Lipid Profile and 5α-Reductase Inhibition Activity of Proprietary Ultrahigh-Pressure Supercritical Carbon Dioxide and Hexane Saw Palmetto Extracts
Abstract
1. Introduction
2. Materials and Methods
2.1. 5αR Enzyme Inhibition Assays
2.1.1. Sample Preparation for 5αR Inhibition Assays
- For each variable, the equivalent of three capsules’ fill weight was transferred to three reaction tubes, respectively, yielding six reaction vessels
- 600 µL of hexane was added to each reaction tube
- Each sample was mixed using a vortex mixer and sonicated until the solids dissolved
- The samples were then centrifuged for 5 min at 4800× g
- The hexane phase of each sample was then transferred to a fresh tube
- The procedure was repeated twice
- The hexane phases for each sample of each were then combined
- The hexane solvent for each sample was then evaporated using a speedvac
- The three samples were pooled using acetone (0.5%) and diluted to a 10 mg/mL stock solution, stored at room temperature
2.1.2. Cell Cultures and Preparation of Cell Homogenates
2.1.3. Preparation of Saw Palmetto Samples and 5αR Enzyme Assays
2.1.4. Data Analysis
2.2. Lipid Profile Assays
2.2.1. Sample Preparation
2.2.2. Analytical Testing/Sample Analysis
2.2.3. Data Analysis
3. Results
3.1. Inhibition of 5αR Enzyme Activity
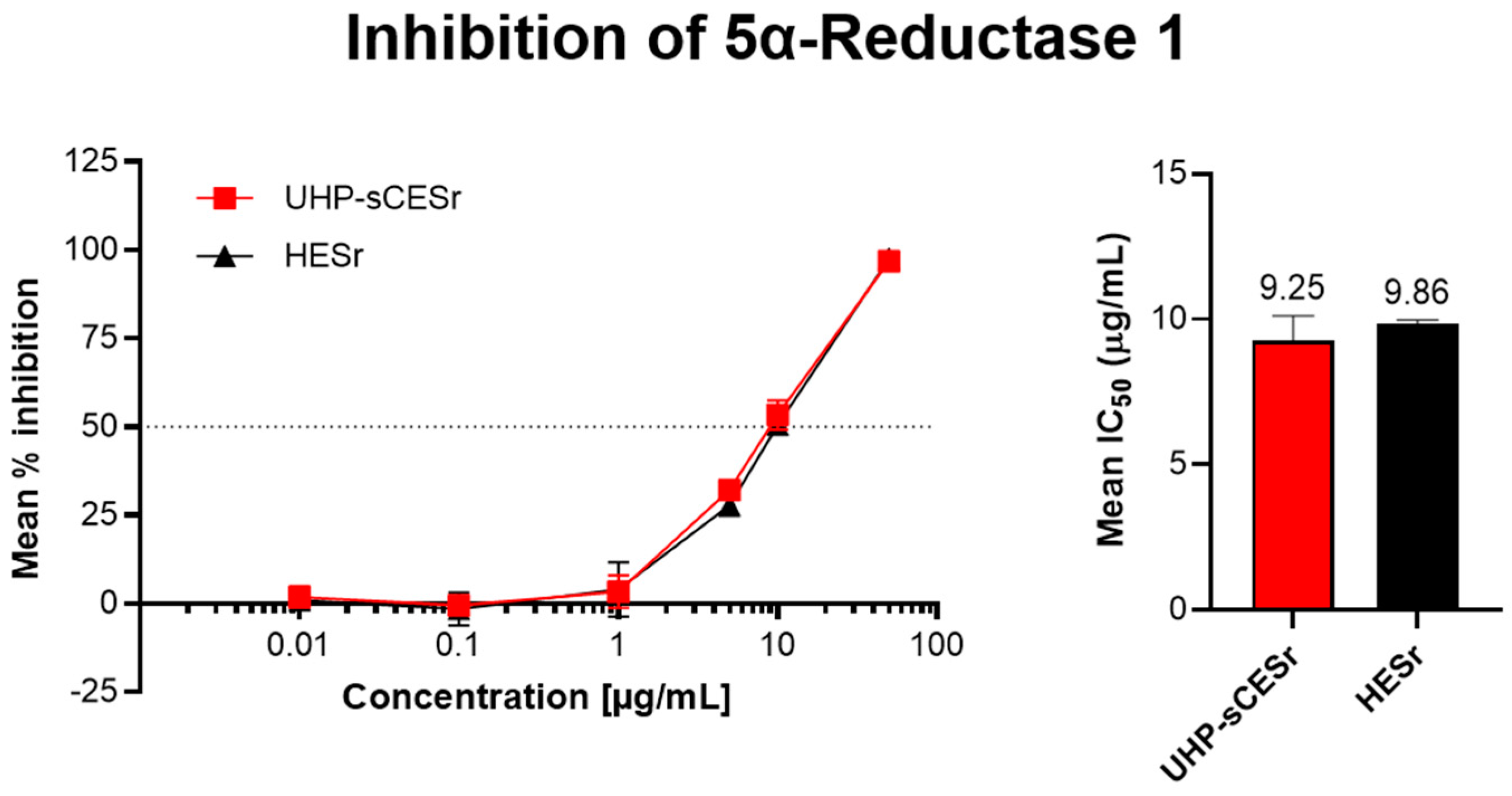
3.1.1. Inhibition of 5αR-1
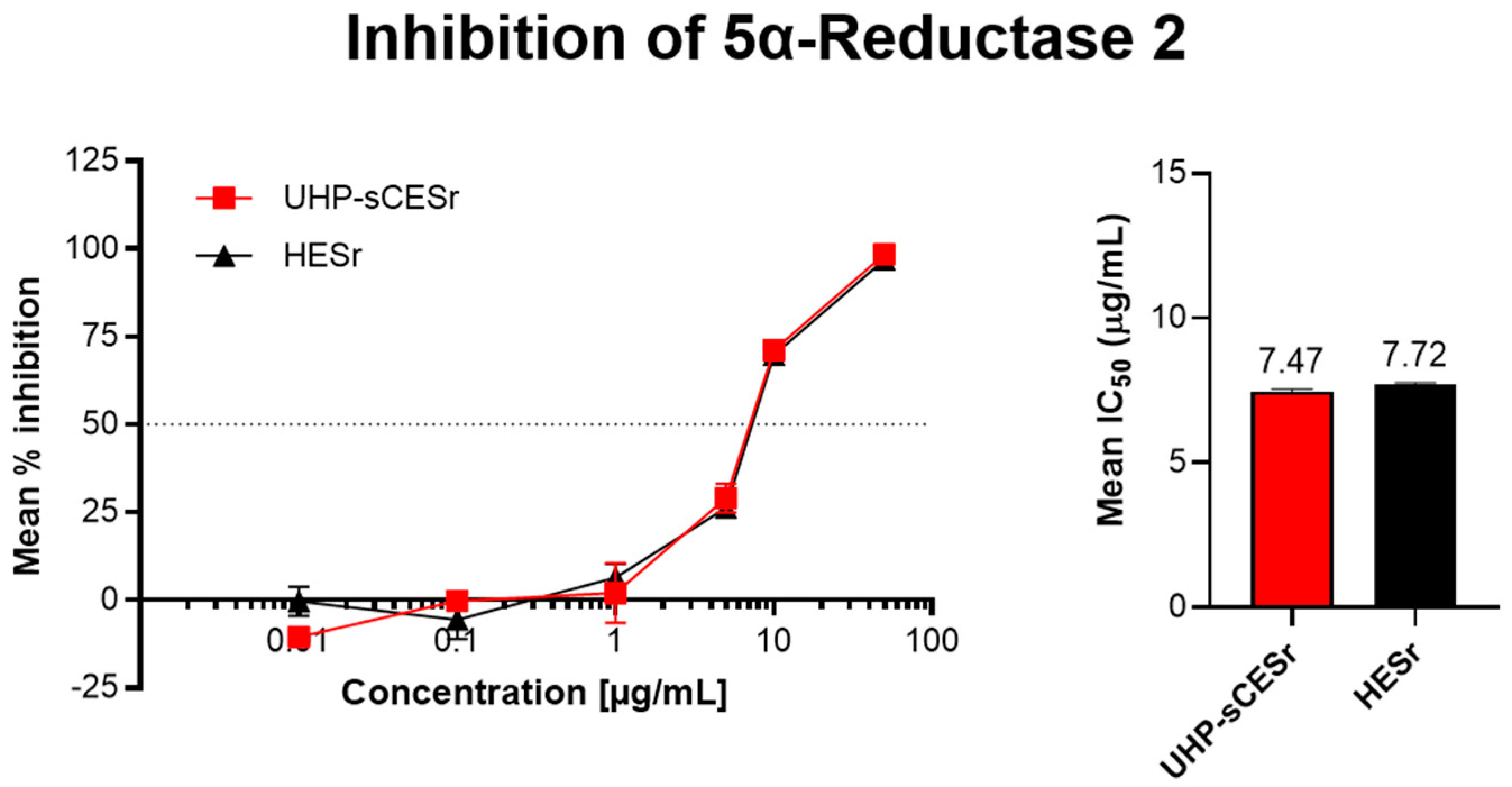
3.1.2. Inhibition of 5αR-2
3.2. Analysis of Lipid Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Nunzio, C.; Salonia, A.; Gacci, M.; Ficarra, V. Inflammation is a target of medical treatment for lower urinary tract symptoms associated with benign prostatic hyperplasia. World J. Urol. 2020, 38, 2771–2779. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, C.; Presicce, F.; Tubaro, A. Inflammatory mediators in the development and progression of benign prostatic hyperplasia. Nat. Rev. Urol. 2016, 13, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Roehrborn, C.G. Pathology of benign prostatic hyperplasia. Int. J. Impot. Res. 2008, 20 (Suppl. S3), S11–S18. [Google Scholar] [CrossRef] [PubMed]
- Wilt, T.J.; N’Dow, J. Benign prostatic hyperplasia. Part 1—Diagnosis. BMJ 2008, 336, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Platz, E.A.; Joshu, C.E.; Mondul, A.M.; Peskoe, S.B.; Willett, W.C.; Giovannucci, E. Incidence and progression of lower urinary tract symptoms in a large prospective cohort of United States men. J. Urol. 2012, 188, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Egan, K.B. The Epidemiology of Benign Prostatic Hyperplasia Associated with Lower Urinary Tract Symptoms: Prevalence and Incident Rates. Urol. Clin. N. Am. 2016, 43, 289–297. [Google Scholar] [CrossRef]
