Abstract
Currently, the challenges that analytical chemistry has to face are ever greater and more complex both from the point of view of the selectivity of analytical methods and their sensitivity. This is especially true in quantitative analysis, where various methods must include the development and validation of new materials, strategies, and procedures to meet the growing need for rapid, sensitive, selective, and green methods. In this context, given the International Guidelines, which over time, are updated and which set up increasingly stringent “limits”, constant innovation is required both in the pre-treatment procedures and in the instrumental configurations to obtain reliable, accurate, and reproducible information. In addition, the environmental field certainly represents the greatest challenge, as analytes are often present at trace and ultra-trace levels. These samples containing analytes at ultra-low concentration levels, therefore, require very labor-intensive sample preparation procedures and involve the high consumption of organic solvents that may not be considered “green”. In the literature, in recent years, there has been a strong development of increasingly high-performing sample preparation techniques, often “solvent-free”, as well as the development of hyphenated instrumental configurations that allow for reaching previously unimaginable levels of sensitivity. This review aims to provide an update of the most recent developments currently in use in sample pre-treatment and instrument configurations in the environmental field, also evaluating the role and future developments of analytical chemistry in light of upcoming challenges and new goals yet to be achieved.
1. Introduction
In recent years, the increase in production activities in all industrial sectors has led to an increase in the production and consumption of xenobiotic compounds [1]. Specifically, their extensive use has led to the pollution of the environment as a major consequence [2], a fact that represents a direct threat to human health. In this perspective, the Stockholm Convention aims to protect human health and the environment from all activities that produce (and often release in an illegal and/or uncontrolled way) persistent organic pollutants (POPs), defined based on four criteria: persistence for a long time, toxicity, bioaccumulation, and wide geographical distribution [3].
Along with POPs, another major problem linked to the widespread use of plastic products has recently emerged. The presence of plastic particles in various environmental compartments (water, soil, and air) [4], even of micrometric dimensions (microplastics, MP, with dimensions < 1–5 mm) as part of industrial or domestic products (scrub, peelings, toothpastes, and make-up), and other products deriving from degradation/fragmentation (secondary microplastics) has reached such a level that considerable concentrations of these compounds have been found even in remote places such as Antarctica [5], the Arctic [6], or the deep ocean [7].
This large diffusion is essentially attributable to the fact that the smaller the particle size, the more these particles spread in the environment [8]. Another fundamental problem related to these MPs is that they are able to retain chemical substances on the surface, especially persistent organic contaminants such as polycyclic aromatic hydrocarbons (PAHs), polychlorinated biphenyls (PCBs), organochloride pesticides (OCPs), and polybrominated diphenyl ethers (PBDE) [9,10,11]. These MPs, therefore, act as a sort of pre-concentrator and modify their interactions and their toxicity in living organisms [12,13].
Among the previous classes, PAHs have been well known for some time and have been reported for decades as the main harmful compounds. Since the 1970s, a list of priority pollutants has been drawn up, leading to the identification of 16 PAH Priority Pollutants by the U.S. EPA [14]. This list does not take into account non-polar high-molecular-weight (HMW) PAHs, alkylated PAHs, or polar polycyclic aromatic compounds (PACs) [15]. In the category of polar PACs, it is mandatory to consider nitrate PAHs (NPAH) and oxygenated PAHs (OPAH), which currently, are the most studied [16,17,18] as their formation can result from reactions between “parent” PAHs and oxidizing agents in the environment. In addition to NPAHs and OPAHs, other polar PACs such as azaarenes (AZA, also referred to as polycyclic aromatic nitrogen heterocycles (PANH)) and polycyclic aromatic sulfur heterocycles (PASH) should also be considered. These compounds, although similar to PAH, NPAH, and OPAH in terms of sources and toxicology [19,20,21], are mainly related to petrogenic emissions [22,23]. It should be emphasized that all of the compounds reported are being studied by IARC (International Agency for Research on Cancer) and some of them have been classified as possible or probable carcinogens [24]. Regarding this category of pollutants, the very complete review paper by Galmiche et al. [25] appears to be very interesting.
Heavy metals also deserve particular attention, which as widely reported in the literature [26,27], not only represent an environmental problem for their presence in soils; superficial, deep, and sea waters; and food (and food supplements and herbal medicines), especially related to the fact that many of them do not have set maximum legal limits and/or are not considered as elements to be monitored and quantified.
As reported by McGregor and Zhao [28], trichlorethylene (TCE), cis-1,2-dichloroethene (cis-1,2-DCE), and vinyl chloride together with per- and polyfluoroalkyl substances (PFAS) have recently been identified as substances of concern in groundwater. Many of these compounds have also been confirmed as carcinogens or suspected carcinogens and are, therefore, of interest at the level of environmental monitoring. Although there is a variety of technologies for the treatment of chlorinated ethenes, technologies for treating PFAS in groundwater are not widespread and/or developed.
In particular, the per- and polyfluorinated alkyl substances (PFAS) are a group of highly stable and degradation-resistant anthropogenic chemicals. These are produced and used in many consumer and industrial products (e.g., food packaging, fire-fighting foams, and textiles) due to their heat-resistant and water-repellent properties. PFAS compounds are persistent, toxic, and potentially harmful to humans, and the leaching and presence of PFAS in our environment have raised serious concerns at a global scale. Exposure to PFAS through drinking water was investigated, leading the European Parliament in December 2020 to adopt the revised Drinking Water Directive (DWD), effective from 12 January 2021, including the “total PFAS” parameter (all per- and polyfluoroalkyl substances limited to a maximum concentration of 0.5 μg/L).
Regarding the analysis by Shimadzu, the application note is very interesting, providing a direct injection analysis of organofluorine compounds (PFAS) by triple–quadrupole LC-MS/MS [29], although to date, the panorama of pre-treatment procedures is still very limited and not very widespread. With regard to this aspect, the recent review by Winchell and collaborators [30] highlights how much has been developed for the analysis of these analytes and how much still remains to be developed, not only at the monitoring level but also at the level of removal processes. This point highlights that their analysis is very difficult as their advisory limits are in sub ppb to sub ppt levels, showing the emerging challenges to analytical chemists.
