Determination of Clobazam and Its Major Metabolite N-desmethylclobazam in Human Plasma with High-Performance Liquid Chromatography
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instrument
2.3. Chromatographic Conditions
2.4. Standard Stock and Working Solutions
2.5. Calibration Standards and Quality Control Samples
2.6. Sample Preparation
2.7. Method Validation
2.7.1. Linearity
2.7.2. Selectivity and Sensitivity
2.7.3. Precision and Accuracy
2.7.4. Recovery
3. Results
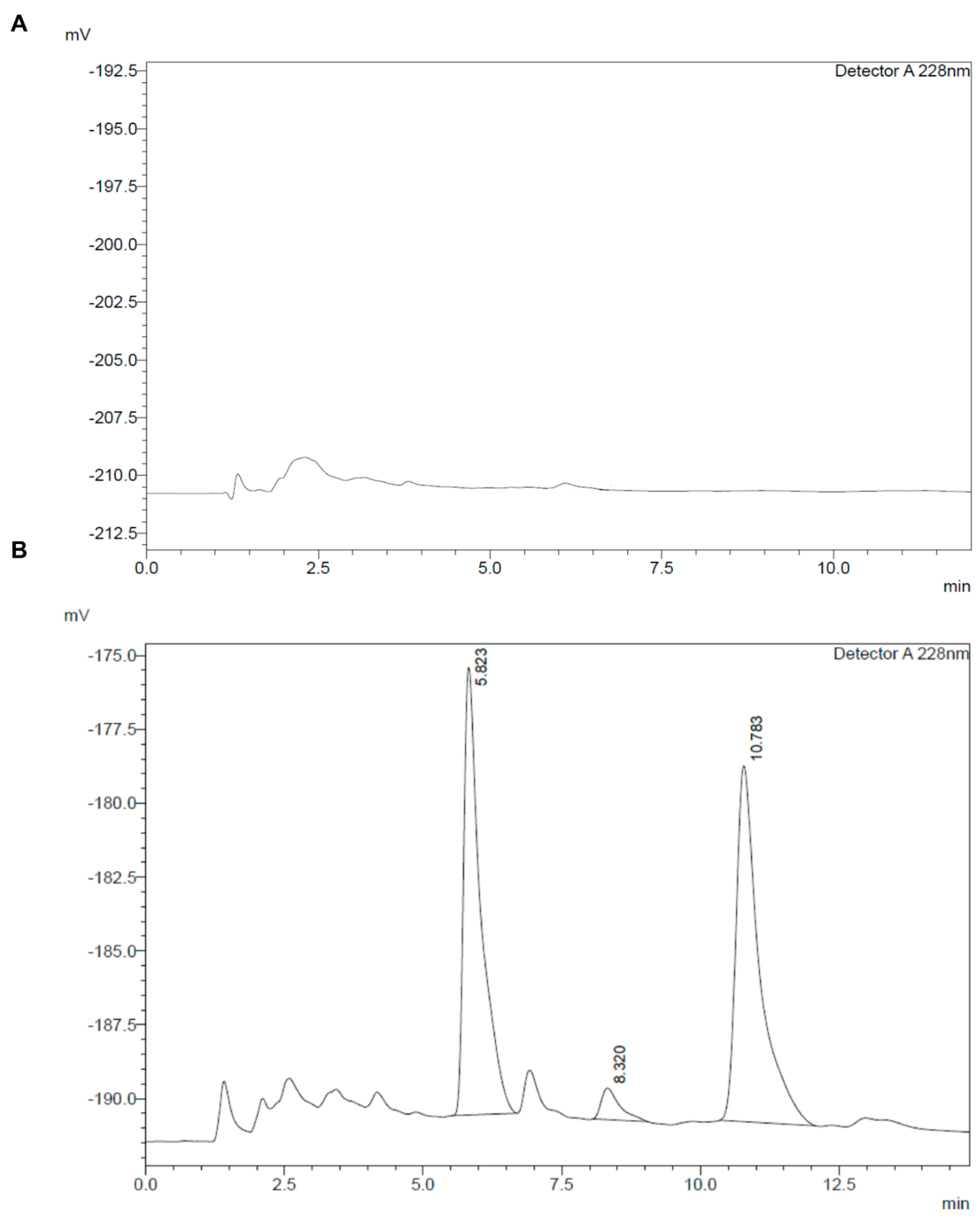
3.1. Liquid Chromatography Method Development
3.2. Method Validation
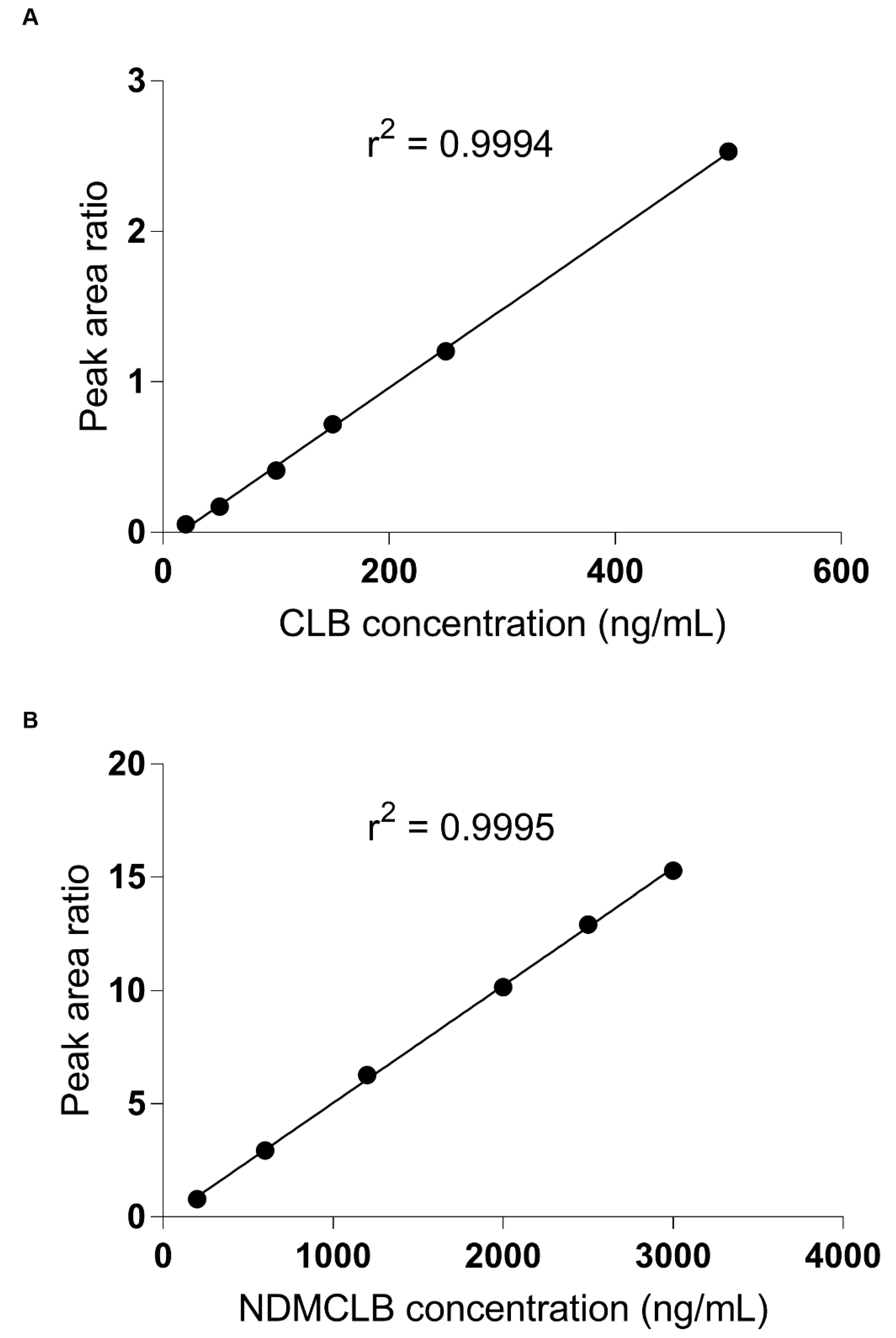
3.2.1. Linearity
3.2.2. Selectivity and Sensitivity
3.2.3. Precision and Accuracy
3.2.4. Recovery
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Robertson, M.M. Current Status of the 1,4- and 1,5-Benzodiazepines in the Treatment of Epilepsy: The Place of Clobazam. Epilepsia 1986, 27 (Suppl. S1), S27–S41. [Google Scholar] [CrossRef]
- Pernea, M.; Sutcliffe, A.G. Clobazam and Its Use in Epilepsy. Pediatr. Rep. 2016, 8, 34–38. [Google Scholar] [CrossRef]
- Mahmoud, S.H.; Rans, C. Systematic review of clobazam use in patients with status epilepticus. Epilepsia Open 2018, 3, 323–330. [Google Scholar] [CrossRef]
- Grigoleit, H.-G.; Hajdú, P.; Hundt, H.K.L.; Koeppen, D.; Malerczyk, V.; Meyer, B.H.; Müller, F.; Witte, P.U. Pharmacokinetic aspects of the interaction between clobazam and cimetidine. Eur. J. Clin. Pharmacol. 1983, 25, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Monjanel-Mouterde, S.; Antoni, M.; Bun, H.; Botta-Frindlund, D.; Gauthier, A.; Durand, A.; Cano, J.P. Pharmacokinetics of a Single Oral Dose of Clobazam in Patients with Liver Disease. Pharmacol. Toxicol. 1994, 74, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Theis, J.G.; Koren, G.; Daneman, R.; Sherwin, A.L.; Menzano, E.; Cortez, M.; Hwang, P. Interactions of clobazam with conventional antiepileptics in children. J. Child Neurol. 1997, 12, 208–213. [Google Scholar] [CrossRef] [PubMed]
