Assessment of Lycopene Levels in Dried Watermelon Pomace: A Sustainable Approach to Waste Reduction and Nutrient Valorization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Proximate Analysis
2.3. Lycopene Analysis
2.4. Statistical Analysis
2.5. Reagents and Standards
3. Results and Discussion
3.1. Proximate Analysis of DWP Samples
3.2. Lycopene Content of DWP Samples
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- UNEP. United Nations Environment Programme UNEP Food Waste Index Report; UNEP: Nairobi, Kenya, 2021. [Google Scholar]
- Un, C. A Sustainable Approach to the Conversion of Waste into Energy: Landfill Gas-to-Fuel Technology. Sustainability 2023, 15, 14782. [Google Scholar] [CrossRef]
- Manivannan, A.; Lee, E.-S.; Han, K.; Lee, H.-E.; Kim, D.-S. Versatile Nutraceutical Potentials of Watermelon—A Modest Fruit Loaded with Pharmaceutically Valuable Phytochemicals. Molecules 2020, 25, 5258. [Google Scholar] [CrossRef]
- Centre for the Promotion of Imports from Developing Countries (CBI). Exporting Fresh Melons to Europe; CBI—The Netherlands Ministry of Foreign Affairs: The Hague, The Netherlands, 2018; Available online: https://www.Cbi.Eu/Market-Information/Fresh-Fruit-Vegetables/Fresh-Melons (accessed on 10 March 2024).
- Arocho, Y.D.; Bellmer, D.; Maness, N.; McGlynn, W.; Rayas-Duarte, P. Watermelon Pomace Composition and the Effect of Drying and Storage on Lycopene Content and Color: Watermelon Pomace. J. Food Qual. 2012, 35, 331–340. [Google Scholar] [CrossRef]
- Fish, W.W.; Bruton, B.D.; Russo, V.M. Watermelon Juice: A Promising Feedstock Supplement, Diluent, and Nitrogen Supplement for Ethanol Biofuel Production. Biotechnol. Biofuels 2009, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Kumar, V.; Yadav, V.; Sarsaiya, S.; Awasthi, S.K.; Sindhu, R.; Binod, P.; Kumar, V.; Pandey, A.; Zhang, Z. Current State of the Art Biotechnological Strategies for Conversion of Watermelon Wastes Residues to Biopolymers Production: A Review. Chemosphere 2022, 290, 133310. [Google Scholar] [CrossRef] [PubMed]
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Aadil, R.M. An Update on Functional, Nutraceutical and Industrial Applications of Watermelon by-Products: A Comprehensive Review. Trends Food Sci. Technol. 2021, 114, 275–291. [Google Scholar] [CrossRef]
- Jahanbakhshi, A.; Salehi, R. Processing Watermelon Waste Using Saccharomyces Cerevisiae Yeast and the Fermentation Method for Bioethanol Production. J. Food Process Eng. 2019, 42, e13283. [Google Scholar] [CrossRef]