In light of what we have seen so far and what is reported in Figure 1, the massive problem inherent in the environmental monitoring of molecules that are harmful (or potentially harmful) both for the environment and for all living organisms is clearly highlighted.
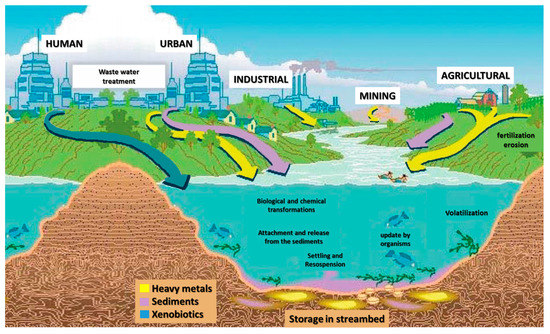
Figure 1.
Connections and sources of xenobiotics that are poured into the environment as result of human activities.
All of these characteristics have been recently reviewed in the literature [31,32], highlighting the great interest in the area of analytical chemistry and the challenges that await this discipline in the near future. In this scenario, the main role of scientists is to check, follow, and resolve the negative impacts of chemical and biological pollutants on people’s health to protect the supply of clean drinking water, thereby managing their effects on the ecosystems and environment. It should be noted that, in recent years, different factors have been taken into consideration in the development of new instrumental configurations and/or new analysis procedures, in particular, the possibilities of performing miniaturized measurements using low-priced and portable sensors for monitoring biological and chemical pollutants in environmental samples, being easy to use, and having low environmental impact (fundamental concept of green analytical chemistry).
The purpose of this review, as a sort of second “act” to a previously published review [33,34], is to highlight the recent applications developed in the environmental field and aimed at the xenobiotics, POPs, pesticides, heavy metals, and other pollutants monitoring, and how these can have positive repercussions on future applications, both from the point of view of pretreatment procedures and instrumental configurations (and portable devices).
2. Pre-Treatment Procedures
The step following sampling and relative to the treatment of the sample in order to make it suitable for instrumental analysis is a crucial point in practically all analytical methods, especially if the concentration of the analytes of interest is extremely low. Another critical element is the presence of interfering substances often present at high concentrations that can lead to matrix effects and problems related to the correct identification and quantification of the analytes [35]. For an accurate (precise and true) and sensitive determination of the elements present at the level of traces and ultra-traces, a pre-concentration phase is normally required before their detection using different instrumental techniques [36]. In this phase, in addition to being an enormous waste of time, there is the risk of not only obtaining an increase in the analyte signal but also the sample treatment procedure not being carried out correctly: an increase in interferents may be encountered with consequent problems in the instrumental analysis phase.
Another very important component in this phase is the use of solvents of various type, especially organic, that can be toxic and high volatile when used in the classic liquid–liquid extraction (LLE) or solid phase extraction (SPE) techniques for the preconcentration of trace elements prior to total or speciation analysis. Modern analytical methods are increasingly focused on the development of simple, fast, sensitive, and environmentally friendly practices that follow the principles of green chemistry (e.g., replacement of toxic reagents, miniaturization and automation, and development of solvent-free procedures) [37].
The development of innovative procedures and materials reflect the actual trend in sample preparation field for analytical applications. Specifically, currently, the application of nanomaterials has become a valuable tool for sample preparation methods, especially in SPE, leading to many advantages, especially with regard to their high surface-volume ratio, chemical stability, and the possibility of functionalization of the sample surface in order to increase selectivity [38]. In this scenario, certainly, the development of new materials, also based on the molecularly imprinted polymer concept, allow for obtaining high performances in terms of sample clean up and interference removal. Coupled with these materials, often based on polymer technologies, the implementation of alternative solvents supported on solid nanomaterials can be considered a further interesting approach (and development) to providing a high surface area by increasing their extraction efficiency [39], with a reduction in solvents/sample volume consumption.
Recently, Oviedo et al. [40] reported an exhaustive and complete overview of the preconcentration and determination of trace elements using alternative solvent systems such as ionic liquids (IL), surfactants, deep eutectic solvent (DES), as well as their combinations with new nanomaterials, summarizing and underlining the progress observed from 2017 to date in the extraction and clean-up procedures with the use of these green solvents.
In Table 1, different and more recent developments related to extraction methods are reported.

Table 1.
Most key developments in extraction methods.
As indicated in Table 1, it is noted that recent literature and scientific research is directed, above all, towards pre-treatment systems based on techniques that exploit the interaction between membranes or chemically functionalized particles in order to maximize the selectivity of the method, while still few are the applications that resort to liquid phases with green solvents (ionic liquids) [40,46,47,48,53].
It should be emphasized that, among the membrane-based techniques, FPSE has recently proved to be a very promising technique as it allows for not only the pre-treatment of a sample but also its conservation for subsequent confirmatory analyses. This technique is also very promising as it has been shown to have analytical performances that allow for the analysis with dated, robust, and well-known instrumental configurations (HPLC-PDA) of xenobiotic traces in complex matrices, obtaining quantification limits comparable with well-known configurations with respect to more complex and expensive and requiring trained personnel (for example, LC interfaced with mass spectrometry). These techniques are also easy to perform since, once the membrane has been activated, it is sufficient to proceed after optimization of the main parameters (sample volume, extraction and back-extraction time, solvent, and back-extraction volume) with sampling and the subsequent instrumental analysis, as highlighted in the literature [54,55].
Currently, only techniques based on solvents and/or functionalized particles have been applied in the analysis of heavy metals, opening up areas of research in the field of membranes capable of selectively adsorbing these analytes for environmental applications.