- De Leon, J.; Spina, E.; Diaz, F.J. Clobazam Therapeutic Drug Monitoring: A Comprehensive Review of the Literature with Proposals to Improve Future Studies. Ther. Drug Monit. 2013, 35, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Caccia, S.; Ballabio, M.; Guiso, G.; Zanini, M.G. Gas-liquid chromatographic determination of clobazam and n-desmethyl-clobazam in plasma. J. Chromatogr. 1979, 164, 100–105. [Google Scholar] [CrossRef]
- Marliac, Y.; Barazi, S. Determination of unchanged clobazam in plasma by gas-liquid chromatography. Ann. Pharm. Françaises 1989, 47, 213–220. [Google Scholar]
- Riva, R.; Tedeschi, G.; Albani, F.; Baruzzi, A. Quantitative determination of clobazam in the plasma of epileptic patients by gas—Liquid chromatography with electron-capture detection. J. Chromatogr. B Biomed. Sci. Appl. 1981, 225, 219–224. [Google Scholar] [CrossRef]
- Simonsen, K.W.; Hermansson, S.; Steentoft, A.; Linnet, K. A Validated Method for Simultaneous Screening and Quantification of Twenty-Three Benzodiazepines and Metabolites Plus Zopiclone and Zaleplone in Whole Blood by Liquid-Liquid Extraction and Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry. J. Anal. Toxicol. 2010, 34, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Mikayelyan, A.; Aleksanyan, A.; Sargsyan, M.; Gevorgyan, A.; Zakaryan, H.; Harutyunyan, A.; Zhamharyan, L.; Armoudjian, Y.; Margaryan, T. Protein Precipitation Method for Determination of Clobazam and N-Desmethylclobazam in Human Plasma by Lc-Ms/Ms. Biomed. Chromatogr. 2020, 34, e4710. [Google Scholar] [CrossRef] [PubMed]
