Green Analytical Chemistry—Recent Innovations
Abstract
1. Introduction
2. Principles and Framework
3. Innovations in Green Analytical Techniques
3.1. Greener Separation Techniques
3.1.1. Green Gas Chromatography (GC) and Liquid Chromatography (LC)
3.1.2. Supercritical Fluid Chromatography (SFC)
3.2. Sustainable Sample Preparation
Ionic Liquids and Eutectic Solvents
3.3. Real-Time and In-Process Monitoring
3.3.1. Advances in Spectroscopic Methods for Real-Time Analysis
3.3.2. Electrochemical Methods
4. Application of Green Solvents
Use of Water, Supercritical Carbon Dioxide, Ionic Liquids, and Bio-Based Solvents in Analytical Processes
5. Application of Green Materials
5.1. Sustainable Materials in Sample Preparation
5.2. Green Chromatographic Columns
5.3. Eco-Friendly Materials in Sensor Fabrication
6. Alternative Energy in Green Chemistry
6.1. Role of Microwave-Assisted and Ultrasound-Assisted Techniques in Reducing Energy Requirements
6.2. Application of Photo-Induced Processes in Analysis
7. Advances in Green Instrumentation
8. Discussion
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mandrioli, R.; Cirrincione, M.; Mladěnka, P.; Protti, M.; Mercolini, L. Green analytical chemistry (GAC) applications in sample preparation for the analysis of anthocyanins in products and by-products from plant sources. Adv. Sample Prep. 2022, 3, 100037. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- de la Guardia, M.; Garrigues, S. Past, present and future of green analytical chemistry. In Challenges in Green Analytical Chemistry; The Royal Society of Chemistry: London, UK, 2020. [Google Scholar]
- Koel, M. Do we need green analytical chemistry? Green Chem. 2016, 18, 923–931. [Google Scholar] [CrossRef]
- Rogers, L.; Jensen, K.F. Continuous manufacturing–the Green Chemistry promise? Green Chem. 2019, 21, 3481–3498. [Google Scholar] [CrossRef]
- Kannaiah, K.P.; Chanduluru, H.K. Exploring sustainable analytical techniques using G score and future innovations in green analytical chemistry. J. Clean. Prod. 2023, 428, 139297. [Google Scholar] [CrossRef]
- Meher, A.K.; Zarouri, A. Environmental Applications of Mass Spectrometry for Emerging Contaminants. Molecules 2025, 30, 364. [Google Scholar] [CrossRef] [PubMed]
- Andraos, J.; Matlack, A.S. Introduction to Green Chemistry; CRC Press: Boca Raton, FL, USA, 2022. [Google Scholar]
- Sajid, M.; Płotka-Wasylka, J. Green analytical chemistry metrics: A review. Talanta 2022, 238, 123046. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Pena-Pereira, F.; Pedersen-Bjergaard, S.; Zuin, V.G.; Ozkan, S.A.; Psillakis, E. The ten principles of green sample preparation. TrAC Trends Anal. Chem. 2022, 148, 116530. [Google Scholar] [CrossRef]
- Ardila-Fierro, K.J.; Hernández, J.G. Sustainability assessment of mechanochemistry by using the twelve principles of green chemistry. ChemSusChem 2021, 14, 2145–2162. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Raccary, B.; Loubet, P.; Peres, C.; Sonnemann, G. Evaluating the environmental impacts of analytical chemistry methods: From a critical review towards a proposal using a life cycle approach. TrAC Trends Anal. Chem. 2022, 147, 116525. [Google Scholar] [CrossRef]
- Abdelrahman, M.M. Green analytical chemistry metrics and life-cycle assessment approach to analytical method development. In Green Chemical Analysis and Sample Preparations: Procedures, Instrumentation, Data Metrics, and Sustainability; Springer: Berlin/Heidelberg, Germany, 2022; pp. 29–99. [Google Scholar]
- Cutillas, V.; García-Gallego, G.; Murcia-Morales, M.; Ferrer, C.; Fernández-Alba, A.R. Beyond helium: Hydrogen as a carrier gas in multiresidue pesticide analysis in fruits and vegetables by GC-MS/MS. Anal. Methods 2024, 16, 1564–1569. [Google Scholar] [CrossRef]
- Dash, S.; Singh, A.; Jose, S.; Elangovan, D.; Surapraraju, S.K.; Natarajan, S.K. Advances in green hydrogen production through alkaline water electrolysis: A comprehensive review. Int. J. Hydrogen Energy 2024, 83, 614–629. [Google Scholar] [CrossRef]