- Alawamlh, O.A.H.; Goueli, R.; Lee, R.K. Lower Urinary Tract Symptoms, Benign Prostatic Hyperplasia, and Urinary Retention. Med. Clin. N. Am. 2018, 102, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Chin, W.Y.; Choi, E.P.H.; Wan, E.Y.F.; Lam, C.L.K. The mediating factors in the relationship between lower urinary tract symptoms and health-related quality of life. BMC Res. Notes 2017, 10, 611. [Google Scholar] [CrossRef]
- Lloyd, G.L.; Marks, J.M.; Ricke, W.A. Benign Prostatic Hyperplasia and Lower Urinary Tract Symptoms: What Is the Role and Significance of Inflammation? Curr. Urol. Rep. 2019, 20, 54. [Google Scholar] [CrossRef]
- Hong, S.J.; Rayford, W.; Valiquette, L.; Emberton, M. The importance of patient perception in the clinical assessment of benign prostatic hyperplasia and its management. BJU Int. 2005, 95, 15–19. [Google Scholar] [CrossRef]
- Chughtai, B.; Forde, J.C.; Thomas, D.D.; Laor, L.; Hossack, T.; Woo, H.H.; Te, A.E.; Kaplan, S.A. Benign prostatic hyperplasia. Nat. Rev. Dis. Prim. 2016, 2, 16031. [Google Scholar] [CrossRef] [PubMed]
- Governa, P.; Giachetti, D.; Biagi, M.; Manetti, F.; De Vico, L. Hypothesis on Serenoa repens (Bartram) small extract inhibition of prostatic 5α-reductase through an in silico approach on 5β-reductase x-ray structure. PeerJ 2016, 4, e2698. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.H.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef]
- Yamana, K.; Labrie, F.; Luu-The, V. Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride. Horm. Mol. Biol. Clin. Investig. 2010, 2, 293–299. [Google Scholar] [CrossRef]
- Nickel, J.C. Inflammation and benign prostatic hyperplasia. Urol. Clin. N. Am. 2008, 35, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Sarma, A.V.; Wei, J.T. Benign prostatic hyperplasia and lower urinary tract symptoms. N. Engl. J. Med. 2012, 367, 248–257. [Google Scholar] [CrossRef] [PubMed]
- McVary, K.T.; Roehrborn, C.G.; Avins, A.L.; Barry, M.J.; Bruskewitz, R.C.; Donnell, R.F.; Foster, H.E., Jr.; Gonzalez, C.M.; Kaplan, S.A.; Penson, D.F.; et al. Update on AUA guideline on the management of benign prostatic hyperplasia. J. Urol. 2011, 185, 1793–1803. [Google Scholar] [CrossRef] [PubMed]
