Electroanalysis Advances in Pharmaceutical Sciences: Applications and Challenges Ahead
Abstract
1. Introduction
Importance of Electroanalysis in the Pharmaceutical Industry
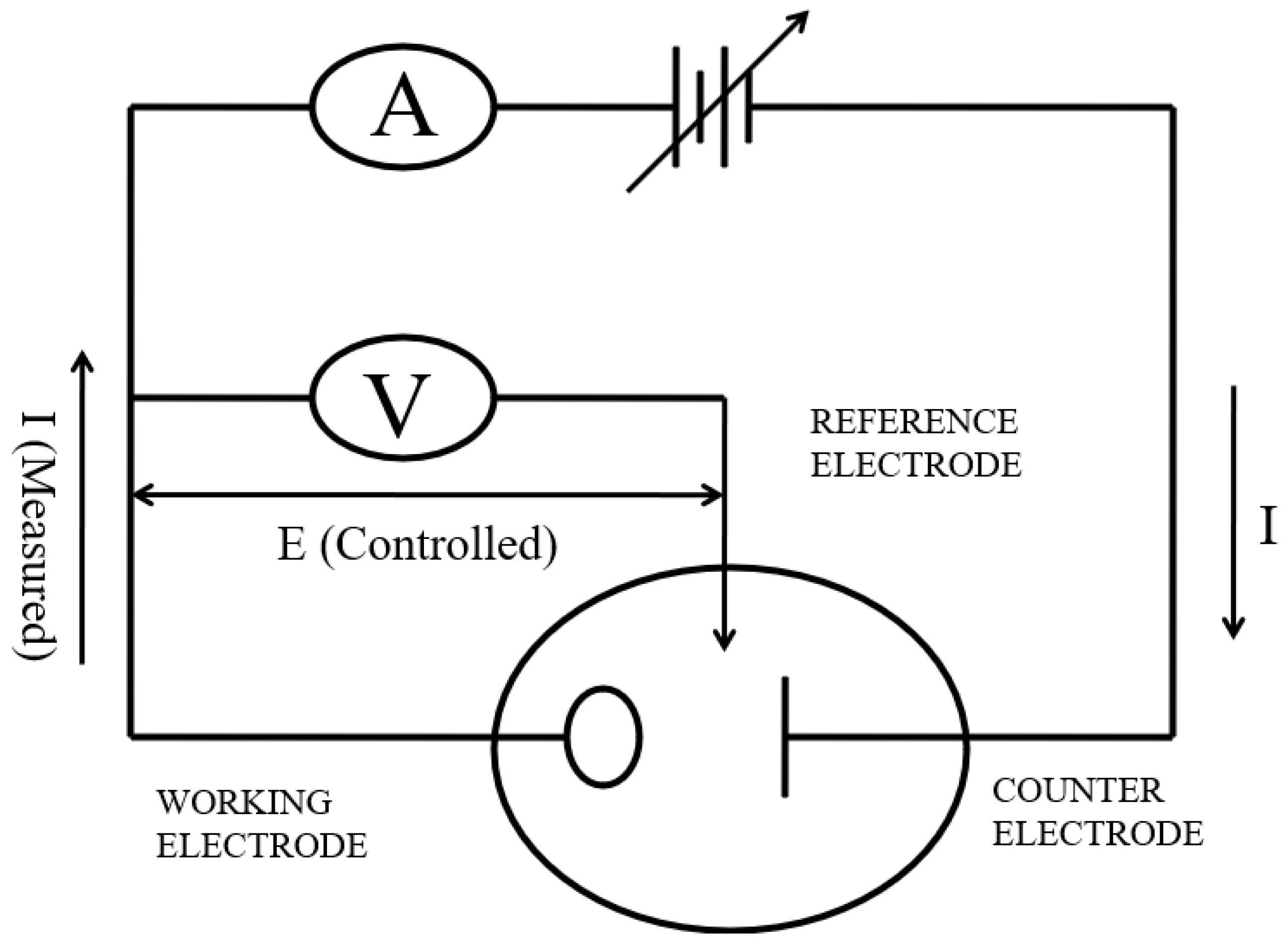
2. Fundamentals of Electroanalysis
2.1. Principles of Electrochemical Methods
2.2. Different Electrochemical Techniques
2.2.1. Voltammetry
2.2.2. Potentiometry
2.2.3. Amperometry
2.3. Electrodes and Sensors Used in Electroanalysis
2.4. Linear Sweep Voltammetry (LSV) and Polarization Methods in Electroanalysis
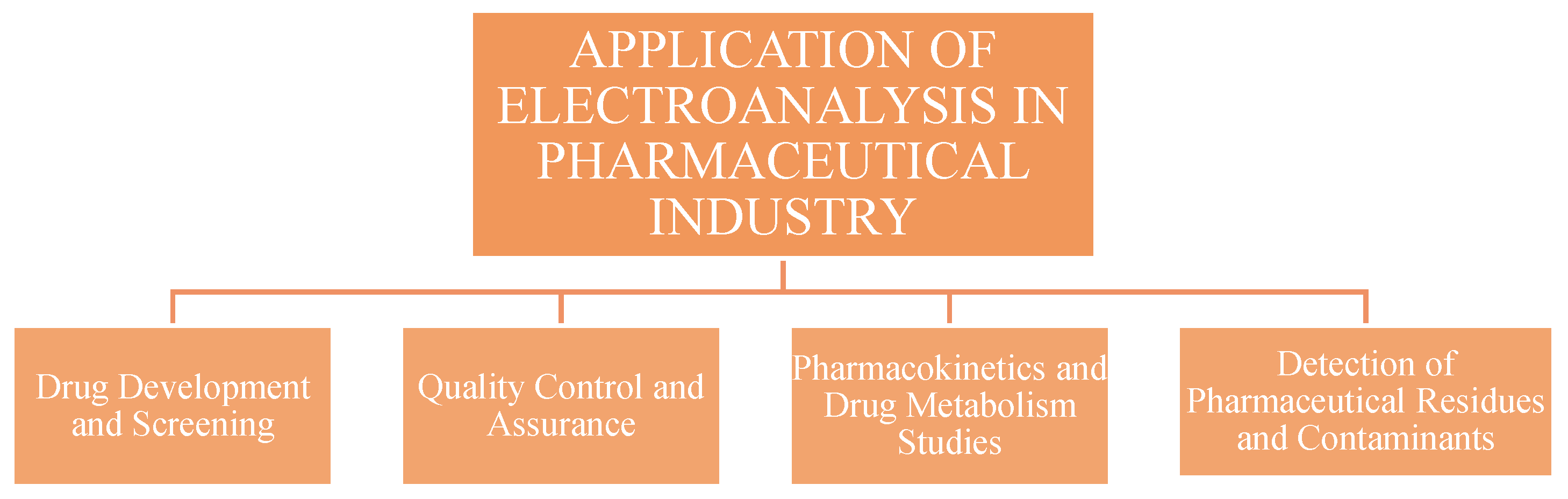
3. Applications of Electroanalysis in Pharmaceutical Industry
3.1. Drug Development and Screening
3.2. Quality Control and Assurance
3.3. Pharmacokinetics and Drug Metabolism Studies
3.4. Detection of Pharmaceutical Residues and Contaminants
4. Electroanalytical Techniques in Pharmaceutical Analysis
4.1. Voltammetric Methods
4.1.1. Cyclic Voltammetry
4.1.2. Differential Pulse Voltammetry
4.1.3. Square Wave Voltammetry
4.2. Potentiometric Methods
4.2.1. Ion-Selective Electrodes
4.2.2. pH Measurement
4.3. Amperometric Methods
4.3.1. Chronoamperometry
4.3.2. Flow Injection Analysis
5. Practical Applications
| S. No. | Properties | Electroanalytical Method | Application | Ref. |
|---|---|---|---|---|
| 1. | Drug Development and Research | Cyclic Voltammetry (CV) | Characterizing redox properties of drug candidates | [62] |
| Electroanalytical Screening | Identifying potential drug candidates | |||
| 2. | Quality Control and Assurance | Differential Pulse Voltammetry (DPV) and Square Wave Voltammetry (SWV) | Determining APIs in pharmaceutical formulations | [63] |
| Electroanalysis | Detecting impurities and degradation products | |||
| 3. | Pharmacokinetics and Drug Metabolism | Electrochemical Sensors | Monitoring drug metabolism in biological fluids | [64] |
| Electroanalysis | Assessing drug bioavailability in plasma and tissues | |||