- Çetintürk, K.; Güzel, B.; Canlı, O. The development of a novel, green, efficient, and eco-friendly GC-MS/MS analytical method for the reliable and rapid determination of dl-PCBs, and PCDD/Fs using hydrogen as a carrier gas and a modified ion source. Talanta 2025, 283, 127180. [Google Scholar] [CrossRef]
- Taylor, T. Health and Safety Concerns of Hydrogen Use with Gas Chromatography. LCGC 2023, 41, 394. [Google Scholar] [CrossRef]
- Bussey, R.O., III. Uses of Portable Gas Chromatography Mass Spectrometers. In Novel Aspects of Gas Chromatography and Chemometrics; IntechOpen: London, UK, 2022. [Google Scholar]
- Junwei, Q.; Kai, X.; Tao, Z.; Huijun, Z.; Shuo, Z.; Xinxin, L.; Xiaoxu, L. Development of a portable gas chromatography linear ion trap mass spectrometer (GC-LIT-MS) for VOCs analysis in water. Int. J. Mass Spectrom. 2024, 497, 117189. [Google Scholar] [CrossRef]
- Zanella, D.; Focant, J.F.; Franchina, F.A. 30th Anniversary of comprehensive two-dimensional gas chromatography: Latest advances. Anal. Sci. Adv. 2021, 2, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Arena, A.; Zoccali, M.; Mondello, L.; Tranchida, P.Q. Direct analysis of phthalate esters in vegetable oils by means of comprehensive two-dimensional gas chromatography combined with triple quadrupole mass spectrometry. Food Chem. 2022, 396, 133721. [Google Scholar] [CrossRef]
- Meher, A.K.; Anandan, P.; Mukhopadhyay, A. Estimation of Isradipine in Human Plasma by LCMS/MS. Asian J. Res. Chem. 2011, 4, 750–753. [Google Scholar]
- Aguilar, M.-I. Reversed-phase high-performance liquid chromatography. In HPLC of Peptides and Proteins: Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2004; pp. 9–22. [Google Scholar]
- Nakov, N.; Acevska, J.; Brezovska, K.; Kavrakovski, Z.; Dimitrovska, A. Green strategies toward eco-friendly HPLC methods in pharma analysis. In High Performance Liquid Chromatography-Recent Advances and Applications; IntechOpen: London, UK, 2023. [Google Scholar]
- Kalisz, O.; Tobiszewski, M.; Nowaczyk, A.; Bocian, S. Exploring the potential of green chemistry in reversed-phase liquid chromatography: A review of sustainable solvents. TrAC Trends Anal. Chem. 2024, 181, 118007. [Google Scholar] [CrossRef]
- Fouad, A.; El-Sayed, D.H.; Salman, B.E.; Bakr, H.H.; Adel, S.E.; Alzarak, T.M.; Mahmoud, A. Macrocyclic antibiotics as effective chiral selectors in liquid chromatography for enantiomeric separation of pharmaceutical compounds: A review. Crit. Rev. Anal. Chem. 2024, 54, 3095–3113. [Google Scholar] [CrossRef] [PubMed]
- Langford, J.B.; Lurie, I.S. Use of micro, capillary, and nano liquid chromatography for forensic analysis. J. Sep. Sci. 2022, 45, 38–50. [Google Scholar] [CrossRef]
- Fang, S.; Huang, Y.; Ruan, Q.; Chen, C.; Liu, S.; Ouyang, G. Recent developments on solid phase microextraction (SPME) coatings for in vivo analysis. Green Anal. Chem. 2023, 6, 100069. [Google Scholar] [CrossRef]
- Aslani, S.; Armstrong, D.W. Ionic liquids as gas chromatography stationary phases. In Ionic Liquids in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; pp. 171–202. [Google Scholar]
- Quintana, A.A.; Sztapka, A.M.; Santos Ebinuma, V.d.C.; Agatemor, C. Enabling sustainable chemistry with ionic liquids and deep eutectic solvents: A fad or the future? Angew. Chem. Int. Ed. 2022, 61, e202205609. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Meher, A.K.; Chen, Y.-C. Automatic sampling and analysis of organics and biomolecules by capillary action-supported contactless atmospheric pressure ionization mass spectrometry. PLoS ONE 2013, 8, e66292. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Krishnamurthy, A.; Chen, S.-H.; Chen, Y.-C. A tapered capillary-based contactless atmospheric pressure ionization mass spectrometry for on-line preconcentration and separation of small organics. Separations 2021, 8, 111. [Google Scholar] [CrossRef]