- Kassim, M.A.; Hussin, A.H.; Meng, T.K.; Kamaludin, R.; Zaki, M.S.I.M.; Zakaria, W.Z.E.W. Valorisation of Watermelon (Citrullus lanatus) Rind Waste into Bioethanol: An Optimization and Kinetic Studies. Int. J. Environ. Sci. Technol. 2022, 19, 2545–2558. [Google Scholar] [CrossRef]
- Naknaen, P.; Itthisoponkul, T.; Sondee, A.; Angsombat, N. Utilization of Watermelon Rind Waste as a Potential Source of Dietary Fiber to Improve Health Promoting Properties and Reduce Glycemic Index for Cookie Making. Food Sci. Biotechnol. 2016, 25, 415–424. [Google Scholar] [CrossRef]
- Al-Sayed, H.M.A.; Ahmed, A.R. Utilization of Watermelon Rinds and Sharlyn Melon Peels as a Natural Source of Dietary Fiber and Antioxidants in Cake. Ann. Agric. Sci. 2013, 58, 83–95. [Google Scholar] [CrossRef]
- Ho, L.-H.; Che Dahri, N. Effect of Watermelon Rind Powder on Physicochemical, Textural, and Sensory Properties of Wet Yellow Noodles. CyTA-J. Food 2016, 14, 465–472. [Google Scholar] [CrossRef]
- Maletti, L.; D’Eusanio, V.; Lancellotti, L.; Marchetti, A.; Pincelli, L.; Strani, L.; Tassi, L. Candying Process for Enhancing Pre-Waste Watermelon Rinds to Increase Food Sustainability. Future Foods 2022, 6, 100182. [Google Scholar] [CrossRef]
- Petchsomrit, A.; McDermott, M.I.; Chanroj, S.; Choksawangkarn, W. Watermelon Seeds and Peels: Fatty Acid Composition and Cosmeceutical Potential. OCL 2020, 27, 54. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Feng, Y.; Duan, Y.; Ma, H.; Zhang, H. Purification and Identification of Novel Antioxidant Peptides from Watermelon Seed Protein Hydrolysates and Their Cytoprotective Effects on H2O2-Induced Oxidative Stress. Food Chem. 2020, 327, 127059. [Google Scholar] [CrossRef] [PubMed]
- Perkins-Veazie, P.; Collins, J.K.; Pair, S.D.; Roberts, W. Lycopene Content Differs among Red-fleshed Watermelon Cultivars. J. Sci. Food Agric. 2001, 81, 983–987. [Google Scholar] [CrossRef]
- Quek, S.Y.; Chok, N.K.; Swedlund, P. The Physicochemical Properties of Spray-Dried Watermelon Powders. Chem. Eng. Process. Process Intensif. 2007, 46, 386–392. [Google Scholar] [CrossRef]
- Rao, A.V.; Ray, M.R.; Rao, L.G. Lycopene. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2006; Volume 51, pp. 99–164. [Google Scholar]
- Heber, D.; Lu, Q.-Y. Overview of Mechanisms of Action of Lycopene. Exp. Biol. Med. 2002, 227, 920–923. [Google Scholar] [CrossRef]