3. Instrumental Configurations and New Materials
Concurrent with the development of the new sample treatment procedures, new materials, and new solvent systems presented above, there has also been the development of new instrumental configurations and new techniques for quantitative measurements. These elements, often combined in order to minimize the manipulation of the original sample (to minimize errors and analyte losses), have recently improved with the development of new carbon-based nanomaterials such as diamond, graphene, amorphous carbon, C60 fullerene, carbon nanotubes, carbon dots (CD), and materials for organic structures that allowed for the simultaneous extraction of the analyte and its fluorimetric detection [56,57,58,59,60]. Such systems could directly or indirectly recognize target analytes by generating fluorescence signals (FRET, IFE, and electron transfer mechanism) [61,62,63] in a single system and simultaneously. At present, however, the structural integrity, chemical/thermal stability, and surface microenvironment of carbon nanomaterials, which greatly influence their practical applications and signal intensity, still need to be “resolved” and/or standardized in order to make the procedure maximally reproducible.
It should be emphasized that, in addition to carbon nanomaterials, other materials for fluorescence (e.g., quantum dots of semiconductors, metallic nanoclusters, and luminescent materials of higher conversion) have seen wide application in the analysis of pesticides through integration with recognition units in the benchtop or portable configuration [64,65] that exploit the use of new sensors [41]. Metal–organic frameworks are a versatile and remarkable class of crystalline functional materials with many unique features and huge potential for numerous applications. There are four main categories of luminescent MOFs: lanthanide-based MOFs, transition metal-based MOFs, heterometal–organic frameworks, and main group metal–organic frameworks. In the field of environmental pollutant analysis, LMOFs have shown their potential as suitable candidates for detecting temperature, pH, nitroaromatic explosives, small molecules, metallic cations, as well as inorganic anions.
Currently, in this scenario, luminescent metal–organic structures (LMOFs) represent an important sub-category of MOFs in which photon emission occurs following the absorption of radiative excitation energy with great potential for practical applications [66,67]. In fact, LMOFs have proven to be a unique basis for chemical detection thanks to their characteristics such as the size, shape, chemical composition, and surface specifications of the pores, which can be finely controlled [68,69]. These elements are reflected in the fact that porosity allows for the adsorption of molecules, increases interactions, preconcentrates the target molecule [69,70], and consequently allows for an increase in sensitivity.
The possibility of monitoring the level of environmental pollution in real time using selective and sensitive techniques is the main objective today in light of a growing introduction of polluting compounds due to anthropogenic activity. There are indeed a large number of organic pollutants and metal ions in water and soil, and there are many volatile organic chemicals in the atmosphere, which pose a serious threat not only to the environment but also, especially, to human health. Many organic molecules show high toxicity values and are able to influence physiological and pathophysiological functions [71,72] to such an extent that their rapid and sensitive detection is of great importance.
At present, the main pollutant detection methods include ultraviolet (UV) spectrophotometry techniques and methods [73], high-performance liquid chromatography (HPLC) [74] coupled with various types of detectors (especially mass spectrometry, MS), Fourier transform infrared spectroscopy (FTIR) [75], near infrared spectroscopy (NIR) [76], and surface-enhanced Raman spectroscopy (SERS) [77]. All of the configurations indicated show high sensitivity and accuracy, but often. the high purchase/maintenance cost and the lack of a “portability” feature limit their practical application.
Surely, the optical detection method, thanks to its high sensitivity, portability, short response times, and low cost, represents the most used configuration in the field of environmental pollutants detection, especially through procedures that increase the luminescence (“ignition”), switch off the luminescence (“switching off”), and measure the luminescence ratio [69,78].
With regard to the development of new devices, currently, analytical chemistry increasingly uses three-dimensional (3D) printing [79], especially for the production of microfluidic systems, electrochemical sensors, and biosensors, as well as the production of instruments applied in the fields of separation sciences, sample pretreatment, and wearable sensors [80,81]. Compared with traditional techniques, microfluidic techniques have many advantages, including the low cost and small footprint that allow for their portability, high analysis speed, reduced consumption of sample and solvents, and integration of various components [82,83].
Regarding microfluidic systems, the application proposed by Li et al. is very interesting. Using a system in a Y-shaped configuration with colorimetric detection, they determined the content of iron in water [84] or nitrates in soil [85]. Mattio et al. developed a similar device in 3D using poly (methyl methacrylate) (PMMA), improving the portability, simplicity, and low-cost determination of lead in natural water [86]. SLA 3D printing has also been employed for the construction of a fourth-generation microflow injection analysis system for metal and glucose trace detection in complex samples and other applications [87], while Fornells et al. exploited different materials integrated on a 3D-printed microfluidic device for the detection of ammonium in ambient water samples [88].
In Table 2, the different key developments in instrument configurations applied in environmental analyses are reported.

Table 2.
List of key developments in instrument configurations.
In this panorama, it should also be highlighted how, in the environmental field, instrumental configurations that resort to electrochemical (bio) sensors based on carbon cloth and carbon paper are increasingly used, combining their characteristics such as their interesting features and their highly efficient, versatile, and disposable design for biomolecules, biomarkers, and hazardous and chemical compound detection in environmental samples. The advantage of an easy integration in miniaturized and portable devices envisages their successful application in point-of-care diagnostics and in situ measurements through the development of portable, space-saving, and high-performance devices in terms of sensitivity and specificity [96,97].
The improvement at the level of the analytical parameters related to the detection of various water pollutants in different ecosystems and/or remote locations is strongly correlated with the development of convenient, portable, simple, and sensitive systems that are easy to use even by untrained personnel. In this context, the possibility of coupling portable devices for measurement (based on sensors) with an efficient and easy-to-use platform, such as a smartphone, have opened a very important line of research for the development of configurations and procedures for in situ analysis [98,99]. Exploiting mobile applications and cameras with very good performance and light sensors, many fascinating biosensors have been developed based on smartphones [100,101,102,103,104,105].
Regarding the techniques/processes based on (bio)sensors, fundamental points in their study and development are based on (i) evaluating the different analytical signals that can be used for quantitative purposes by means of different electrochemical and optical sensors, (ii) developing different molecularly imprinted probes to increase the selectivity of the procedure, and (iii) exploiting the possibility of nanomaterials being incorporated into and used in portable sensors.