- Foster, S.W.; Gates, E.P.; Peaden, P.A.; Calugaru, S.V.; West, W.R.; Lee, M.L.; Grinias, J.P. Column selection considerations in compact capillary liquid chromatography. J. Chromatogr. A 2023, 1701, 464067. [Google Scholar] [CrossRef]
- Kaplitz, A.S.; Berger, T.A.; Berger, B.K.; Schug, K.A. A review of fraction collection technology for supercritical fluid chromatography. TrAC Trends Anal. Chem. 2022, 151, 116588. [Google Scholar] [CrossRef]
- Deidda, R.; Schelling, C.; Roussel, J.; Dispas, A.; De Bleye, C.; Ziemons, É.; Hubert, P.; Veuthey, J. The analysis of cannabinoids in cannabis samples by supercritical fluid chromatography and ultra-high-performance liquid chromatography: A comparison study. Anal. Sci. Adv. 2021, 2, 2–14. [Google Scholar] [CrossRef]
- Pilařová, V.; Hadysová, Z.; Švec, F.; Nováková, L. Supercritical fluids in analysis of cannabinoids in various Cannabis products. Anal. Chim. Acta 2022, 1232, 340452. [Google Scholar] [CrossRef]
- Rovetto, L.J.; Aieta, N.V. Supercritical carbon dioxide extraction of cannabinoids from Cannabis sativa L. J. Supercrit. Fluids 2017, 129, 16–27. [Google Scholar] [CrossRef]
- Beres, M. Expanding the boundaries of SFC: Analysis of biomolecules. In Separation Science and Technology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 14, pp. 257–297. [Google Scholar]
- Jiang, D.; Yang, J.; Chen, Y.; Jin, Y.; Fu, Q.; Ke, Y.; Liang, X. An attempt to apply a subtraction model for characterization of non-polar stationary phase in supercritical fluid chromatography. J. Chromatogr. A 2023, 1701, 464071. [Google Scholar] [CrossRef] [PubMed]
- Ganzera, M.; Zwerger, M. Analysis of natural products by SFC–applications from 2015 to 2021. TrAC Trends Anal. Chem. 2021, 145, 116463. [Google Scholar] [CrossRef]
- Peterka, O.; Wolrab, D.; Jirásko, R.; Kanásová, M.; Dolečková, Z.; Holčapek, M. Ultrahigh-Performance Supercritical Fluid Chromatography–Mass Spectrometry in the Clinical Lipidomic Analysis. In Clinical Metabolomics: Methods and Protocols; Giera, M., Sánchez-López, E., Eds.; Springer: New York, NY, USA, 2025; pp. 305–314. [Google Scholar]
- Plachká, K.; Pilařová, V.; Horáček, O.; Gazárková, T.; Vlčková, H.K.; Kučera, R.; Nováková, L. Columns in analytical-scale supercritical fluid chromatography: From traditional to unconventional chemistries. J. Sep. Sci. 2023, 46, 2300431. [Google Scholar] [CrossRef]
- Toribio, L.; Bernal, J.; Martín, M.T.; Ares, A.M. Supercritical fluid chromatography coupled to mass spectrometry: A valuable tool in food analysis. TrAC Trends Anal. Chem. 2021, 143, 116350. [Google Scholar] [CrossRef]
- Guillarme, D.; Desfontaine, V.; Heinisch, S.; Veuthey, J.-L. What are the current solutions for interfacing supercritical fluid chromatography and mass spectrometry? J. Chromatogr. B 2018, 1083, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Prothmann, J.; Sun, M.; Spégel, P.; Sandahl, M.; Turner, C. Ultra-high-performance supercritical fluid chromatography with quadrupole-time-of-flight mass spectrometry (UHPSFC/QTOF-MS) for analysis of lignin-derived monomeric compounds in processed lignin samples. Anal. Bioanal. Chem. 2017, 409, 7049–7061. [Google Scholar] [CrossRef]
- Hunt, A.J.; Sin, E.H.; Marriott, R.; Clark, J.H. Generation, capture, and utilization of industrial carbon dioxide. ChemSusChem Chem. Sustain. Energy Mater. 2010, 3, 306–322. [Google Scholar] [CrossRef]
- Dugheri, S.; Mucci, N.; Cappelli, G.; Trevisani, L.; Bonari, A.; Bucaletti, E.; Squillaci, D.; Arcangeli, G. Advanced Solid-Phase Microextraction Techniques and Related Automation: A Review of Commercially Available Technologies. J. Anal. Methods Chem. 2022, 2022, 8690569. [Google Scholar] [CrossRef]
- Li, N.; Zhang, T.; Chen, G.; Xu, J.; Ouyang, G.; Zhu, F. Recent advances in sample preparation techniques for quantitative detection of pharmaceuticals in biological samples. TrAC Trends Anal. Chem. 2021, 142, 116318. [Google Scholar] [CrossRef]
- Amini, S.; Ebrahimzadeh, H.; Seidi, S.; Jalilian, N. Preparation of Polyacrylonitrile/Ni-MOF electrospun nanofiber as an efficient fiber coating material for headspace solid-phase microextraction of diazinon and chlorpyrifos followed by CD-IMS analysis. Food Chem. 2021, 350, 129242. [Google Scholar] [CrossRef] [PubMed]