- Gerster, H. The Potential Role of Lycopene for Human Health. J. Am. Coll. Nutr. 1997, 16, 109–126. [Google Scholar] [CrossRef]
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simões, S. Lycopene in Human Health. LWT 2020, 127, 109323. [Google Scholar] [CrossRef]
- Bramley, P.M. Is Lycopene Beneficial to Human Health? Phytochemistry 2000, 54, 233–236. [Google Scholar] [CrossRef]
- Mein, J.R.; Lian, F.; Wang, X.-D. Biological Activity of Lycopene Metabolites: Implications for Cancer Prevention. Nutr. Rev. 2008, 66, 667–683. [Google Scholar] [CrossRef] [PubMed]
- Palozza, P.; Simone, R.E.; Catalano, A.; Mele, M.C. Tomato Lycopene and Lung Cancer Prevention: From Experimental to Human Studies. Cancers 2011, 3, 2333–2357. [Google Scholar] [CrossRef] [PubMed]
- Arab, L.; Steck, S. Lycopene and Cardiovascular Disease123. Am. J. Clin. Nutr. 2000, 71, 1691S–1695S. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.K.; Rengasamy, K.R.R.; Mahomoodally, F.M.; Keum, Y.-S. Protective Effects of Lycopene in Cancer, Cardiovascular, and Neurodegenerative Diseases: An Update on Epidemiological and Mechanistic Perspectives. Pharmacol. Res. 2020, 155, 104730. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.L.; Schwartz, S.J. Lycopene Stability During Food Processing. Proc. Soc. Exp. Biol. Med. 1998, 218, 101–105. [Google Scholar] [CrossRef]
- Anguelova, T.; Warthesen, J. Lycopene Stability in Tomato Powders. J. Food Sci. 2000, 65, 67–70. [Google Scholar] [CrossRef]
- Xianquan, S.; Shi, J.; Kakuda, Y.; Yueming, J. Stability of Lycopene During Food Processing and Storage. J. Med. Food 2005, 8, 413–422. [Google Scholar] [CrossRef]
- Oberoi, D.P.S.; Sogi, D.S. Effect of Drying Methods and Maltodextrin Concentration on Pigment Content of Watermelon Juice Powder. J. Food Eng. 2015, 165, 172–178. [Google Scholar] [CrossRef]
- Milczarek, R.R.; Sedej, I. Enhancing Nutritional Quality of Spray-dried Concentrated Watermelon Juice Using Watermelon By-product Carrier Blends. eFood 2023, 4, e72. [Google Scholar] [CrossRef]
- Oberoi, D.P.S.; Sogi, D.S. Prediction of Lycopene Degradation during Dehydration of Watermelon Pomace (Cv Sugar Baby). J. Saudi Soc. Agric. Sci. 2017, 16, 97–103. [Google Scholar] [CrossRef]
- Lingayat, A.; Chandramohan, V.P.; Raju, V.R.K.; Kumar, A. Development of Indirect Type Solar Dryer and Experiments for Estimation of Drying Parameters of Apple and Watermelon. Therm. Sci. Eng. Prog. 2020, 16, 100477. [Google Scholar] [CrossRef]