- Strum, S.B. Serenoa repens (Saw Palmetto) for Lower Urinary Tract Symptoms (LUTS): The Evidence for Efficacy and Safety of Lipidosterolic Extracts. Part II. Uro 2021, 1, 139–154. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Allkanjari, O.; Busetto, G.M.; Cai, T.; Larganà, G.; Magri, V.; Perletti, G.; Robustelli Della Cuna, F.S.; Russo, G.I.; Stamatiou, K.; et al. Nutraceutical treatment and prevention of benign prostatic hyperplasia and prostate cancer. Arch. Ital. Urol. Androl. 2019, 91. [Google Scholar] [CrossRef]
- Strum, S.B. Serenoa repens (Saw Palmetto) for Lower Urinary Tract Symptoms (LUTS): The Evidence for Efficacy and Safety of Lipidosterolic Extracts. Part III. Uro 2021, 1, 155–179. [Google Scholar] [CrossRef]
- Fornara, P.; Madersbacher, S.; Vahlensieck, W.; Bracher, F.; Romics, I.; Kil, P. Phytotherapy Adds to the Therapeutic Armamentarium for the Treatment of Mild-To-Moderate Lower Urinary Tract Symptoms in Men. Urol. Int. 2020, 104, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Fusco, F.; Arcaniolo, D.; Creta, M.; Piccinocchi, G.; Arpino, G.; Laringe, M.; Piccinocchi, R.; Longo, N.; Verze, P.; Mangiapia, F.; et al. Demographic and comorbidity profile of patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia in a real-life clinical setting: Are 5-alpha-reductase inhibitor consumers different? World J. Urol. 2015, 33, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Capogrosso, P.; Serino, A.; Ventimiglia, E.; Boeri, L.; Dehò, F.; Damiano, R.; Briganti, A.; Montorsi, F.; Salonia, A. Effects of silodosin on sexual function—Realistic picture from the everyday clinical practice. Andrology 2015, 3, 1076–1081. [Google Scholar] [CrossRef] [PubMed]
- Pais, P.; Villar, A.; Rull, S. Determination of the potency of a novel saw palmetto supercritical CO2 extract (SPSE) for 5α-reductase isoform II inhibition using a cell-free in vitro test system. Res. Rep. Urol. 2016, 8, 41–49. [Google Scholar] [CrossRef]
- Perry, R.; Milligan, G.; Anderson, P.; Gillon, A.; White, M. Real-world use of Permixon® in benign prostatic hyperplasia—Determining appropriate monotherapy and combination treatment. Adv. Ther. 2012, 29, 538–550. [Google Scholar] [CrossRef]
- Scaglione, F.; Lucini, V.; Pannacci, M.; Caronno, A.; Leone, C. Comparison of the potency of different brands of Serenoa repens extract on 5alpha-reductase types I and II in prostatic co-cultured epithelial and fibroblast cells. Pharmacology 2008, 82, 270–275. [Google Scholar] [CrossRef]