- Laloup, M.; Fernandez, M.D.M.R.; De Boeck, G.; Wood, M.; Maes, V.; Samyn, N. Validation of a Liquid Chromatography-Tandem Mass Spectrometry Method for the Simultaneous Determination of 26 Benzodiazepines and Metabolites, Zolpidem and Zopiclone, in Blood, Urine, and Hair. J. Anal. Toxicol. 2005, 29, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Knapp, J.; Boknik, P.; Gumbinger, H.G.; Linck, B.; Luss, H.; Muller, F.U.; Schmitz, W.; Vahlensieck, U.; Neumann, J. Quantitation of Clobazam in Human Plasma Using High-Performance Liquid Chromatography. J. Chromatogr. Sci. 1999, 37, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Bolner, A.; Tagliaro, F.; Lomeo, A. Optimised determination of clobazam in human plasma with extraction and high-performance liquid chromatography analysis. J. Chromatogr. B Biomed. Sci. Appl. 2001, 750, 177–180. [Google Scholar] [CrossRef]
- Rouini, M.; Ardakani, Y.H.; Hakemi, L.; Mokhberi, M.; Badri, G. Simultaneous determination of clobazam and its major metabolite in human plasma by a rapid HPLC method. J. Chromatogr. B 2005, 823, 167–171. [Google Scholar] [CrossRef]
- Ratnaraj, N.; Goldberg, V.; Lascelles, P.T. Determination of clobazam and desmethylclobazam in serum using high-performance liquid chromatography. Analyst 1984, 109, 813–815. [Google Scholar] [CrossRef]
- Pistos, C.; Stewart, J.T. Direct injection HPLC method for the determination of selected benzodiazepines in plasma using a Hisep column. J. Pharm. Biomed. Anal. 2003, 33, 1135–1142. [Google Scholar] [CrossRef]
- Tomasini, J.-L.; Bun, H.; Coassolo, P.; Aubert, C.; Cano, J.-P. Determination of Clobazam, n-desmethylclobazam and their hydroxy metabolites in plasma and urine by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1985, 343, 369–377. [Google Scholar] [CrossRef]