- Hasturk Sahin, F.; Aktas, T.; Orak, H.; Ulger, P. Influence of Pretreatments and Different Drying Methods on Color Parameters and Lycopene Content of Dried Tomato. Bulg. J. Agric. Sci. 2011, 17, 867–881. [Google Scholar]
- Davoodi, M.G.; Vijayanand, P.; Kulkarni, S.G.; Ramana, K.V.R. Effect of Different Pre-Treatments and Dehydration Methods on Quality Characteristics and Storage Stability of Tomato Powder. LWT-Food Sci. Technol. 2007, 40, 1832–1840. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Methods of Analysis of the Association of Official’s Analytical Chemists, 14th ed.; Associataion of Official Analytical Chemist: Washington, DC, USA, 1990. [Google Scholar]
- Anthon, G.; Barrett, D.M. Standardization of a Rapid Spectrophotometric Method for Lycopene Analysis. Acta Hortic. 2007, 758, 111–128. [Google Scholar] [CrossRef]
- Buczkowska, H.; Sałata, A.; Nurzyńska-Wierdak, R. Melon (Cucumis melo L.) Fruit Yield under Irrigation and Mycorrhiza Conditions. Agronomy 2023, 13, 1559. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Leskovar, D.I.; Colla, G.; Rouphael, Y. Watermelon and Melon Fruit Quality: The Genotypic and Agro-Environmental Factors Implicated. Sci. Hortic. 2018, 234, 393–408. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Massa, D.; Salerno, A.; Rea, E. Yield, Fruit Quality and Mineral Composition of Grafted Melon Plants Grown under Saline Conditions. J. Hortic. Sci. Biotechnol. 2006, 81, 146–152. [Google Scholar] [CrossRef]
- Cabello, M.J.; Castellanos, M.T.; Romojaro, F.; Martínez-Madrid, C.; Ribas, F. Yield and Quality of Melon Grown under Different Irrigation and Nitrogen Rates. Agric. Water Manag. 2009, 96, 866–874. [Google Scholar] [CrossRef]
- Schmidt, D.A.; Kerley, M.S.; Porter, J.H.; Dempsey, J.L. Structural and Nonstructural Carbohydrate, Fat, and Protein Composition of Commercially Available, Whole Produce. Zoo Biol. 2005, 24, 359–373. [Google Scholar] [CrossRef]
- Wani, A.A.; Sogi, D.S.; Singh, P.; Wani, I.A.; Shivhare, U.S. Characterisation and Functional Properties of Watermelon (Citrullus lanatus) Seed Proteins. J. Sci. Food Agric. 2011, 91, 113–121. [Google Scholar] [CrossRef]