- Scaglione, F.; Lucini, V.; Pannacci, M.; Dugnani, S.; Leone, C. Comparison of the potency of 10 different brands of Serenoa repens extracts. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 569–574. [Google Scholar]
- Vela-Navarrete, R.; Alcaraz, A.; Rodríguez-Antolín, A.; Miñana López, B.; Fernández-Gómez, J.M.; Angulo, J.C.; Castro Díaz, D.; Romero-Otero, J.; Brenes, F.J.; Carballido, J.; et al. Efficacy and safety of a hexanic extract of Serenoa repens (Permixon(®)) for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH): Systematic review and meta-analysis of randomised controlled trials and observational studies. BJU Int. 2018, 122, 1049–1065. [Google Scholar] [CrossRef]
- Alcaraz, A.; Rodríguez-Antolín, A.; Carballido-Rodríguez, J.; Castro-Díaz, D.; Esteban-Fuertes, M.; Cózar-Olmo, J.M.; Ficarra, V.; Medina-López, R.; Fernández-Gómez, J.M.; Angulo, J.C.; et al. Clinical Benefit of Tamsulosin and the Hexanic Extract of Serenoa repens, in Combination or as Monotherapy, in Patients with Moderate/Severe LUTS-BPH: A Subset Analysis of the QUALIPROST Study. J. Clin. Med. 2020, 9, 2909. [Google Scholar] [CrossRef]
- Boeri, L.; Capogrosso, P.; Ventimiglia, E.; Cazzaniga, W.; Pederzoli, F.; Moretti, D.; Dehò, F.; Montanari, E.; Montorsi, F.; Salonia, A. Clinically Meaningful Improvements in LUTS/BPH Severity in Men Treated with Silodosin Plus Hexanic Extract of Serenoa repens or Silodosin Alone. Sci. Rep. 2017, 7, 15179. [Google Scholar] [CrossRef]
- MacDonald, R.; Tacklind, J.W.; Rutks, I.; Wilt, T.J. Serenoa repens monotherapy for benign prostatic hyperplasia (BPH): An updated Cochrane systematic review. BJU Int. 2012, 109, 1756–1761. [Google Scholar] [CrossRef] [PubMed]
- Fusco, F.; Creta, M.; De Nunzio, C.; Gacci, M.; Li Marzi, V.; Finazzi Agrò, E. Alpha-1 adrenergic antagonists, 5-alpha reductase inhibitors, phosphodiesterase type 5 inhibitors, and phytotherapic compounds in men with lower urinary tract symptoms suggestive of benign prostatic obstruction: A systematic review and meta-analysis of urodynamic studies. Neurourol. Urodyn. 2018, 37, 1865–1874. [Google Scholar] [CrossRef]
- Committee on Herbal Medicinal Products (HMPC) (Ed.) Assessment Report on Serenoa repens (W. Bartram): Small, Fructus; European Medicines Agency: London, UK, 2015.
- Scaglione, F. How to Choose the Right Serenoa repens Extract. Eur. Urol. Suppl. 2015, 14, e1464–e1469. [Google Scholar] [CrossRef]
- EAU Guidelines. Edition Presented at the EAU Annual Congress Amsterdam 2022. Available online: https://uroweb.org/guidelines/management-of-non-neurogenic-male-luts (accessed on 25 July 2022).