4. Conclusions
In this article, only the most recent developments in the field of environmental analysis that have led to a rapid increase in the application potential of analytical techniques in this sector have been reported. It should be noted that many developments observed in a specific application field are, however, used as ideas for other sectors, and this leads to a greater choice of procedures that can be applied to analytical problems that are often very “distant” from the original intent. Specifically, however, it can be observed that, in recent years, more and more attention has been paid to all of the techniques that allow for a reduction in the impact of human activities on the environment and to the development of green procedures and of devices for in situ analyzes.
As depicted in Figure 1, the close connection between the environment, human activity and health is even more evident. These are sectors that, even if at first glance could be limited in their “boundaries”, in reality involve strong reciprocal influences linked to the life cycle of the molecules and their degradation/metabolism processes. It can be fairly concluded that it is possible to apply, after appropriate verification of the analytical performance (and in this case, a new validation process in the new matrix), methods created for different purposes. If we combine this element with the continuous interest in green and high throughput procedures, we can understand how analytical chemistry plays a predominant role in all fields where chemical processes are involved.
Author Contributions
Writing: A.K., H.I.U., G.M.M., I.A., F.S. (Francesco Santavenere), M.B., S.R. and E.R.; conceptualization, supervision, writing and project administration: A.K., H.I.U., I.A., F.S. (Fabio Savini), M.L., C.D. and U.d.G. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was obtained for this review paper.
Institutional Review Board Statement
No Institutional Review Board was necessary for this review paper.
Informed Consent Statement
No Informed Consent was necessary for this review paper.
Data Availability Statement
Data are available to the Authors.
Conflicts of Interest
The authors declare that there are no conflicts of interests.
References
- Badea, S.L.; Geana, E.I.; Niculescu, V.C.; Ionete, R.E. Recent progresses in analytical GC and LC mass spectrometric based-methods for the detection of emerging chlorinated and brominated contaminants and their transformation products in aquatic environment. Sci. Total Environ. 2020, 722, 137914. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Bouwman, H.; Uren, R.C.; van der Lingen, C.D.; Vetter, W. Halogenated natural products and anthropogenic persistent organic pollutants in chokka squid (Loligo reynaudii) from three sites along the South Atlantic and Indian Ocean coasts of South Africa. Environ. Pollut. 2019, 255, 113282. [Google Scholar] [CrossRef] [PubMed]
- Cariou, R.; Omer, E.; Leon, A.; Dervilly-Pinel, G.; Le Bizec, B. Screening halogenated environmental contaminants in biota based on isotopic pattern and mass defect provided by high resolution mass spectrometry profiling. Anal. Chim. Acta 2016, 936, 130–138. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, S.; Allen, S.; Allen, D.; Gao, T.; Sillanpaa, M. Atmospheric microplastics: A review on current status and perspectives. Earth Sci. Rev. 2020, 203, 103118. [Google Scholar] [CrossRef]
- Waller, C.L.; Griffiths, H.J.; Waluda, C.M.; Thorpe, S.E.; Loaiza, I.; Moreno, B.; Pacherres, C.O.; Hughes, K.A. Microplastics in the Antarctic marine system: An emerging area of research. Sci. Total Environ. 2017, 598, 220–227. [Google Scholar] [CrossRef]
- Gonzalez-Pleiter, M.; Velazquez, D.; Edo, C.; Carretero, O.; Gago, J.; Baron-Sola, A.; Hernandez, L.E.; Yousef, I.; Quesada, A.; Leganes, F.; et al. Fibers spreading worldwide: Microplastics and other anthropogenic litter in an Arctic freshwater lake. Sci. Total Environ. 2020, 722, 137904. [Google Scholar] [CrossRef]
- Courtene-Jones, W.; Quinn, B.; Gary, S.F.; Mogg, A.O.M.; Narayanaswamy, B.E. Microplastic pollution identified in deep-sea water and ingested by benthic invertebrates in the Rockall Trough, North Atlantic Ocean. Environ. Pollut. 2017, 231, 271–280. [Google Scholar] [CrossRef]
- Huang, Y.; Qing, X.; Wang, W.; Han, G.; Wang, J. Mini-review on current studies of airborne microplastics: Analytical methods, occurrence, sources, fate and potential risk to human beings. TrAC 2020, 125, 115821. [Google Scholar] [CrossRef]
- Tourinho, P.S.; Kocí, V.; Loureiro, S.; van Gestel, C.A.M. Partitioning of chemical contaminants to microplastics: Sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation. Environ. Pollut. 2019, 252, 1246–1256. [Google Scholar] [CrossRef]
- Rodrigues, J.P.; Duarte, A.C.; Santos-Echeandía, J.; Rocha-Santos, T. Significance of interactions between microplastics and POPs in the marine environment: A critical overview. TrAC 2019, 111, 252–260. [Google Scholar] [CrossRef]
- Hüffer, T.; Wagner, S.; Reemtsma, T.; Hofmann, T. Sorption of organic substances to tire wear materials: Similarities and differences with other types of microplastic. TrAC 2019, 113, 392–401. [Google Scholar] [CrossRef]
