10,959 (24.9%) | 35.6% (0.12) | ||
| Diagnosis included | |||||
| Only SCZ | 84 (56.8%) | 27,733 (63.1%) | 35.1% (0.14) | p = 0.23 | |
| SCZ spectrum | 50 (33.8%) | 13,875 (31.6%) | 35.8% (0.12) | ||
| Broad psychosis | 14 (9.5%) | 23,53 (5.4%) | 28.9% (0.13) | ||
| Use of risperidone | |||||
| Yes | 66 (44.9%) | 18,732 (42.6%) | 37.2% (0.15) | p = 0.06 | |
| No | 81 (55.1%) | 24,740 (56.3%) | 32.9% (0.11) | ||
| Use of olanzapine | |||||
| Yes | 69 (46.9%) | 19,016 (43.3%) | 38.1% (0.14) | p = 0.01 * | Yes vs. No: p < 0.01 |
| No | 78 (53.1%) | 24,456 (55.6%) | 31.9% (0.12) | ||
| Use of quetiapine | |||||
| Yes | 29 (19.7%) | 8523 (19.4%) | 35% (0.12) | p = 0.94 | |
| No | 118 (80.3%) | 34,949 (79.5%) | 34.8% (0.14) | ||
| Use of ziprasidone | |||||
| Yes | 27 (18.4%) | 7895 (18.0%) | 30.4% (0.12) | p = 0.06 | |
| No | 120 (81.6%) | 35,577 (80.9%) | 35.8% (0.14) | ||
| Use of aripiprazole | |||||
| Yes | 29 (19.6%) | 10,448 (23.8%) | 37.5% (0.10) | p = 0.22 | |
| No | 119 (80.4%) | 33,513 (76.2%) | 34.1% (0.14) | ||
| Use of FGA | |||||
| Yes | 52 (35.4%) | 16,550 (37.6%) | 31.6% (0.12) | p = 0.03 * | Yes vs. No: p = 0.03 |
| No | 95 (64.6%) | 26,922 (61.2%) | 36.6% (0.14) | ||
| Use of additional SGAs †† | |||||
| Yes | 29 (19.7%) | 9372 (21.3%) | 36.8% (0.11) | p = 0.38 | |
| No | 118 (80.3%) | 34,100 (77.6%) | 34.3% (0.14) | ||
| Use of placebo | |||||
| Yes | 37 (25.0%) | 13,070 (29.7%) | 28.3% (0.11) | p = 0.01 * | Yes vs. No: p < 0.01 |
| No | 111 (75.0%) | 30891 (70.3%) | 36.9% (0.14) | ||
| Type of administration | |||||
| Oral | 121 (81.8%) | 34,361 (78.2%) | 35.3% (0.14) | p = 0.49 | |
| Injectable | 13 (8.8%) | 5320 (12.1%) | 34% (0.11) | ||
| Both | 14 (9.5%) | 4280 (9.7%) | 30.8% (0.14) | ||
| Antipsychotic of interest | |||||
| Multiple SGAs | 77 (52.0%) | 21,687 (49.3%) | 38.5% (0.14) | p = 0.01 * | Single SGA vs. Mult. SGAs: p < 0.01 |
| Single SGA | 71 (48.0%) | 22,274 (50.7%) | 30.7% (0.12) | ||
| Comparator arm | |||||
| Only SGA | 66 (44.6%) | 16,555 (37.7%) | 40% (0.14) | p = 0.01 * | FGA vs. Only SGAs: p < 0.01 Placebo vs. Only SGAs: p < 0.01 FGA + placebo vs. Only SGAs: p < 0.01 |
| FGA | 45 (54.9%) | 14,336 (32.6%) | 32.4% (0.12) | ||
| Placebo | 21 (25.6%) | 6862 (15.6%) | 28.9% (0.11) | ||
| FGA + placebo | 16 (19.5%) | 6208 (14.1%) | 27.5% (0.10) |
| B | SE | β | t | p | |
|---|---|---|---|---|---|
| Year of publication | 0.004 | 0.002 | 0.183 | 2.251 | 0.026 |
| Sample size | 0.000 | 0.000 | −0.049 | −0.588 | 0.557 |
| Duration of trial | 0.000 | 0.000 | 0.057 | 0.685 | 0.494 |
| Mean age | 0.004 | 0.002 | 0.228 | 2.788 | 0.006 |
| B | SE | β | t | p | |
|---|---|---|---|---|---|
| Constant | 0.302 | 0.074 | 4.057 | 0.000 | |
| Location | −0.025 | 0.004 | −0.494 | −5.848 | 0.000 |
| Funding | −0.002 | 0.000 | −0.398 | −4.656 | 0.000 |
| Inclusion criteria for women | 0.001 | 0.000 | 0.221 | 2.691 | 0.008 |
| Mean age | 0.004 | 0.002 | 0.166 | 2.017 | 0.046 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, L.; Machado, V.; Luersen, Y.C.; Paraventi, F.; Doretto, L.; Chaves, A.C. Sex Selection Bias in Schizophrenia Antipsychotic Trials—An Update Systematic Review. Women 2021, 1, 97-108. https://doi.org/10.3390/women1020009
Fonseca L, Machado V, Luersen YC, Paraventi F, Doretto L, Chaves AC. Sex Selection Bias in Schizophrenia Antipsychotic Trials—An Update Systematic Review. Women. 2021; 1(2):97-108. https://doi.org/10.3390/women1020009
Chicago/Turabian StyleFonseca, Lais, Viviane Machado, Yaskara C. Luersen, Felipe Paraventi, Larissa Doretto, and Ana Cristina Chaves. 2021. "Sex Selection Bias in Schizophrenia Antipsychotic Trials—An Update Systematic Review" Women 1, no. 2: 97-108. https://doi.org/10.3390/women1020009
APA StyleFonseca, L., Machado, V., Luersen, Y. C., Paraventi, F., Doretto, L., & Chaves, A. C. (2021). Sex Selection Bias in Schizophrenia Antipsychotic Trials—An Update Systematic Review. Women, 1(2), 97-108. https://doi.org/10.3390/women1020009