- Gafner, S.; Baggett, S. Bulletin on Saw Palmetto (Serenoa repens) Adulteration; American Botanical Council: Austin, TX, USA, 2017. [Google Scholar]
- Nickel, J.C.; Chughtai, B.; De Nunzio, C.; Brahmbhatt, J.; Shore, N.; Te, A.E.; Djavan, B. Rethinking the Role of Saw Palmetto Extract for Men with Lower Urinary Tract Symptoms in North America. Uro 2022, 2, 137–150. [Google Scholar] [CrossRef]
- Pais, P. Potency of a novel saw palmetto ethanol extract, SPET-085, for inhibition of 5alpha-reductase II. Adv. Ther. 2010, 27, 555–563. [Google Scholar] [CrossRef]
- Reichert, W.; Hartmann, R.W.; Jose, J. Stable expression of the human 5alpha-reductase isoenzymes type I and type II in HEK293 cells to identify dual and selective inhibitors. J. Enzym. Inhib. 2001, 16, 47–53. [Google Scholar] [CrossRef]
- USP 40-NF 35; Saw Palmetto Extract. United States Pharmacopeia and National Formulary: Rockville, MD, USA, 2017; pp. 7179–7181.
- Habib, F.K.; Wyllie, M.G. Not all brands are created equal: A comparison of selected components of different brands of Serenoa repens extract. Prostate Cancer Prostatic Dis. 2004, 7, 195–200. [Google Scholar] [CrossRef]
- McCarty, L.T. Sustainability metrics for GEMS supply chain excellence: 2021 report. 2 September 2021; p. 6. [Google Scholar]
- Arruzazabala, M.L.; Molina, V.; Carbajal, D.; Mas, R.; Gonzalez, V.; Rodriguez, E.; Marrero, D. Different ripening stages of Roystonea regia fruits influence their effects on testosterone-induced prostate enlargement in rats. Lat. Am. J. Pharm. 2008, 27, 41–45. [Google Scholar]
- Boccafoschi, C.; Annoscia, S. Comparison of Serenoa repens extract and placebo in a controlled clinical trial in patients with prostatic adenomatosis. Urologiia 1983, 50, 1257–1268. [Google Scholar] [CrossRef]
- Cirillo-Marucco, E.; Pagliarulo, A.; Tritto, G.; Piccinno, A.; Di Rienzo, U. Serenoa repens extract (Permixon®) in the early treatment of prostatic hypertrophy. Urologia 1983, 50, 1269–1277. [Google Scholar] [CrossRef]
- Emili, E.; Lo Cigno, M.; Petrone, U. Clinical results on a new drug in prostate hypertrophy therapy (Permixon). Urologia 1983, 50, 1042–1048. [Google Scholar] [CrossRef]
- Mandressi, A.; Tarallo, U.; Maggioni, A.; Tombolini, P.; Rocco, F.; Quadraccia, S. Medical treatment of benign prostatic hyperplasia: Efficacy of the extract of Serenoa repens (Permixon) compared to that of the extract of Pygeum africanum and a placebo. Urologia 1983, 50, 752–758. [Google Scholar] [CrossRef]
- Champault, G.; Patel, J.C.; Bonnard, A.M. A double-blind trial of an extract of the plant Serenoa repens in benign prostatic hyperplasia. Br. J. Clin. Pharmacol. 1984, 18, 461–462. [Google Scholar] [CrossRef]
- Cukier, J.; Ducassou, J.; Le Guillou, M.; Leriche, A.; Lobel, B.; Toubol, J. Permixon versus placebo: Results of a multicenter study. C. R. Ther. Pharmacol. Clin. 1985, 4, 15–21. [Google Scholar]
- Tasca, A.; Barulli, M.; Cavazzana, A.; Zattoni, F.; Artibani, W.; Pagano, F. Treatment of obstructive symptomatology caused by prostatic adenoma with an extract of Serenoa repens: Double-blind clinical test v. placebo. Minerva Urol. Nefrol. 1985, 37, 87–91. [Google Scholar]
- Tosto, A.; Rovereto, B.; Paoletti, M.C.; Rizzo, M.; Nicolucci, A.; Costantini, A. Serenoa repens extract in the treatment of functional disorders secondary to adenoma of the prostate: Considerations on 20 cases. Urologia 1985, 52, 536–542. [Google Scholar] [CrossRef]
- Cabasino, S.; Puddu, A.; Spiga, E. Evaluation of the efficacy of the Serenoa repens extract in the medical therapy of benign prostatic hypertrophy. Urologia 1986, 53, 535–538. [Google Scholar] [CrossRef]
- Mancuso, G.; Guillot, F.; Migaleddu, V.; Satta, U. Serenoa repens in the medical treatment of benign prostatic hypertrophy: Our experience. Urologia 1986, 53, 709–714. [Google Scholar] [CrossRef]