- Müntz, K.; Belozersky, M.A.; Dunaevsky, Y.E.; Schlereth, A.; Tiedemann, J. Stored Proteinases and the Initiation of Storage Protein Mobilization in Seeds during Germination and Seedling Growth. J. Exp. Bot. 2001, 52, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Tze, N.L.; Han, C.P.; Yusof, Y.A.; Ling, C.N.; Talib, R.A.; Taip, F.S.; Aziz, M.G. Physicochemical and Nutritional Properties of Spray-Dried Pitaya Fruit Powder as Natural Colorant. Food Sci. Biotechnol. 2012, 21, 675–682. [Google Scholar] [CrossRef]
- Jarquín-Enríquez, L.; Mercado-Silva, E.M.; Maldonado, J.L.; Lopez-Baltazar, J. Lycopene Content and Color Index of Tomatoes Are Affected by the Greenhouse Cover. Sci. Hortic. 2013, 155, 43–48. [Google Scholar] [CrossRef]
- Brandt, S.; Pék, Z.; Barna, É.; Lugasi, A.; Helyes, L. Lycopene Content and Colour of Ripening Tomatoes as Affected by Environmental Conditions. J. Sci. Food Agric. 2006, 86, 568–572. [Google Scholar] [CrossRef]
- Ribeiro, H.S.; Ax, K.; Schubert, H. Stability of Lycopene Emulsions in Food Systems. J. Food Sci. 2003, 68, 2730–2734. [Google Scholar] [CrossRef]
- Brul, S.; Coote, P. Preservative Agents in Foods: Mode of Action and Microbial Resistance Mechanisms. Int. J. Food Microbiol. 1999, 50, 1–17. [Google Scholar] [CrossRef]
- Coban, H.B. Organic Acids as Antimicrobial Food Agents: Applications and Microbial Productions. Bioprocess Biosyst. Eng. 2020, 43, 569–591. [Google Scholar] [CrossRef]
- Salehi, F. Recent Applications of Powdered Fruits and Vegetables as Novel Ingredients in Biscuits: A Review. Nutrire 2020, 45, 1. [Google Scholar] [CrossRef]
- Salehi, F. Quality, Physicochemical, and Textural Properties of Dairy Products Containing Fruits and Vegetables: A Review. Food Sci. Nutr. 2021, 9, 4666–4686. [Google Scholar] [CrossRef]
- Salehi, F.; Aghajanzadeh, S. Effect of Dried Fruits and Vegetables Powder on Cakes Quality: A Review. Trends Food Sci. Technol. 2020, 95, 162–172. [Google Scholar] [CrossRef]
- D’Eusanio, V.; Genua, F.; Marchetti, A.; Morelli, L.; Tassi, L. Characterization of Some Stilbenoids Extracted from Two Cultivars of Lambrusco—Vitis Vinifera Species: An Opportunity to Valorize Pruning Canes for a More Sustainable Viticulture. Molecules 2023, 28, 4074. [Google Scholar] [CrossRef] [PubMed]
- D’Eusanio, V.; Malferrari, D.; Marchetti, A.; Roncaglia, F.; Tassi, L. Waste By-Product of Grape Seed Oil Production: Chemical Characterization for Use as a Food and Feed Supplement. Life 2023, 13, 326. [Google Scholar] [CrossRef] [PubMed]