- Mansour, F.R.; Bedair, A.; Hamed, M.; Magdy, G.; Ali, I.; Locatelli, M. Applications of (natural) deep eutectic solvents in liquid phase microextraction: A review. Microchem. J. 2024, 198, 110178. [Google Scholar] [CrossRef]
- Faraji, H. Advancements in overcoming challenges in dispersive liquid-liquid microextraction: An overview of advanced strategies. TrAC Trends Anal. Chem. 2024, 170, 117429. [Google Scholar] [CrossRef]
- Notardonato, I.; Avino, P. Dispersive Liquid–Liquid Micro Extraction: An Analytical Technique Undergoing Continuous Evolution and Development—A Review of the Last 5 Years. Separations 2024, 11, 203. [Google Scholar] [CrossRef]
- Gjelstad, A. Three-phase hollow fiber liquid-phase microextraction and parallel artificial liquid membrane extraction. TrAC Trends Anal. Chem. 2019, 113, 25–31. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, J.; Guo, R.; Nie, Q.; Zhu, G. Acid induced dispersive liquid–liquid microextraction based on in situ formation of hydrophobic deep eutectic solvents for the extraction of bisphenol A and alkylphenols in water and beverage samples. Food Chem. 2024, 442, 138425. [Google Scholar] [CrossRef] [PubMed]
- Rageh, A.H.; Abdel-Aal, F.A.; Farrag, S.A.; Ali, A.-M.B.H. A surfactant-based quasi-hydrophobic deep eutectic solvent for dispersive liquid-liquid microextraction of gliflozins from environmental water samples using UHPLC/fluorescence detection. Talanta 2024, 266, 124950. [Google Scholar] [CrossRef]
- Lorenzo-Parodi, N.; Kaziur-Cegla, W.; Gjelstad, A.; Schmidt, T.C. Liquid-phase microextraction of aromatic amines: Hollow fiber–liquid-phase microextraction and parallel artificial liquid membrane extraction comparison. Anal. Bioanal. Chem. 2023, 415, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Do-Thanh, C.-L.; Luo, H.; Dai, S. A perspective on task-specific ionic liquids for the separation of rare earth elements. RSC Sustain. 2023, 1, 1168–1176. [Google Scholar] [CrossRef]
- Lu, C.; Sun, S.; Li, H.; Du, Z.; Li, B.; Zhu, L. New residue analysis method for four task-specific ionic liquids in water, soil and plants. Bull. Environ. Contam. Toxicol. 2022, 109, 338–343. [Google Scholar] [CrossRef]
- Volpatto, F.; Vitali, L. Development of a new method using dispersive liquid–liquid microextraction with hydrophobic natural deep eutectic solvent for the analysis of multiclass emerging contaminants in surface water by liquid chromatography-mass spectrometry. Anal. Methods 2025, 17, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Sholokhova, A.Y.; Matyushin, D.D.; Shashkov, M.V. Quantitative structure-retention relationships for pyridinium-based ionic liquids used as gas chromatographic stationary phases: Convenient software and assessment of reliability of the results. J. Chromatogr. A 2024, 1730, 465144. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ángel, M.J.; Carda-Broch, S.; García-Álvarez-Coque, M. Ionic liquids as mobile phase additives and immobilized on stationary phases in liquid chromatography. In Ionic Liquids in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2022; pp. 203–234. [Google Scholar]
- Meher, A.K.; Chen, Y.-C. Combination of Raman spectroscopy and mass spectrometry for online chemical analysis. Anal. Chem. 2016, 88, 9151–9157. [Google Scholar] [CrossRef] [PubMed]
- Mangam, V.T.; Narla, D.; Konda, R.K.; Sarella, P.N.K. Beyond the spectrum: Exploring unconventional applications of fourier transform infrared (FTIR) spectroscopy. Asian J. Pharm. Anal. 2024, 14, 86–94. [Google Scholar] [CrossRef]
- Abdolkarimi-Mahabadi, M.; Bayat, A.; Mohammadi, A. Use of UV-Vis spectrophotometry for characterization of carbon nanostructures: A review. Theor. Exp. Chem. 2021, 57, 191–198. [Google Scholar] [CrossRef]