- Martorana, G.; Giberti, C.; Pizzorno, R.; Natta, G.D.; Brancadoro, M.T.; Barreca, T.; Rolandi, E.; Isotta, A.; Neumaier, C.E. Long-term study with Serenoa repens extract in patients with prostatic adenoma. Urologia 1986, 53, 366–369. [Google Scholar] [CrossRef]
- Paoletti, P.P.; Francalanci, R.; Tenti, S.; Paoletti, G.; Pedaccini, P. Medical treatment of prostatic hypertrophy: Experience with the therapeutic use of Serenoa repens. Urologia 1986, 53, 182–187. [Google Scholar] [CrossRef]
- Pannunzio, E.; D’Ascenzo, R.; Giardinetti, F.; Civili, P.; Persichelli, E. Serenoa repens vs. gestonorone caproate in the treatment of benign prostatic hypertrophy: Randomized study. Urologia 1986, 53, 696–705. [Google Scholar] [CrossRef]
- Pescatore, D.; Calvi, P.; Michelotti, P. Urodynamic assessment of treatment in patients with prostatic adenoma with Serenoa repens extract. Urologia 1986, 53, 894–897. [Google Scholar] [CrossRef]
- Reece Smith, H.; Memon, A.; Smart, C.J.; Dewbury, K. The value of Permixon in benign prostatic hypertrophy. Br. J. Urol. 1986, 58, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Authie, D.; Cauquil, J. Assessment of the effectiveness of Permixon* in daily practice: A multicentric study. C. R. Ther. Pharmacol. Clin. 1987, 5, 3–13. [Google Scholar]
- Ollé Carreras, J. Our experience with hexane extract from Serenoa repens in the treatment of benign prostatic hypertrophy. Arch. Esp. Urol. 1987, 40, 310–313. [Google Scholar]
- Vespasiani, G.; Cesaroni, M.; Parziani, S.; Rosi, P.; Valentini, P.; Porena, M. Serenoa repens in the treatment of benign prostatic hypertrophy. Urologia 1987, 54, 145–149. [Google Scholar] [CrossRef]
- Orfei, S.; Grumelli, B.; Galetti, G. Clinical and uroflowimetric evaluation of Permixon® in geriatrics. Urologia 1988, 55, 373–381. [Google Scholar] [CrossRef]
- Dathe, G.; Schmid, H. Phytotherapy for benign prostatic hyperplasia (BPH) with an extract of Serenoa repens (Permixon). Urol. B 1991, 31, 223–330. [Google Scholar]
- Hanuš, M.; Matoušková, M. Alternative therapy of benign prostatic hypertrophy—Permixon (Capistan). Rozhl. Chir. 1993, 72, 75–79. [Google Scholar]
- Descotes, J.L.; Rambeaud, J.J.; Deschaseaux, P.; Faure, G. Placebo-controlled evaluation of the efficacy and tolerability of Permixon® in benign prostatic hyperplasia after exclusion of placebo responders. Clin. Drug Investig. 1995, 9, 291–297. [Google Scholar] [CrossRef]
- Ebbinghaus, K. Effectiveness of Permixon for the treatment of benign prostatic hyperplasia. J. Urol. Urogynäkol. 1995, 2, 17–21. [Google Scholar]
- Gorilovsky, L.M. Permixon in the treatment of benign prostatic hyperplasia. Ter. Arkh. 1995, 67, 62–64. [Google Scholar]
- Carraro, J.C.; Raynaud, J.P.; Koch, G.; Chisholm, G.D.; Di Silverio, F.; Teillac, P.; Da Silva, F.C.; Cauquil, J.; Chopin, D.K.; Hamdy, F.C.; et al. Comparison of phytotherapy (Permixon) with finasteride in the treatment of benign prostate hyperplasia: A randomized international study of 1,098 patients. Prostate 1996, 29, 231–240. [Google Scholar] [CrossRef]
- Foroutan, F. Effectiveness and tolerability of Permixon in a larger patient population (592 patients) under practical conditions. J. Urol. Urogynäkol. 1997, 2, 17–21. [Google Scholar]
- Stepanov, V.N.; Siniakova, L.A.; Sarrazin, B.; Raynaud, J.P. Efficacy and tolerability of the lipidosterolic extract of Serenoa repens (Permixon) in benign prostatic hyperplasia: A double-blind comparison of two dosage regimens. Adv. Ther. 1999, 16, 231–241. [Google Scholar] [PubMed]
- Al-Shukri, S.H.; Deschaseaux, P.; Kuzmin, I.V.; Amdiy, R.R. Early urodynamic effects of the lipido-sterolic extract of Serenoa repens (Permixon®) in patients with lower urinary tract symptoms due to benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2000, 3, 195–199. [Google Scholar] [CrossRef]