- Kunicki, P. Simple and sensitive high-performance liquid chromatographic method for the determination of 1,5-benzodiazepine clobazam and its active metabolite N-desmethylclobazam in human serum and urine with application to 1,4-benzodiazepines analysis. J. Chromatogr. B Biomed. Sci. Appl. 2001, 750, 41–49. [Google Scholar] [CrossRef]
- Akerman, K.K. Analysis of clobazam and its active metabolite norclobazam in plasma and serum using HPLC/DAD. Scand. J. Clin. Lab. Investig. 1996, 56, 609–614. [Google Scholar] [CrossRef]
- Dusci, L.J.; Hackett, L.P.; Dusci, L.J.; Hackett, L.P. Simultaneous Determination of Clobazam, N-Desmethyl Clobazam and Clonazepam in Plasma by High Performance Liquid Chromatography. Ther. Drug Monit. 1987, 9, 113–116. [Google Scholar] [CrossRef]
- Brachet-Liermain, A.; Jarry, C.; Faure, O.; Guyot, M.; Loiseau, P. Liquid Chromatography Determination of Clobazam and Its Major Metabolite N-Desmethylclobazam in Human Plasma. Ther. Drug Monit. 1982, 4, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Zilli, M.A.; Nisi, G. Simple and sensitive method for the determination of clobazam, clonazepam and nitrazepam in human serum by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1986, 378, 492–497. [Google Scholar] [CrossRef]
- Tolbert, D.; Larsen, F. A Comprehensive Overview of the Clinical Pharmacokinetics of Clobazam. J. Clin. Pharmacol. 2019, 59, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Schulz, M.; Schmoldt, A. Therapeutic and toxic blood concentrations of more than 500 drugs. Die Pharm. 1997, 52, 895–911. [Google Scholar]

| Concentration (ng/mL) | Observed Concentration (ng/mL)—Mean ± SD | CV (%) | Percent Error | |
|---|---|---|---|---|
| Inter-day (n = 19) | 20 | 19.2 ± 3.0 | 15.4 | −4.5 |
| 60 | 55.9 ± 6.4 | 11.4 | −6.8 | |
| 150 | 151.5 ± 8.3 | 5.4 | 1.0 | |
| 400 | 388.5 ± 32.6 | 8.4 | −2.9 | |
| Intra-day (n = 5) | 20 | 18.6 ± 1.1 | 6.1 | −6.9 |
| 60 | 65.5 ± 5.1 | 7.8 | 9.2 | |
| 150 | 159.6 ± 5.8 | 3.7 | 5.5 | |
| 400 | 401.1 ± 9.5 | 2.4 | 0.3 |