- Li, C.; Huang, Y.; Li, X.; Zhang, Y.; Chen, Q.; Ye, Z.; Alqarni, Z.; Bell, S.E.J.; Xu, Y. Towards practical and sustainable SERS: A review of recent developments in the construction of multifunctional enhancing substrates. J. Mater. Chem. C 2021, 9, 11517–11552. [Google Scholar] [CrossRef]
- Lillotte, T.D.; Joester, M.; Frindt, B.; Berghaus, A.; Lammens, R.F.; Wagner, K.G. UV–VIS spectra as potential process analytical technology (PAT) for measuring the density of compressed materials: Evaluation of the CIELAB color space. Int. J. Pharm. 2021, 603, 120668. [Google Scholar] [CrossRef]
- Amirvaresi, A.; Nikounezhad, N.; Amirahmadi, M.; Daraei, B.; Parastar, H. Comparison of near-infrared (NIR) and mid-infrared (MIR) spectroscopy based on chemometrics for saffron authentication and adulteration detection. Food Chem. 2021, 344, 128647. [Google Scholar] [CrossRef]
- Tsuchikawa, S.; Ma, T.; Inagaki, T. Application of near-infrared spectroscopy to agriculture and forestry. Anal. Sci. 2022, 38, 635–642. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, M.; Mujumdar, A.S.; Wang, D. Non-destructive quality determination of frozen food using NIR spectroscopy-based machine learning and predictive modelling. J. Food Eng. 2023, 343, 111374. [Google Scholar] [CrossRef]
- Akhtar, M.; Ahmed, N.; Mahmood, S.; Jabbar, A.; Ahmed, R.; Umar, Z.A.; Iqbal, J.; Baig, M.A. Elemental analysis of cement and its components by laser-induced breakdown spectroscopy (LIBS) and laser ablation time of flight mass spectrometry (LA-TOF-MS). Anal. Lett. 2022, 55, 904–916. [Google Scholar] [CrossRef]
- Senesi, G.S.; Harmon, R.S.; Hark, R.R. Field-portable and handheld laser-induced breakdown spectroscopy: Historical review, current status and future prospects. Spectrochim. Acta Part B At. Spectrosc. 2021, 175, 106013. [Google Scholar] [CrossRef]
- Downs, A.M.; Plaxco, K.W. Real-time, in vivo molecular monitoring using electrochemical aptamer based sensors: Opportunities and challenges. ACS Sens. 2022, 7, 2823–2832. [Google Scholar] [CrossRef]
- Antonacci, A.; Scognamiglio, V.; Mazzaracchio, V.; Caratelli, V.; Fiore, L.; Moscone, D.; Arduini, F. based electrochemical devices for the pharmaceutical field: State of the art and perspectives. Front. Bioeng. Biotechnol. 2020, 8, 339. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Qi, H.; Ma, Y.; Deng, Y.; Liu, S.; Jie, Y.; Jing, J.; He, J.; Zhang, X.; Wheatley, L.; et al. A flexible and physically transient electrochemical sensor for real-time wireless nitric oxide monitoring. Nat. Commun. 2020, 11, 3207. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Keçili, R.; Ghorbani-Bidkorbeh, F.; Hussain, C.M. Green miniaturized technologies in analytical and bioanalytical chemistry. TrAC Trends Anal. Chem. 2021, 143, 116383. [Google Scholar] [CrossRef]
- Merone, G.M.; Tartaglia, A.; Rosato, E.; D’ovidio, C.; Kabir, A.; Ulusoy, H.I.; Savini, F.; Locatelli, M. Ionic liquids in analytical chemistry: Applications and recent trends. Curr. Anal. Chem. 2021, 17, 1340–1355. [Google Scholar] [CrossRef]
- Bahgat, E.A.; Hafez, H.M.; El-Sayed, H.M.; Kabil, N.A.S. Development of a solvent-free micellar HPLC method for determination of five antidiabetic drugs using response surface methodology. Microchem. J. 2022, 179, 107446. [Google Scholar] [CrossRef]
- Tomikj, M.; Božinovska, M.; Anevska-Stojanovska, N.; Lazova, J.; Acevska, J.; Brezovska, K.; Tonich-Ribarska, J.; Nakov, N. Sustainable and white HPLC method for simultaneous determination of amlodipine and atorvastatin in film-coated tablet. Green Anal. Chem. 2024, 8, 100103. [Google Scholar] [CrossRef]
- Plachká, K.; Pilařová, V.; Kosturko, Š.; Škop, J.; Svec, F.; Nováková, L. Ultrahigh-Performance Supercritical Fluid Chromatography–Multimodal Ionization–Tandem Mass Spectrometry as a Universal Tool for the Analysis of Small Molecules in Complex Plant Extracts. Anal. Chem. 2024, 96, 2840–2848. [Google Scholar] [CrossRef]