- Bhagat, J.; Nishimura, N.; Shimada, Y. Toxicological interactions of microplastics/nanoplastics and environmental contaminants: Current knowledge and future perspectives. J. Hazard Mater. 2020, 405, 123913. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Skrzypek, G.; Hernandez-Sanchez, C.; Ortega-Zamora, C.; Gonzalez-Salamo, J.; Gonzalez-Curbelo, M.A.; Hernandez-Borges, J. Microplastic-adsorbed organic contaminants: Analytical methods and occurrence. TrAC 2021, 136, 116186. [Google Scholar] [CrossRef]
- Keith, L.H. The source of U.S. EPA’s sixteen PAH priority pollutants. Polycycl. Aromat. Comp. 2015, 35, 147–160. [Google Scholar] [CrossRef]
- Andersson, J.T.; Achten, C. Time to say goodbye to the 16 EPA PAHs? Toward an up-to-date use of PACs for environmental purposes. Polycycl. Aromat. Comp. 2015, 35, 330–354. [Google Scholar] [CrossRef]
- Bandowe, B.A.M.; Meusel, H. Nitrated polycyclic aromatic hydrocarbons (nitro-PAHs) in the environment e a review. Sci. Total Environ. 2017, 581–582, 237–257. [Google Scholar] [CrossRef]
- Lammel, G. Polycyclic aromatic compounds in the atmosphere e a review identifying research needs. Polycycl. Aromat. Comp. 2015, 35, 316–329. [Google Scholar] [CrossRef]
- Walgraeve, C.; Demeestere, K.; Dewulf, J.; Zimmermann, R.; Van Langenhove, H. Oxygenated polycyclic aromatic hydrocarbons in atmospheric particulate matter: Molecular characterization and occurrence. Atmos. Environ. 2010, 44, 1831–1846. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Preston, M.R. Azaarenes in the aerosol of an urban atmosphere. Environ. Sci. Technol. 1998, 32, 577–583. [Google Scholar] [CrossRef]
- Collins, J.F.; Brown, J.P.; Alexeeff, G.V.; Salmon, A.G. Potency equivalency factors for some polycyclic aromatic hydrocarbons and polycyclic aromatic hydrocarbon derivatives. Regul. Toxicol. Pharmacol. 1998, 28, 45–54. [Google Scholar] [CrossRef]
- Delhomme, O.; Millet, M. Azaarenes in atmospheric particulate matter samples of three different urban sites in east of France. Atmos. Environ. 2012, 47, 541–545. [Google Scholar] [CrossRef]
- Lee, M.L.; Novotny, M.; Bartle, K.D. Gas chromatography/mass spectrometric and nuclear magnetic resonance determination of polynuclear aromatic hydrocarbons in airborne particulates. Anal. Chem. 1976, 48, 1566–1572. [Google Scholar] [CrossRef]
- Mossner, S.G.; Wise, S.A. Determination of polycyclic aromatic sulfur heterocycles in fossil fuel-related samples. Anal. Chem. 1999, 71, 58–69. [Google Scholar] [CrossRef] [PubMed]
- IARC, Bitumens and Bitumen Emissions, and Some N- and S-Heterocyclic Polycyclic Aromatic Hydrocarbons. International Agency for Research on Cancer, Lyon, 2013. Available online: https://www.ncbi.nlm.nih.gov/books/NBK294234/ (accessed on 22 August 2022).
- Galmiche, M.; Delhomme, O.; François, Y.-N.; Millet, M. Environmental analysis of polar and non-polar Polycyclic Aromatic Compounds in airborne particulate matter, settled dust and soot: Part II: Instrumental analysis and occurrence. TrAC 2021, 134, 116146. [Google Scholar] [CrossRef]
- Melucci, D.; Locatelli, M.; Casolari, S.; Locatelli, C. New polluting metals. Quantification in herbal medicines by voltammetric and spectroscopic analytical methods. J. Pharm. Biomed. Anal. 2022, 211, 114599. [Google Scholar] [CrossRef] [PubMed]
- Melucci, D.; Casolari, S.; Locatelli, M.; Locatelli, C. Thallium: A polluting metal of new generation. Its voltammetric determination in herbal medicines in the presence of metal interferences. Analytica 2021, 2, 76–83. [Google Scholar] [CrossRef]
- McGregor, R.; Zhao, Y. The in situ treatment of TCE and PFAS in groundwater within a silty sand aquifer. Remediation 2021, 31, 7–17. [Google Scholar] [CrossRef]
- Shimadzu, Application Note No. C225. Available online: https://www.shimadzu.it/sites/shimadzu.seg/files/SEG-images/industries/food/PFAS/AppNote_PFAS_LCMS_8060_C225.pdf (accessed on 23 August 2022).
- Winchell, L.J.; Wells, M.J.M.; Ross, J.J.; Fonoll, X.; Norton, J.W., Jr.; Kuplicki, S.; Khan, M.; Bell, K.Y. Analyzes of per- and polyfluoroalkyl substances (PFAS) through the urban water cycle: Toward achieving an integrated analytical workflow across aqueous, solid, and gaseous matrices in water and wastewater treatment. Sci. Total Environ. 2021, 774, 145257. [Google Scholar] [CrossRef]
- Alygizakis, N.; Markou, A.N.; Rousis, N.I.; Galani, A.; Avgeris, M.; Adamopoulos, P.G.; Scorilas, A.; Lianidou, E.S.; Paraskevis, D.; Tsiodras, S.; et al. Analytical methodologies for the detection of SARS-CoV-2 in wastewater: Protocols and future perspectives. TrAC 2021, 134, 116125. [Google Scholar] [CrossRef]
- Gul, I.; Le, W.; Jie, Z.; Ruiqin, F.; Bilal, M.; Tang, L. Recent advances on engineered enzyme-conjugated biosensing modalities and devices for halogenated compounds. TrAC 2021, 134, 116145. [Google Scholar] [CrossRef]
- Merone, G.M.; Tartaglia, A.; Locatelli, M.; D’Ovidio, C.; Rosato, E.; de Grazia, U.; Santavenere, F.; Rossi, S.; Savini, F. Analytical Chemistry in the 21st Century: Challenges, Solutions, and Future Perspectives of Complex Matrices Quantitative Analyses in Biological/Clinical Field. Analytica 2020, 1, 44–59. [Google Scholar] [CrossRef]