| Concentration (ng/mL) | Observed Concentration (ng/mL)—Mean ± SD | CV (%) | Percent Error | |
|---|---|---|---|---|
| Inter-day (n = 15) | 200 | 201.3 ± 9.5 | 4.7 | 0.7 |
| 600 | 564.8 ± 16.3 | 2.9 | −5.9 | |
| 1600 | 1567.0 ± 56.7 | 3.6 | −2.1 | |
| 2500 | 2456.9 ± 132.4 | 5.4 | −1.7 | |
| Intra-day (n = 5) | 200 | 189.7 ± 5.7 | 3.0 | −5.2 |
| 600 | 546.5 ± 29.6 | 5.4 | −8.9 | |
| 1600 | 1517.6 ± 58.4 | 3.8 | −5.1 | |
| 2500 | 2526.6 ± 90.6 | 3.6 | 1.1 |
| Drug | Concentration (ng/mL) | Recovery, Mean ± SD (%) | CV (%) |
|---|---|---|---|
| CLB | 60 | 95.7 ± 5.2 (n = 3) | 5.4 |
| 150 | 98.0 ± 3.8 (n = 3) | 3.8 | |
| 400 | 97.7 ± 1.5 (n = 3) | 1.6 | |
| NDMCLB | 600 | 102.1 ± 1.2 (n = 3) | 1.2 |
| 1600 | 92.5 ± 1.9 (n = 3) | 2.1 | |
| 2500 | 96.6 ± 5.8 (n = 3) | 6.0 | |
| Diazepam | 500 | 102.3 ± 1.7 (n = 3) | 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isse, F.A.; Mahmoud, S.H. Determination of Clobazam and Its Major Metabolite N-desmethylclobazam in Human Plasma with High-Performance Liquid Chromatography. Analytica 2021, 2, 57-65. https://doi.org/10.3390/analytica2030007
Isse FA, Mahmoud SH. Determination of Clobazam and Its Major Metabolite N-desmethylclobazam in Human Plasma with High-Performance Liquid Chromatography. Analytica. 2021; 2(3):57-65. https://doi.org/10.3390/analytica2030007
Chicago/Turabian StyleIsse, Fadumo Ahmed, and Sherif Hanafy Mahmoud. 2021. "Determination of Clobazam and Its Major Metabolite N-desmethylclobazam in Human Plasma with High-Performance Liquid Chromatography" Analytica 2, no. 3: 57-65. https://doi.org/10.3390/analytica2030007
APA StyleIsse, F. A., & Mahmoud, S. H. (2021). Determination of Clobazam and Its Major Metabolite N-desmethylclobazam in Human Plasma with High-Performance Liquid Chromatography. Analytica, 2(3), 57-65. https://doi.org/10.3390/analytica2030007