- Majzoobi, M.; Vosooghi Poor, Z.; Mesbahi, G.; Jamalian, J.; Farahnaky, A. Effects of Carrot Pomace Powder and a Mixture of Pectin and Xanthan on the Quality of Gluten-Free Batter and Cakes. J. Texture Stud. 2017, 48, 616–623. [Google Scholar] [CrossRef]
- Maletti, L.; D’Eusanio, V.; Durante, C.; Marchetti, A.; Tassi, L. VOCs Analysis of Three Different Cultivars of Watermelon (Citrullus lanatus L.) Whole Dietary Fiber. Molecules 2022, 27, 8747. [Google Scholar] [CrossRef] [PubMed]
- D’Eusanio, V.; Maletti, L.; Marchetti, A.; Roncaglia, F.; Tassi, L. Volatile Aroma Compounds of Gavina® Watermelon (Citrullus lanatus L.) Dietary Fibers to Increase Food Sustainability. AppliedChem 2023, 3, 66–88. [Google Scholar] [CrossRef]
- Maletti, L.; D’Eusanio, V.; Durante, C.; Marchetti, A.; Pincelli, L.; Tassi, L. Comparative Analysis of VOCs from Winter Melon Pomace Fibers before and after Bleaching Treatment with H2O2. Molecules 2022, 27, 2336. [Google Scholar] [CrossRef]


| Sample Name | Cultivar | Pre-Treatment | Storage Condition |
|---|---|---|---|
| PN | Perla Nera® | No | Vial-sealed, RT, dark |
| PN_A | Perla Nera® | Solution A | Vial-sealed, RT, dark |
| PN_B | Perla Nera® | Solution B | Vial-sealed, RT, dark |
| AM | Asahi Miyako | No | Vial-sealed, RT, dark |
| AM_A | Asahi Miyako | Solution A | Vial-sealed, RT, dark |
| AM_B | Asahi Miyako | Solution B | Vial-sealed, RT, dark |
| G | Gavina® | No | Vial-sealed, RT, dark |
| G_A | Gavina® | Solution A | Vial-sealed, RT, dark |
| G_B | Gavina® | Solution B | Vial-sealed, RT, dark |
| C | Crimson Sweet | No | Vial-sealed, RT, dark |
| C_A | Crimson Sweet | Solution A | Vial-sealed, RT, dark |
| C_B | Crimson Sweet | Solution B | Vial-sealed, RT, dark |
| PNV | Perla Nera® | No | Vaacum stored, RT, dark |
| PN_AV | Perla Nera® | Solution A | Vaacum stored, RT, dark |
| PN_BV | Perla Nera® | Solution B | Vaacum stored, RT, dark |
| AMV | Asahi Miyako | No | Vaacum stored, RT, dark |
| AM_AV | Asahi Miyako | Solution A | Vaacum stored, RT, dark |
| AM_BV | Asahi Miyako | Solution B | Vaacum stored, RT, dark |
| GV | Gavina® | No | Vaacum stored, RT, dark |
| G_AV | Gavina® | Solution A | Vaacum stored, RT, dark |
| G_BV | Gavina® | Solution B | Vaacum stored, RT, dark |
| CV | Crimson Sweet | No | Vaacum stored, RT, dark |
| C_AV | Crimson Sweet | Solution A | Vaacum stored, RT, dark |
| C_BV | Crimson Sweet | Solution B | Vaacum stored, RT, dark |
| PN | AM | G | C | |
|---|---|---|---|---|
| Moisture content (%) | 6.14 ± 0.12 a | 6.68 ± 0.14 b | 5.93 ± 0.11 a | 6.91 ± 0.16 b |