- Kabir, A.; Locatelli, M.; Ulusoy, H.I. Recent Trends in Microextraction Techniques Employed in Analytical and Bioanalytical Sample Preparation. Separations 2017, 4, 36. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Mohebbi, A.; Pazhohan, A.; Nemati, M.; Mogaddam, M.R.A. Air-assisted liquid-liquid microextraction; principles and applications with analytical instruments. TrAC 2020, 122, 115734. [Google Scholar] [CrossRef]
- Fiorentini, E.F.; Escudero, L.B.; Wuilloud, R.G. Magnetic ionic liquid-based dispersive liquid-liquid microextraction technique for preconcentration and ultra-trace determination of Cd in honey. Anal. Bioanal. Chem. 2018, 410, 4715–4723. [Google Scholar] [CrossRef] [PubMed]
- Makos, P.; Słupek, E.; Gebicki, J. Hydrophobic deep eutectic solvents in microextraction techniques—A review. Microchem. J. 2020, 152, 104384. [Google Scholar] [CrossRef]
- Martinis, E.M.; Grijalba, A.C.; Perez, M.B.; Llaver, M.; Wuilloud, R.G. Synergistic analytical preconcentration with ionic liquid enanomaterial hybrids. TrAC 2017, 97, 333–344. [Google Scholar] [CrossRef]
- Sotolongo, A.C.; Messina, M.M.; Ibanez, F.J.; Wuilloud, R.G. Hybrid ionic liquid- 3D graphene-Ni foam for on-line preconcentration and separation of Hg species in water with atomic fluorescence spectrometry detection. Talanta 2020, 210, 120614. [Google Scholar] [CrossRef]
- Oviedo, M.N.; Fiorentini, E.F.; Llaver, M.; Wuilloud, R.G. Alternative solvent systems for extraction and preconcentration of trace elements. TrAC 2021, 137, 116227. [Google Scholar] [CrossRef]
- Su, D.; Li, H.; Yan, X.; Lin, Y.; Lu, G. Biosensors based on fluorescence carbon nanomaterials for detection of pesticides. TrAC 2021, 134, 116126. [Google Scholar] [CrossRef]
- Belka, M.; Ulenberg, S.; Baczek, T. Fused deposition modeling enables the lowcost fabrication of porous, customized-shape sorbents for small-molecule extraction. Anal. Chem. 2017, 89, 4373–4376. [Google Scholar] [CrossRef]
- Su, C.K.; Chen, W.C. 3D-printed, TiO2 NP-incorporated mini column coupled with ICP-MS for speciation of inorganic arsenic and selenium in high-salt content samples. Mikrochim. Acta 2018, 185, 268. [Google Scholar] [CrossRef] [PubMed]
- Calderilla, C.; Maya, F.; Cerda, V.; Leal, L.O. Direct photoimmobilization of extraction disks on “green state” 3D printed devices. Talanta 2019, 202, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Calderilla, C.; Maya, F.; Cerda, V.; Leal, L.O. 3D printed device including diskbased solid-phase extraction for the automated speciation of iron using the multisyringe flow injection analysis technique. Talanta 2017, 175, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Cacho, J.I.; Campillo, N.; Vinas, P.; Hernández-Córdoba, M. In situ ionic liquid dispersive liquid-liquid microextraction coupled to gas chromatography-mass spectrometry for the determination of organophosphorus pesticides. J. Chromatogr. A 2018, 1559, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Nowak, I.; Rykowska, I.; Ziemblińska-Bernart, J. Orthogonal array design optimisation of an in situ ionic liquid dispersive liquid–liquid microextraction for the detection of phenol and endocrine-disrupting phenols in aqueous samples. J. Iran. Chem. Soc. 2020, 17, 825–838. [Google Scholar] [CrossRef]
- Hui, Y.; Xiong, C.; Bian, C.; Gui, S.; Tong, J.; Li, Y.; Gao, C.; Huang, Y.; Tang, W.C.; Xia, S. Temperature-controlled ionic liquid dispersive liquid–liquid microextraction combined with fluorescence detection of ultra-trace Hg2+ in water. Anal. Methods 2019, 11, 2669–2676. [Google Scholar] [CrossRef]
- Sarıkaya, M.; Ulusoy, H.I.; Morgul, U.; Ulusoy, S.; Tartaglia, A.; Yılmaz, E.; Soylak, M.; Locatelli, M.; Kabir, A. Sensitive determination of Fluoxetine and Citalopram antidepressants in urine and wastewater samples by liquid chromatography coupled with photodiode array detector. J. Chromatogr. A 2021, 1648, 462215. [Google Scholar] [CrossRef]
- Ulusoy, H.I.; Koseoglu, K.; Kabir, A.; Ulusoy, S.; Locatelli, M. Fabric phase sorptive extraction followed by HPLC-PDA detection for the monitoring of Pirimicarb and Fenitrothion pesticide residues. Microchim. Acta 2020, 187, 337. [Google Scholar] [CrossRef]
- Canlı, A.G.; Sürücü, B.; Ulusoy, H.I.; Yılmaz, E.; Kabir, A.; Locatelli, M. Analytical Methodology for Trace Determination of Propoxur and Fenitrothion Pesticide Residues by Decanoic Acid Modified Magnetic Nanoparticles. Molecules 2019, 24, 4621. [Google Scholar] [CrossRef]
- ALOthman, Z.A.; Badjah, A.Y.; Locatelli, M.; Ali, I. Multi-walled carbon nanotubes solid phase extraction and capillary electrophoresis methods for the analysis of 4-cyano- and 3-nitro-phenols in water. Molecules 2020, 25, 3893. [Google Scholar] [CrossRef]
- Orazbayeva, D.; Koziel, J.A.; Trujillo-Rodríguez, M.J.; Anderson, J.L.; Kenessov, B. Polymeric ionic liquid sorbent coatings in headspace solid-phase microextraction: A green sample preparation technique for the determination of pesticides in soil. Microchem. J. 2020, 157, 104996. [Google Scholar] [CrossRef]
- Kabir, A.; Mesa, R.; Jurmain, J.; Furton, K.G. Fabric Phase Sorptive Extraction Explained. Separations 2017, 4, 21. [Google Scholar] [CrossRef]
- Zilfidou, E.; Kabir, A.; Furton, K.G.; Samanidou, V. Fabric Phase Sorptive Extraction: Current State of the Art and Future Perspectives. Separations 2018, 53, 40. [Google Scholar] [CrossRef]