- Axente, R.E.; Stan, M.; Chitescu, C.L.; Nitescu, V.G.; Vlasceanu, A.-M.; Baconi, D.L. Application of Ionic Liquids as Mobile Phase Additives for Simultaneous Analysis of Nicotine and Its Metabolite Cotinine in Human Plasma by HPLC–DAD. Molecules 2023, 28, 1563. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Guo, Y.; Liu, Y.; Liu, Z.; Zhang, L. Rapid and enhanced detection of sulfonamide antibiotic using task-specific ionic liquids nanoconfined in tunable nanoporous carbons. Talanta 2025, 285, 127396. [Google Scholar] [CrossRef]
- Habib, A.; Mabrouk, M.M.; Fekry, M.; Mansour, F.R. Glycerol as a novel green mobile phase modifier for reversed phase liquid chromatography. Microchem. J. 2021, 169, 106587. [Google Scholar] [CrossRef]
- Azadeh, A.M.; Heydar, K.T.; Amini, M.H. Investigation and characterization of deep eutectic solvent (DES) based stationary phase in gas chromatography. J. Chromatogr. A 2025, 1739, 465511. [Google Scholar] [CrossRef]
- Rana, A.K.; Mishra, Y.K.; Gupta, V.K.; Thakur, V.K. Sustainable materials in the removal of pesticides from contaminated water: Perspective on macro to nanoscale cellulose. Sci. Total Environ. 2021, 797, 149129. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhang, B.; Li, J.; Sun, Y.; Wang, X.; Ma, P.; Song, D. One-step fabrication of hydrophilic MIL-68 (Al)/Chitosan-coated melamine sponge for vortex-assisted solid-phase extraction of parabens in water samples. Talanta 2021, 224, 121799. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Zhao, S.; Yuan, B.; Gao, Y.; Liu, R. In-situ growth of metal coordination-synergistic imprinted polymer onto shrimp shell-derived magnetic FeNi biochar for specific recognition of monocrotaline in herbal medicine. Mater. Today Sustain. 2023, 24, 100599. [Google Scholar] [CrossRef]
- Sultan, U.; Städtke, K.; Göpfert, A.; Lemmen, D.; Metwalli, E.; Maiti, S.; Schlumberger, C.; Yokosawa, T.; Zubiri, B.A.; Spiecker, E.; et al. Substituting fossil-based with bio-based chemicals: The case of limonene as a greener pore expander for micellar templated silica. RSC Sustain. 2023, 1, 1449–1461. [Google Scholar] [CrossRef]
- Dembek, M.; Bocian, S. Stationary phases for green liquid chromatography. Materials 2022, 15, 419. [Google Scholar] [CrossRef]
- Rahmani, H.; Shetty, D.; Wagih, M.; Ghasempour, Y.; Palazzi, V.; Carvalho, N.B.; Correia, R.; Costanzo, A.; Vital, D.; Alimenti, F.; et al. Next-generation IoT devices: Sustainable eco-friendly manufacturing, energy harvesting, and wireless connectivity. IEEE J. Microw. 2023, 3, 237–255. [Google Scholar] [CrossRef]
- Teixeira, S.C.; Gomes, N.O.; de Oliveira, T.V.; Fortes-Da-Silva, P.; Soares, N.d.F.F.; Raymundo-Pereira, P.A. Review and Perspectives of sustainable, biodegradable, eco-friendly and flexible electronic devices and (Bio) sensors. Biosens. Bioelectron. X 2023, 14, 100371. [Google Scholar] [CrossRef]
- Nonglait, D.L.; Gokhale, J.S. Review insights on the demand for natural pigments and their recovery by emerging microwave-assisted extraction (MAE). Food Bioprocess Technol. 2024, 17, 1681–1705. [Google Scholar] [CrossRef]
- Milutinović, M.; Miladinović, M.; Gašić, U.; Dimitrijević-Branković, S.; Rajilić-Stojanović, M. Recovery of bioactive molecules from Hypericum perforatum L. dust using microwave-assisted extraction. Biomass Convers. Biorefinery 2024, 14, 7111–7123. [Google Scholar] [CrossRef]
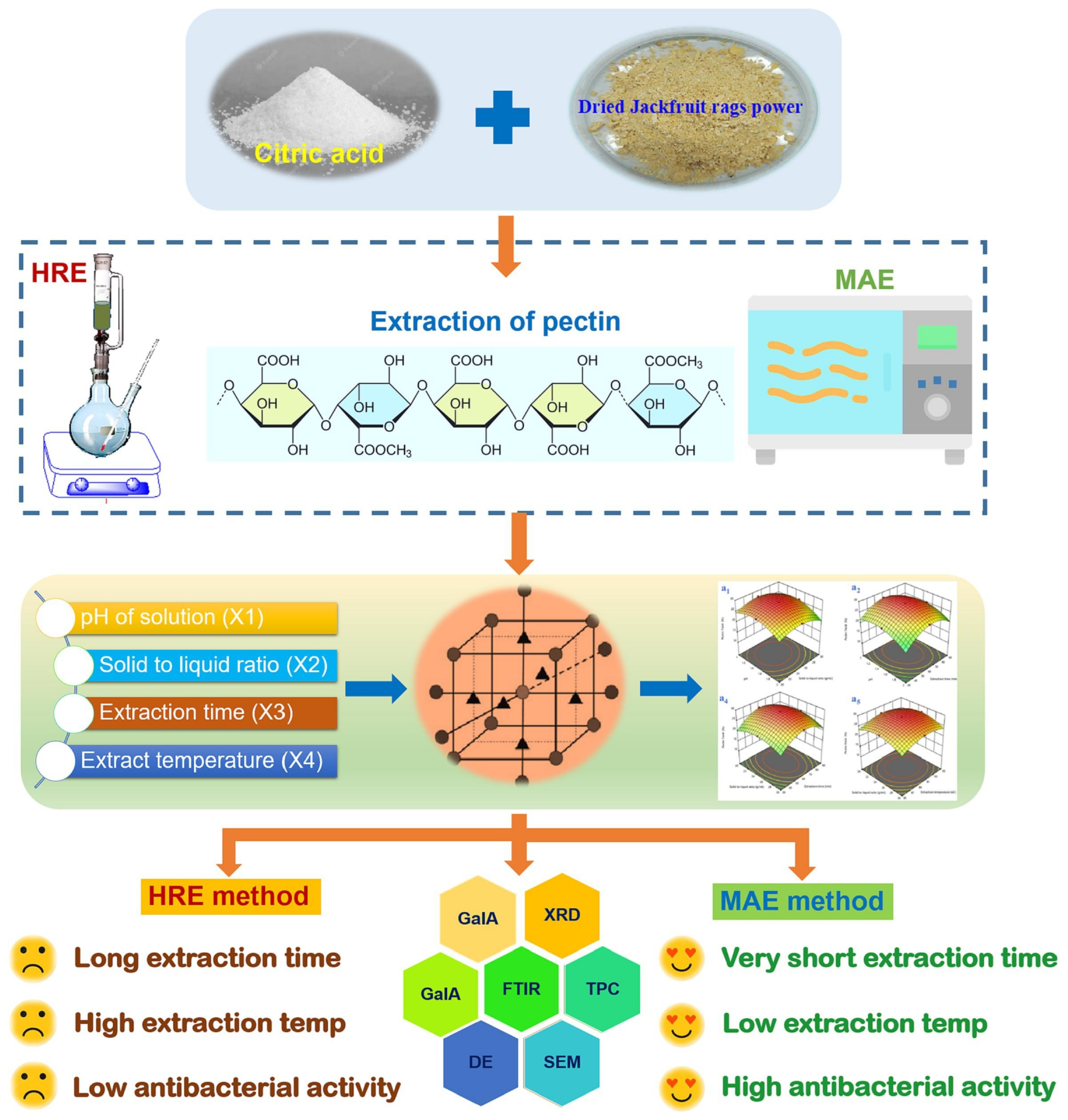
- Tran, N.T.K.; Nguyen, V.B.; Van Tran, T.; Nguyen, T.T.T. Microwave-assisted extraction of pectin from jackfruit rags: Optimization, physicochemical properties and antibacterial activities. Food Chem. 2023, 418, 135807. [Google Scholar] [CrossRef] [PubMed]
- Biale, G.; La Nasa, J.; Fiorentini, L.; Ceccarini, A.; Carnaroglio, D.; Mattonai, M.; Modugno, F. Characterization and quantification of microplastics and organic pollutants in mussels by microwave-assisted sample preparation and analytical pyrolysis. Environ. Sci. Adv. 2024, 3, 76–84. [Google Scholar] [CrossRef]
- Rao, M.V.; Sengar, A.S.; Sunil, C.; Rawson, A. Ultrasonication-A green technology extraction technique for spices: A review. Trends Food Sci. Technol. 2021, 116, 975–991. [Google Scholar] [CrossRef]