| Total fat (%) | 0.66 ± 0.08 a | 0.59 ± 0.11 a | 0.46 ± 0.09 a | 0.62 ± 0.10 a |
| Ashes (%) | 3.10 ± 0.02 a | 2.91 ± 0.03 a | 4.65 ± 0.02 a | 3.59 ± 0.04 a |
| Carbohydrate (%) * | 82.1 ± 0.7 a | 75.1 ± 0.4 b | 80.9 ± 0.5 a | 78.6 ± 0.8 d |
| Total dietary fiber (%) | 16.2 ± 0.6 a | 15.3 ± 0.5 ab | 13.3 ± 0.5 c | 14.1 ± 0.6 bc |
| Glucose (%) | 10.4 ± 0.14 a | 10.6 ± 0.11 a | 9.17 ± 0.13 b | 10.4 ± 0.14 a |
| Fructose (%) | 30.9 ± 0.8 a | 31.9 ± 0.6 a | 33.6 ± 0.5 b | 29.4 ± 0.4 a |
| Sucrose (%) | 8.53 ± 0.41 a | 8.85 ± 0.40 a | 8.01 ± 0.39 a | 6.89 ± 0.37 b |
| Total Sugars (%) | 49.8 ± 0.6 a | 51.3 ± 0.7 a | 50.8 ± 0.8 a | 46.7 ± 0.4 b |
| Proteins (%) | 8.04 ± 0.68 a | 14.7 ± 0.8 b | 8.04 ± 0.45 a | 10.3 ± 0.6 c |
| C% | 40.0 ± 0.6 a | 42.3 ± 0.5 b | 38.9 ± 0.9 a | 39.6 ± 0.8 a |
| H% | 6.44 ± 0.12 a | 6.33 ± 0.22 a | 6.49 ± 0.17 ab | 6.92 ± 0.17 b |
| N% | 1.34 ± 0.10 a | 2.41 ± 0.11 b | 1.35 ± 0.14 a | 1.73 ± 0.11 c |
| S% | <0.1% | <0.1% | <0.1% | <0.1% |
| Days of Storage | 0 | 7 | 14 | 21 | 28 | 90 |
|---|---|---|---|---|---|---|
| PN | 1.135 ± 0.051 b | 0.934 ± 0.030 c | 0.734 ± 0.052 d | 0.544 ± 0.041 e | 0.129 ± 0.012 f | |
| PN_A | 1.445 ± 0.051 a | 1.437 ± 0.054 a | 1.347 ± 0.063 ab | 1.252 ± 0.048 b | 1.066 ± 0.070 c | 0.549 ± 0.035 d |
| PN_B | 1.446 ± 0.059 a | 1.442 ± 0.061 a | 1.409 ± 0.051 a | 1.376 ± 0.075 a | 1.151 ± 0.052 b | 0.641 ± 0.034 c |
| AM | 1.337 ± 0.050 a | 0.932 ± 0.034 b | 0.758 ± 0.071 c | 0.435 ± 0.062 d | 0.244 ± 0.057 e | 0.013 ± 0.006 f |
| AM_A | 1.274 ± 0.042 a | 1.234 ± 0.059 a | 1.050 ± 0.075 b | 0.888 ± 0.067 c | 0.809 ± 0.061 c | 0.237 ± 0.014 d |
| AM_B | 1.261 ± 0.047 a | 1.243 ± 0.048 a | 1.125 ± 0.021 b | 0.924 ± 0.045 c | 0.855 ± 0.044 c | 0.295 ± 0.044 d |
| G | 1.568 ± 0.042 a | 1.339 ± 0.039 b | 1.168 ± 0.056 c | 0.835 ± 0.059 d | 0.630 ± 0.063 e | 0.147 ± 0.013 f |
| G_A | 1.571 ± 0.060 a | 1.509 ± 0.061 ab | 1.382 ± 0.066 bc | 1.236 ± 0.051 c | 1.049 ± 0.054 d | 0.615 ± 0.025 e |
| G_B | 1.544 ± 0.047 a | 1.542 ± 0.048 a | 1.474 ± 0.083 ab | 1.343 ± 0.064 bc | 1.246 ± 0.054 c | 0.731 ± 0.030 d |
| C | 0.809 ± 0.069 a | 0.314 ± 0.046 b | 0.234 ± 0.060 bc | 0.152 ± 0.042 cd | 0.141 ± 0.051 cd | 0.037 ± 0.0013 d |
| C_A | 0.734 ± 0.050 a | 0.561 ± 0.037 b | 0.423 ± 0.077 c | 0.379 ± 0.046 c | 0.354 ± 0.049 c | 0.140 ± 0.018 d |
| C_B | 0.757 ± 0.052 a | 0.735 ± 0.063 a | 0.564 ± 0.047 b | 0.427 ± 0.079 bc | 0.366 ± 0.043 c | 0.154 ± 0.008 d |