- Medeiros, A.S.; Verona, C.B.M.; Mattos, D., Jr.; Silva, E.G.; Fonseca, G.N.; Begliomini, H.; Pous, J.H.; Cury, J.; Costa, M.M.; Prado, M.J.; et al. Efficacy and tolerability of the extract of Serenoa repens in a multicentric study in patients with symptomatic benign prostatic hyperplasia. Rev. Bras. Med. 2000, 57, 321–324. [Google Scholar]
- Aliaev, Y.G.; Vinarov, A.Z.; Lokshin, K.L.; Spivak, L.G. Five-year experience in treating patients with prostatic hyperplasia patients with Permixon (Serenoa repens “Pierre Fabre Medicament”). Urologiia 2002, 7, 23–25. [Google Scholar]
- Debruyne, F.; Koch, G.; Boyle, P.; Da Silva, F.C.; Gillenwater, J.G.; Hamdy, F.C.; Perrin, P.; Teillac, P.; Vela-Navarrete, R.; Raynaud, J.P. Comparison of a phytotherapeutic agent (Permixon) with an alpha-blocker (tamsulosin) in the treatment of benign prostatic hyperplasia: A 1-year randomized international study. Eur. Urol. 2002, 41, 497–506. [Google Scholar] [CrossRef]
- Giannakopoulos, X.; Baltogiannis, D.; Giannakis, D.; Tasos, A.; Sofikitis, N.; Charalabopoulos, K.; Evangelou, A. The lipidosterolic extract of Serenoa repens in the treatment of benign prostatic hyperplasia: A comparison of two dosage regimens. Adv. Ther. 2002, 19, 285–296. [Google Scholar] [CrossRef]
- Glemain, P.; Coulange, C.; Billebaud, T.; Gattegno, B.; Muszynski, R.; Loeb, G. Tamsulosin with or without Serenoa repens in benign prostatic hyperplasia: The OCOS trial. Prog. Urol. 2002, 12, 395–403. [Google Scholar] [PubMed]
- Pytel, Y.A.; Vinarov, A.; Lopatkin, N.; Sivkov, A.; Gorilovsky, L.; Raynaud, J.P. Long-term clinical and biologic effects of the lipidosterolic extract of Serenoa repens in patients with symptomatic benign prostatic hyperplasia. Adv. Ther. 2002, 19, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Vela Navarrete, R.; Garcia Cardoso, J.V.; Barat, A.; Manzarbeitia, F.; López Farré, A. BPH and inflammation: Pharmacological effects of Permixon on histological and molecular inflammatory markers. Results of a double blind pilot clinical assay. Eur. Urol. 2003, 44, 549–555. [Google Scholar] [CrossRef]
- Debruyne, F.; Boyle, P.; Calais Da Silva, F.; Gillenwater, J.G.; Hamdy, F.C.; Perrin, P.; Teillac, P.; Vela-Navarrete, R.; Raynaud, J.P.; Schulman, C.C. Evaluation of the clinical benefit of Permixon and tamsulosin in severe BPH patients—PERMAL study subset analysis. Eur. Urol. 2004, 45, 773–779. [Google Scholar] [CrossRef] [PubMed]
- El-Demiry, M. Serenoa repens in the treatment of patients with symptomatic benign prostatic hyperplasia. BJU Int. 2004, 94, 146–147. [Google Scholar] [CrossRef]
- Djavan, B.; Fong, Y.K.; Chaudry, A.; Reissigl, A.; Anagnostou, T.; Bagheri, F.; Waldert, M.; Marihart, S.; Harik, M.; Marberger, M. Progression delay in men with mild symptoms of bladder outlet obstruction: A comparative study of phytotherapy and watchful waiting. World J. Urol. 2005, 23, 253–256. [Google Scholar] [CrossRef]
- Giulianelli, R.; Pecoraro, S.; Sepe, G.; Leonardi, R.; Gentile, B.C.; Albanesi, L.; Brunori, S.; Mavilla, L.; Pisanti, F.; Giannella, R.; et al. Multicenter study on the efficacy and tolerability of an extract of Serenoa repens in patients with chronic benign prostate conditions associated with inflammation. Arch. Ital. Urol. Androl. 2012, 84, 94–98. [Google Scholar]
- Latil, A.; Petrissans, M.T.; Rouquet, J.; Robert, G.; de la Taille, A. Effects of hexanic extract of Serenoa repens (Permixon® 160 mg) on inflammation biomarkers in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Prostate 2015, 75, 1857–1867. [Google Scholar] [CrossRef]
- Alcaraz, A.; Carballido-Rodriguez, J.; Unda-Urzaiz, M.; Medina-Lopez, R.; Ruiz-Cerda, J.L.; Rodriguez-Rubio, F.; Garcia-Rojo, D.; Brenes-Bermudez, F.J.; Cozar-Olmo, J.M.; Baena-Gonzalez, V.; et al. Quality of life in patients with lower urinary tract symptoms associated with BPH: Change over time in real-life practice according to treatment—The QUALIPROST study. Int. Urol. Nephrol. 2016, 48, 645–656. [Google Scholar] [CrossRef]