- Zimmermann, R.; Hanley, L. Photoionization and Photo-Induced Processes in Mass Spectrometry: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Moradeeya, P.G.; Sharma, A.; Kumar, M.A.; Basha, S. Titanium dioxide based nanocomposites–current trends and emerging strategies for the photocatalytic degradation of ruinous environmental pollutants. Environ. Res. 2022, 204, 112384. [Google Scholar] [CrossRef]
- Chen, Q.; Qin, L.; Shi, C.; Kang, S.-Z.; Li, X. A stable and plug-and-play aluminium/titanium dioxide/metal-organic framework/silver composite sheet for sensitive Raman detection and photocatalytic removal of 4-aminothiophenol. Chemosphere 2021, 282, 131000. [Google Scholar] [CrossRef]
- Araújo, K.C.; Barreto, M.C.; Siqueira, A.S.; Freitas, A.C.P.; Oliveira, L.G.; Bastos, M.E.P.; Rocha, M.E.P.; Silva, L.A.; Fragoso, W.D. Oil spill in northeastern Brazil: Application of fluorescence spectroscopy and PARAFAC in the analysis of oil-related compounds. Chemosphere 2021, 267, 129154. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.; Sun, J.; Ji, J.; Ye, Y.; Lu, X.; Zhang, Y.; Sun, X. Rapid, on-site, and sensitive detection of aflatoxin M1 in milk products by using time-resolved fluorescence microsphere test strip. Food Control 2021, 121, 107616. [Google Scholar] [CrossRef]
- Shihan, M.H.; Novo, S.G.; Le Marchand, S.J.; Wang, Y.; Duncan, M.K. A simple method for quantitating confocal fluorescent images. Biochem. Biophys. Rep. 2021, 25, 100916. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Macedo, R.C.; da Cunha, A.L.; Toloza, C.A.; Aucélio, R.Q. Determination of trifloxystrobin in soy grape juice and natural water by photo-induced fluorescence and high-performance liquid chromatography. J. Braz. Chem. Soc. 2023, 34, 1877–1886. [Google Scholar] [CrossRef]
- Trimpin, S.; Inutan, E.D.; Karki, S.; Elia, E.A.; Zhang, W.-J.; Weidner, S.M.; Marshall, D.D.; Hoang, K.; Lee, C.; Davis, E.T.J.; et al. Fundamental studies of new ionization technologies and insights from IMS-MS. J. Am. Soc. Mass Spectrom. 2019, 30, 1133–1147. [Google Scholar] [CrossRef]
- Trimpin, S.; Marshall, D.D.; Karki, S.; Madarshahian, S.; Hoang, K.; Meher, A.K.; Pophristic, M.; Richards, A.L.; Lietz, C.B.; Fischer, J.L.; et al. An overview of biological applications and fundamentals of new inlet and vacuum ionization technologies. Rapid Commun. Mass Spectrom. 2021, 35, e8829. [Google Scholar] [CrossRef]
- Trimpin, S.; Inutan, E.D.; McEwen, C.N.; Meher, A.K.; Karki, S.; Madarshahian, S.; Fenner, M.A.; Marshall, D.D. Combining novel and traditional ionization methods for mass spectrometry for more comprehensive analyses. Cut. Edge At. Spectrosc. 2018, 33, 12–17. [Google Scholar]
- Trimpin, S.; Moreno-Pedraza, A.; Hoang, K.; Pophristic, M.; Inutan, E.D.; Marshall, D.D.; McEwen, C.N. Multi-functional Vacuum Ionization Source for MAI, LSI, and MALDI: Operational from AP for Comprehensive, Low-Cost Data-Mining in Mass Spectrometry. In Proceedings of the 68th Annual ASMS Conference on Mass Spectrometry and Allied Topics 2020, Houston, TX, USA, 31 May–4 June 2020. [Google Scholar]
- Karki, S.; Meher, A.K.; Inutan, E.D.; Pophristic, M.; Marshall, D.D.; Rackers, K.; Trimpin, S.; McEwen, C.N. Development of a robotics platform for automated multi-ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2021, 35, e8449. [Google Scholar] [CrossRef]
- Meher, A.K.; Chen, Y.-C. Electrospray modifications for advancing mass spectrometric analysis. Mass Spectrom. 2017, 6, S0057-S. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meher, A.K.; Chen, Y.C. Polarization induced electrospray ionization mass spectrometry for the analysis of liquid, viscous and solid samples. J. Mass Spectrom. 2015, 50, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Meher, A.K.; Chen, Y.-C. Tissue paper assisted spray ionization mass spectrometry. RSC Adv. 2015, 5, 94315–94320. [Google Scholar] [CrossRef]