- Zhou, J.; Chizhik, A.I.; Chu, S.; Jin, D. Single-particle spectroscopy for functional nanomaterials. Nature 2020, 579, 41–50. [Google Scholar] [CrossRef]
- Aragay, G.; Pino, F.; Merkoci, A. Nanomaterials for sensing and destroying pesticides. Chem. Rev. 2012, 112, 5317–5338. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Talapaneni, S.N.; Joseph, S.; Ramadass, K.; Singh, G.; Scaranto, J.; Ravon, U.; Al-Bahily, K.; Vinu, A. Recent advances in functionalized micro and mesoporous carbon materials: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 2680–2721. [Google Scholar] [CrossRef]
- Clancy, A.J.; Bayazit, M.K.; Hodge, S.A.; Skipper, N.T.; Howard, C.A.; Shaffer, M.S.P. Charged carbon nanomaterials: Redox chemistries of fullerenes, carbon nanotubes, and graphenes. Chem. Rev. 2018, 118, 7363–7408. [Google Scholar] [CrossRef]
- Semeniuk, M.; Yi, Z.; Poursorkhabi, V.; Tjong, J.; Jaffer, S.; Lu, Z.H.; Sain, M. Future perspectives and review on organic carbon dots in electronic applications. ACS Nano 2019, 13, 6224–6255. [Google Scholar] [CrossRef]
- Wu, X.; Song, Y.; Yan, X.; Zhu, C.; Ma, Y.; Du, D.; Lin, Y. Carbon quantum dots as fluorescence resonance energy transfer sensors for organophosphate pesticides determination. Biosens. Bioelectron. 2017, 94, 292–297. [Google Scholar] [CrossRef]
- Li, H.; Sun, C.; Vijayaraghavan, R.; Zhou, F.; Zhang, X.; MacFarlane, D.R. Long lifetime photoluminescence in N, S co-doped carbon quantum dots from an ionic liquid and their applications in ultrasensitive detection of pesticides. Carbon 2016, 104, 33–39. [Google Scholar] [CrossRef]
- Huang, S.; Yao, J.; Chu, X.; Liu, Y.; Xiao, Q.; Zhang, Y. One-step facile synthesis of nitrogen-doped carbon dots: A ratiometric fluorescent probe for evaluation of acetylcholinesterase activity and detection of organophosphorus pesticides in tap water and food. J. Agric. Food Chem. 2019, 67, 11244–11255. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chen, L. Quantum dots coated with molecularly imprinted polymer as fluorescence probe for detection of cyphenothrin. Biosens. Bioelectron. 2015, 64, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhou, T.; Wang, Y.; Gong, L.; Liu, J. Transformation from gold nanoclusters to plasmonic nanoparticles: A general strategy towards selective detection of organophosphorothioate pesticides. Biosens. Bioelectron. 2018, 99, 274–280. [Google Scholar] [CrossRef]
- Zhao, S.N.; Wang, G.; Poelman, D.; Voort, P.V. Luminescent lanthanide MOFs: A unique platform for chemical sensing. Materials 2018, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Li, N.; Wang, D.; Fan, M.; Zhang, S.; Gong, Z. Environmental pollution analysis based on the luminescent metal organic frameworks: A review. TrAC 2021, 134, 116131. [Google Scholar] [CrossRef]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metaleorganic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metaleorganic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Yang, J.; Liu, F.; Dai, F.; Wang, R.; Sun, D. Lanthanide metaleorganic frameworks containing a novel flexible ligand for luminescence sensing of small organic molecules and selective adsorption. J. Mater. Chem. 2015, 3, 12777–12785. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, L.; Jiang, F.; Wu, M.; Pang, J.; Wan, X.; Hong, M. A water-stable 3D Eu-MOF based on a metallacyclodimeric secondary building unit for sensitive fluorescent detection of acetone molecules. CrystEngComm 2019, 21, 321–328. [Google Scholar] [CrossRef]
- Huelsmann, R.D.; Martendal, E. A simple and effective liquid-liquid-liquid microextraction method with ultraviolet spectrophotometric detection for the determination of bisphenol A in aqueous matrices and plastic leachates. J. Braz. Chem. Soc. 2020, 31, 1575–1584. [Google Scholar] [CrossRef]
- Lee, K.J.; Dabrowski, K. High-performance liquid chromatographic determination of gossypol and gossypolone enantiomers in fish tissues using simultaneous electrochemical and ultraviolet detectors. J. Chromatogr. B 2002, 779, 313–319. [Google Scholar] [CrossRef]
- Mirghani, M.E.S.; Man, Y.B.C. A new method for determining gossypol in cottonseed oil by FTIR spectroscopy. J. Am. Oil Chem. Soc. 2003, 80, 625–628. [Google Scholar] [CrossRef]
- Martinez, Y.M.; Munoz-Ortuno, M.; Herraez-Hernandez, R.; Campins-Falco, P. Rapid analysis of effluents generated by the dairy industry for fat determination by preconcentration in nylon membranes and attenuated total reflectance infrared spectroscopy measurement. Talanta 2014, 119, 11–16. [Google Scholar] [CrossRef]
- Emamian, S.; Eshkeiti, A.; Narakathu, B.B.; Avuthu, S.G.R.; Atashbar, M.Z. Gravure printed flexible surface enhanced Raman spectroscopy (SERS) substrate for detection of 2,4-dinitrotoluene (DNT) vapor. Sens. Actuators B Chem. 2015, 217, 129–135. [Google Scholar] [CrossRef]
- Hu, Z.; Deibert, B.J.; Li, J. Luminescent metal-organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef]
- Zhang, C.; Bills, B.J.; Manicke, N.E. Rapid prototyping using 3D printing in bioanalytical research. Bioanalysis 2017, 9, 329–331. [Google Scholar] [CrossRef]