- de la Taille, A.; Bardin, L.; Castagné, C.; Auges, M.; Capronnier, O.; Chalret du Rieu, Q. Alpha-blockers or phytotherapy as first-line treatment of LUTS/BPH in general medicine: The PERSAT non-interventional study. Prog. Urol. 2020, 30, 522–531. [Google Scholar] [CrossRef]
- Alcaraz, A.; Rodríguez-Antolín, A.; Carballido-Rodríguez, J.; Castro-Díaz, D.; Medina-Polo, J.; Fernández-Gómez, J.M.; Ficarra, V.; Palou, J.; Ponce de León Roca, J.; Angulo, J.C.; et al. Efficacy and tolerability of the hexanic extract of Serenoa repens compared to tamsulosin in moderate-severe LUTS-BPH patients. Sci. Rep. 2021, 11, 19401. [Google Scholar] [CrossRef]
- Alcaraz, A.; Gacci, M.; Ficarra, V.; Medina-Polo, J.; Salonia, A.; Fernández-Gómez, J.M.; Ciudin, A.; Castro-Díaz, D.; Rodríguez-Antolín, A.; Carballido-Rodríguez, J.; et al. Efficacy and Safety of the hexanic extract of Serenoa repens vs. watchful waiting in men with moderate to severe LUTS-BPH: Results of a paired matched clinical study. J. Clin. Med. 2022, 11, 967. [Google Scholar] [CrossRef] [PubMed]
- de la Taille, A.; Chalret du Rieu, Q.; Dialla, O.; Bardin, L. Alpha-blockers or hexanic extract of Serenoa repens for 6 months: Sub-analysis of the PERSAT study. Prog. Urol. 2022. [Google Scholar] [CrossRef]
- Champault, G.; Bonnard, A.M.; Cauquil, J.; Patel, J.C. Medical treatment of prostatic adenoma. Controlled trial: PA 109 vs. placebo in one hundred and ten patients. Ann. Urol. 1984, 18, 407–410. [Google Scholar]
- Robert, G.Y. Comparison of the effects of hexanic extract of Serenoa repens (Permixon) and tamsulosin on inflammatory biomarkers in the treatment of benign prostatic hyperplasia-related lower urinary tract symptoms. Eur. Urol. Suppl. 2015, 14, e1470–e1474. [Google Scholar] [CrossRef]
- Hizli, F.; Uygur, M.C. A prospective study of the efficacy of Serenoa repens, tamsulosin, and Serenoa repens plus tamsulosin treatment for patients with benign prostate hyperplasia. Int. Urol. Nephrol. 2007, 39, 879–886. [Google Scholar] [CrossRef] [PubMed]

| Ratio of Lauric Acid (%) to Individual Fatty Acids (%) | ||||
|---|---|---|---|---|
| Fatty Acid | HESr (lot G06743) | HESr-T (n = 5) | UHP-sCESr (lot 170710) | UHP-sCESr-T (n = 81) |
| Oleic | 1.2 | 1.1 | 1.1 | 1.0 |
| Myristic | 2.7 | 2.7 | 2.8 | 2.6 |
| Palmitic | 3.7 | 3.6 | 3.6 | 3.5 |
| Linoleic | 6.2 | 6.9 | 5.9 | 6.1 |
| Capric | 12.3 | 12.5 | 11.4 | 11.3 |
| Caprylic | 17.6 | 16.5 | 15.4 | 14.4 |
| Stearic | 17.7 | 18.2 | 17.4 | 17.5 |
| Caproic | 37.8 | 27.7 | 31.4 | 23.4 |
| Linolenic | 42.5 | 50.8 | 34.8 | 43.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cartwright, E.J.; Dohnalek, M.H.; Hill, W.S. Lipid Profile and 5α-Reductase Inhibition Activity of Proprietary Ultrahigh-Pressure Supercritical Carbon Dioxide and Hexane Saw Palmetto Extracts. Uro 2023, 3, 27-39. https://doi.org/10.3390/uro3010005
Cartwright EJ, Dohnalek MH, Hill WS. Lipid Profile and 5α-Reductase Inhibition Activity of Proprietary Ultrahigh-Pressure Supercritical Carbon Dioxide and Hexane Saw Palmetto Extracts. Uro. 2023; 3(1):27-39. https://doi.org/10.3390/uro3010005
Chicago/Turabian StyleCartwright, Elizabeth J., Margaret H. Dohnalek, and W. Stephen Hill. 2023. "Lipid Profile and 5α-Reductase Inhibition Activity of Proprietary Ultrahigh-Pressure Supercritical Carbon Dioxide and Hexane Saw Palmetto Extracts" Uro 3, no. 1: 27-39. https://doi.org/10.3390/uro3010005
APA StyleCartwright, E. J., Dohnalek, M. H., & Hill, W. S. (2023). Lipid Profile and 5α-Reductase Inhibition Activity of Proprietary Ultrahigh-Pressure Supercritical Carbon Dioxide and Hexane Saw Palmetto Extracts. Uro, 3(1), 27-39. https://doi.org/10.3390/uro3010005