- Malik, A.K.; Setia, A.; Mehata, A.K.; Priya, V.; Suseela, M.N.L.; Gokul, P.; Fredo, A.J.; Jain, S.K.; Selvin, J.; Muthu, M.S. Green analytical chemistry: Experimental and chemometric methods for the detection of therapeutics using liquid chromatography in wastewater samples. Anal. Chem. Lett. 2024, 14, 1–28. [Google Scholar] [CrossRef]
- Paul, A.; de Boves Harrington, P. Chemometric applications in metabolomic studies using chromatography-mass spectrometry. TrAC Trends Anal. Chem. 2021, 135, 116165. [Google Scholar] [CrossRef]
- Cadeado, A.N.; Machado, C.C.; Oliveira, G.C.; Silva DASe Muñoz, R.A.; Silva, S.G. Internet of things as a tool for sustainable analytical chemistry: A review. J. Braz. Chem. Soc. 2022, 33, 681–692. [Google Scholar] [CrossRef]
- Ayres, L.B.; Gomez, F.J.; Linton, J.R.; Silva, M.F.; Garcia, C.D. Taking the leap between analytical chemistry and artificial intelligence: A tutorial review. Anal. Chim. Acta 2021, 1161, 338403. [Google Scholar] [CrossRef] [PubMed]
- Rial, R.C. AI in analytical chemistry: Advancements, challenges, and future directions. Talanta 2024, 274, 125949. [Google Scholar] [CrossRef]
- Kowtharapu, L.P.; Katari, N.K.; Muchakayala, S.K.; Marisetti, V.M. Green metric tools for analytical methods assessment critical review, case studies and crucify. TrAC Trends Anal. Chem. 2023, 166, 117196. [Google Scholar] [CrossRef]


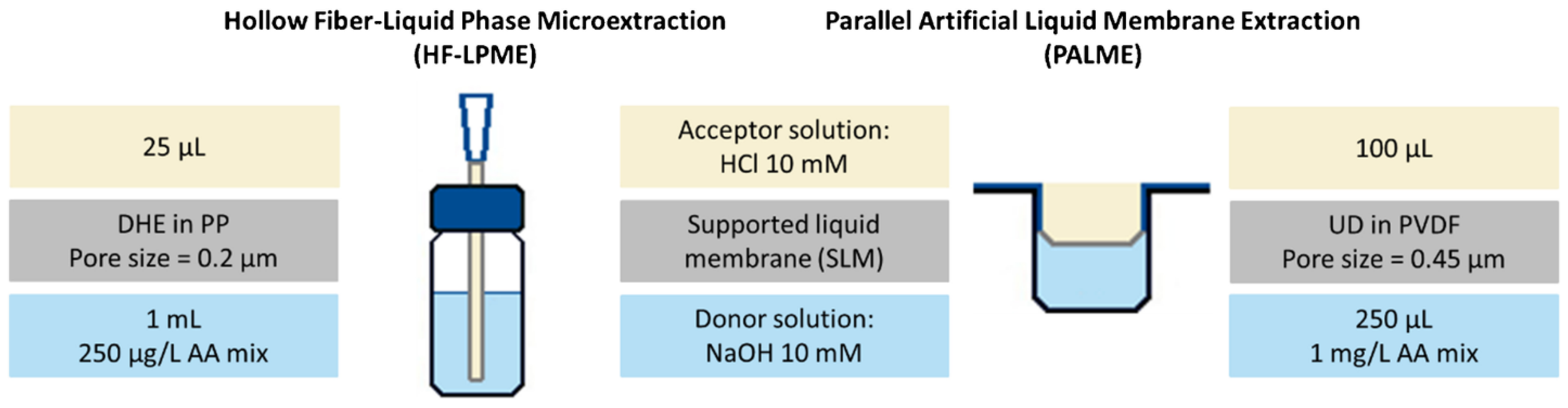
| Aspect | Traditional LC | Green LC |
|---|---|---|
| Retention Time | Shorter, due to higher elution strength | Longer, especially for hydrophobic analytes |
| Resolution (Rs) | Typically high (e.g., Rs > 1.5) | Comparable with optimization |
| Peak Symmetry | Sharp peaks, good symmetry | May broaden, but comparable with polar-embedded phases |
| Sensitivity (LOD) | Higher (e.g., 0.05 µg/mL) | Slightly lower (e.g., 0.08 µg/mL) |
| Environmental Impact | High waste, hazardous solvents | Reduced waste, eco-friendly |
| Optimization Needs | Standard, less complex | Requires specialized columns, temperature adjustments |
| Aspect | Standard LC Columns (4.6 mm i.d.) | Capillary LC Columns (10–100 µm i.d.) |
|---|---|---|
| Column Efficiency (N) | ~50,000 plates/m | >100,000 plates/m, due to smaller particles |
| Resolution (Rs) | Moderate to high (e.g., Rs = 1.8 for enantiomers) | Higher (e.g., Rs = 2.5 for enantiomers) |
| Solvent Consumption | High (1–1.5 mL/min flow rate) | Low (e.g., 300 nL/min, 100–1000× reduction) |
| Sample Capacity | High, microgram range | Low, nanogram range, limits preparative use |
| Sensitivity | Standard, may require larger injection volumes | Enhanced, due to reduced dilution, better with MS |
| Trade-offs | Lower efficiency, higher waste | Higher efficiency, but lower capacity, higher cost |
| Industry | Application | Example Companies | Green Alignment |
|---|---|---|---|
| Environmental Monitoring | Detect heavy metals, greenhouse gases, air quality | Sensorix, Figaro USA Inc., Cambridge Sensotec | Reduces pollution, real-time monitoring |
| Agriculture | Soil moisture, nutrient levels, plant health | Multi Nano Sense Technologies | Optimizes resource use, reduces waste |
| Medical/Pharmaceutical | Sweat chloride, drug degradation kinetics | Sensirion AG, Cambridge Sensotec | Supports sustainable healthcare, low waste |
| Food Industry | Detect contaminants like dyes, monitor storage conditions | Figaro USA Inc., Sensirion AG | Enhances food safety, reduces chemical use |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meher, A.K.; Zarouri, A. Green Analytical Chemistry—Recent Innovations. Analytica 2025, 6, 10. https://doi.org/10.3390/analytica6010010
Meher AK, Zarouri A. Green Analytical Chemistry—Recent Innovations. Analytica. 2025; 6(1):10. https://doi.org/10.3390/analytica6010010
Chicago/Turabian StyleMeher, Anil Kumar, and Akli Zarouri. 2025. "Green Analytical Chemistry—Recent Innovations" Analytica 6, no. 1: 10. https://doi.org/10.3390/analytica6010010
APA StyleMeher, A. K., & Zarouri, A. (2025). Green Analytical Chemistry—Recent Innovations. Analytica, 6(1), 10. https://doi.org/10.3390/analytica6010010