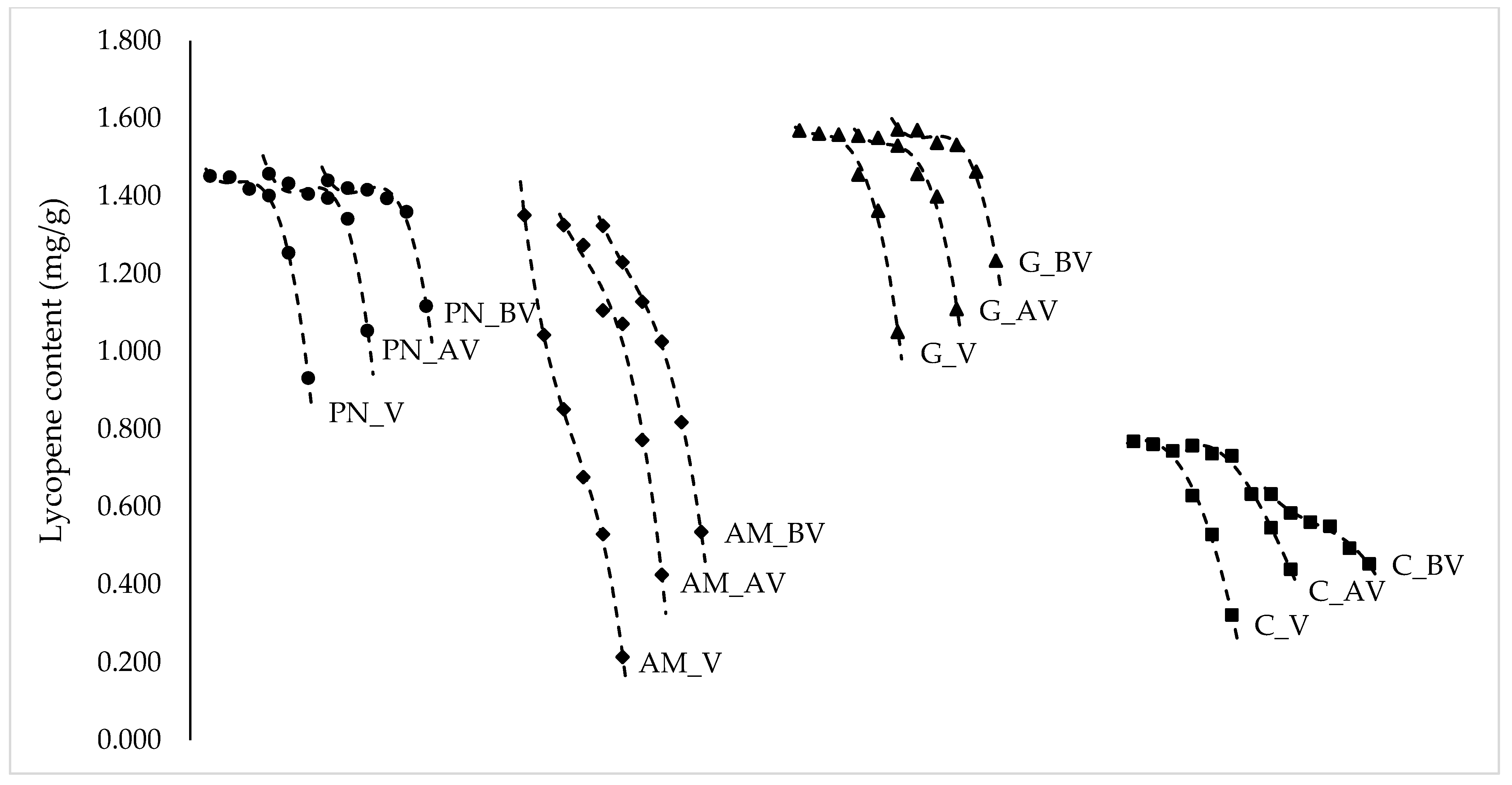
| PN_V | 1.452 ± 0.055 a | 1.449 ± 0.045 a | 1.419 ± 0.058 a | 1.402 ± 0.070 ab | 1.254 ± 0.045 b | 0.932 ± 0.046 c |
| PN_AV | 1.458 ± 0.060 a | 1.433 ± 0.041 a | 1.406 ± 0.056 a | 1.395 ± 0.040 a | 1.342 ± 0.046 a | 1.054 ± 0.040 b |
| PN_BV | 1.441 ± 0.048 a | 1.421 ± 0.067 a | 1.417 ± 0.071 a | 1.395 ± 0.051 a | 1.360 ± 0.045 a | 1.117 ± 0.030 b |
| AM_V | 1.351 ± 0.045 a | 1.042 ± 0.056 b | 0.851 ± 0.039 c | 0.677 ± 0.051 d | 0.530 ± 0.052 e | 0.214 ± 0.019 f |
| AM_AV | 1.326 ± 0.058 a | 1.274 ± 0.052 a | 1.106 ± 0.034 b | 1.071 ± 0.052 b | 0.772 ± 0.042 c | 0.425 ± 0.027 d |
| AM_BV | 1.324 ± 0.052 a | 1.230 ± 0.047 ab | 1.128 ± 0.049 bc | 1.026 ± 0.042 c | 0.818 ± 0.055 d | 0.535 ± 0.028 e |
| G_V | 1.569 ± 0.042 a | 1.562 ± 0.055 a | 1.559 ± 0.046 a | 1.456 ± 0.031 ab | 1.363 ± 0.047 b | 1.050 ± 0.051 c |
| G_AV | 1.555 ± 0.060 a | 1.550 ± 0.043 a | 1.530 ± 0.037 a | 1.457 ± 0.039 ab | 1.399 ± 0.023 b | 1.109 ± 0.019 c |
| G_BV | 1.572 ± 0.048 a | 1.570 ± 0.064 a | 1.532 ± 0.062 a | 1.516 ± 0.024 a | 1.463 ± 0.047 a | 1.234 ± 0.031 b |
| C_V | 0.769 ± 0.042 a | 0.761 ± 0.049 a | 0.744 ± 0.054 ab | 0.629 ± 0.050 bc | 0.529 ± 0.051 c | 0.322 ± 0.030 d |
| C_AV | 0.758 ± 0.053 a | 0.737 ± 0.055 a | 0.731 ± 0.047 a | 0.633 ± 0.059 ab | 0.546 ± 0.046 bc | 0.439 ± 0.052 c |
| C_BV | 0.633 ± 0.057 a | 0.584 ± 0.056 ab | 0.561 ± 0.038 ac | 0.550 ± 0.043 ac | 0.494 ± 0.030 bc | 0.453 ± 0.011 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Eusanio, V. Assessment of Lycopene Levels in Dried Watermelon Pomace: A Sustainable Approach to Waste Reduction and Nutrient Valorization. Analytica 2024, 5, 311-321. https://doi.org/10.3390/analytica5030020
D’Eusanio V. Assessment of Lycopene Levels in Dried Watermelon Pomace: A Sustainable Approach to Waste Reduction and Nutrient Valorization. Analytica. 2024; 5(3):311-321. https://doi.org/10.3390/analytica5030020
Chicago/Turabian StyleD’Eusanio, Veronica. 2024. "Assessment of Lycopene Levels in Dried Watermelon Pomace: A Sustainable Approach to Waste Reduction and Nutrient Valorization" Analytica 5, no. 3: 311-321. https://doi.org/10.3390/analytica5030020
APA StyleD’Eusanio, V. (2024). Assessment of Lycopene Levels in Dried Watermelon Pomace: A Sustainable Approach to Waste Reduction and Nutrient Valorization. Analytica, 5(3), 311-321. https://doi.org/10.3390/analytica5030020