- Zhang, Y.; Ge, S.; Yu, J. Chemical and biochemical analysis on lab-on-a-chip devices fabricated using three-dimensional printing. TrAC 2016, 85, 166–180. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Wang, L.; Pumera, M. Recent advances of 3D printing in analytical chemistry: Focus on microfluidic, separation, and extraction devices. TrAC 2021, 135, 116151. [Google Scholar] [CrossRef]
- Li, F.; Macdonald, N.P.; Guijt, R.M.; Breadmore, M.C. Using printing orientation for tuning fluidic behavior in microfluidic chips made by fused deposition modeling 3D printing. Anal. Chem. 2017, 89, 12805–12811. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Smejkal, P.; Macdonald, N.P.; Guijt, R.M.; Breadmore, M.C. One-step fabrication of a microfluidic device with an integrated membrane and embedded reagents by multimaterial 3D printing. Anal. Chem. 2017, 89, 4701–4707. [Google Scholar] [CrossRef] [PubMed]
- Mattio, E.; Robert-Peillard, F.; Branger, C.; Puzio, K.; Margaillan, A.; Brach-Papa, C.; Knoery, J.; Boudenne, J.L.; Coulomb, B. 3D-printed flow system for determination of lead in natural waters. Talanta 2017, 168, 298–302. [Google Scholar] [CrossRef]
- Cocovi-Solberg, D.J.; Rosende, M.; Michalec, M.; Miro, M. 3D Printing: The second dawn of lab-on-valve fluidic platforms for automatic (bio)chemical assays. Anal. Chem. 2019, 91, 1140–1149. [Google Scholar] [CrossRef]
- Fornells, E.; Murray, E.; Waheed, S.; Morrin, A.; Diamond, D.; Paull, B.; Breadmore, M. Integrated 3D printed heaters for microfluidic applications: Ammonium analysis within environmental water. Anal. Chim. Acta 2020, 1098, 94–101. [Google Scholar] [CrossRef]
- ALOthman, Z.A.; Alsheetan, K.M.; AL-Anazy, M.M.; Locatelli, M.; Ali, I. Metformin residue analysis in water by MWCNTs-based solid-phase micromembrane tip extraction and capillary electrophoresis methods. Int. J. Environ. Sci. Technol. 2021, 18, 3419–3426. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar] [CrossRef]
- Leeuw, T.; Boss, E.S.; Wright, D.L. In situ Measurements of Phytoplankton Fluorescence Using Low Cost Electronics. Sensors 2013, 13, 7872. [Google Scholar] [CrossRef] [Green Version]
- Saeed, A.A.; Singh, B.; Nooredeen Abbas, M.; Dempsey, E. Evaluation of bismuth modified carbon thread electrode for simultaneous and highly sensitive Cd (II) and Pb (II) determination. Electroanalysis 2016, 28, 2205–2213. [Google Scholar] [CrossRef]
- Liu, R.; Wang, Y.; Li, D.; Dong, L.; Li, B.; Liu, B.; Ma, H.; Li, F.; Yin, X.; Chen, X. A simple, low-cost and efficient β-CD/MWCNTs/CP-based electrochemical sensor for the Rapid and sensitive detection of methyl parathion. Int. J. Electrochem. Sci. 2019, 14, 9785–9795. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.; Wang, Y.; Ma, Y.; Sun, J.; Li, T.; Wang, J.; Yue, X.; Ouyang, S.; Ji, Y. High-performance electrochemical nitrite sensing enabled using commercial carbon fiber cloth. Inorg. Chem. Front. 2019, 6, 1501–1506. [Google Scholar] [CrossRef]
- Torrinha, A.; Morais, S. Electrochemical (bio)sensors based on carbon cloth and carbon paper: An overview. TrAC 2021, 142, 116324. [Google Scholar] [CrossRef]
- Sohrabi, H.; Hemmati, A.; Reza Majidi, M.; Eyvazi, S.; Jahanban-Esfahlan, A.; Baradaran, B.; Adlpour-Azar, R.; Mokhtarzadeh, A.; de la Guardia, M. Recent advances on portable sensing and biosensing assays applied for detection of main chemical and biological pollutant agents in water samples: A critical review. TrAC 2021, 143, 116344. [Google Scholar] [CrossRef]
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, A.; Luong, J.H. Emerging Technologies for Next-Generation Point-of-Care Testing. Trends Biotechnol. 2015, 33, 692–705. [Google Scholar] [CrossRef]
- Coskun, A.F.; Wong, J.; Khodadadi, D.; Nagi, R.; Tey, A.; Ozcan, A. A personalized food allergen testing platform on a cellphone. Lab Chip 2013, 13, 636–640. [Google Scholar] [CrossRef]
- Preechaburana, P.; Suska, A.; Filippini, D. Biosensing with cell phones. Trends Biotechnol. 2014, 32, 351–355. [Google Scholar] [CrossRef]
- Gopinath, S.C.; Tang, T.-H.; Chen, Y.; Citartan, M.; Lakshmipriya, T. Bacterial detection: From microscope to smartphone. Biosens. Bioelectron. 2014, 60, 332–342. [Google Scholar] [CrossRef]
- Erickson, D.; O’Dell, D.; Jiang, L.; Oncescu, V.; Gumus, A.; Lee, S.; Mancuso, M.; Mehta, S. Smartphone technology can be transformative to the deployment of lab-on-chip diagnostics. Lab Chip 2014, 14, 3159–3164. [Google Scholar] [CrossRef] [Green Version]
- Vashist, S.K.; van Oordt, T.; Schneider, E.M.; Zengerle, R.; von Stetten, F.; Luong, J.H. A smartphone-based colorimetric reader for bioanalytical applications using the screen-based bottom illumination provided by gadgets. Biosens. Bioelectron. 2015, 67, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jiang, J.; Chen, J.; Zhang, Q.; Lu, Y.; Yao, Y.; Li, S.; Liu, G.L.; Liu, Q. Smartphone-based portable biosensing system using impedance measurement with printed electrodes for 2,4,6-trinitrotoluene (TNT) detection. Biosens. Bioelectron. 2015, 70, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.-C.; Peng, J.; Mauk, M.G.; Awasthi, S.; Song, J.; Friedman, H.; Bau, H.H.; Liu, C. Smart Cup: A Minimally-Instrumented, Smartphone-Based Point-of-Care Molecular Diagnostic Device. Sens. Actuators B Chem. 2016, 229, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).