| 4. | Therapeutic Drug Monitoring (TDM) | Electrochemical Sensors | Real-time monitoring of drug levels in patients | [65] |
| Electrochemical Devices | Point-of-care testing for rapid drug measurement | |||
| 5. | Environmental Monitoring | Electroanalysis | Detecting pharmaceutical residues in environmental samples | [66] |
| 6. | Counterfeit Drug Detection | Electrochemical Techniques | Authenticating pharmaceuticals and detecting counterfeit drugs | |
| 7. | Electrochemical Biosensors | Enzyme-Based Sensors | Detecting drug molecules and metabolites | [67] |
| 8. | Electrochemical Impedance Spectroscopy (EIS) | EIS | Studying drug-target interactions (proteins, DNA) | [68] |
| EIS | Characterizing drug delivery systems (nanoparticles, hydrogels) | |||
| Real-world Examples in Drug Analysis | ||||
| 9. | Detection of Pharmaceutical Compounds | Cyclic Voltammetry (CV) | Detecting paracetamol in tablets and biological fluids | [69] |
| Differential Pulse Voltammetry (DPV) | Detecting propranolol in pharmaceutical tablets | |||
| 10. | Analysis of Antibiotics | Square Wave Voltammetry (SWV) | Determining ciprofloxacin in milk samples | [70] |
| Amperometric Detection (with HPLC) | Analyzing amoxicillin in urine samples | |||
| 11. | Analysis of Illicit Drugs | Electrochemical Sensors | On-site detection of cocaine and methamphetamine | [71] |
| Potentiometric Methods | Measuring morphine metabolites in urine samples | |||
| 12. | Quality Control in Drug Manufacturing | Electrochemical Impedance Spectroscopy (EIS) | Studying drug release profiles in controlled-release tablets | [72] |
| Chronoamperometry | Monitoring degradation and stability of pharmaceutical compounds | |||
5.1. Drug Development and Research
5.1.1. Electrochemical Characterization in Drug Development
- (i)
- Stability Assessment—CV helps determine whether a drug candidate undergoes oxidation or reduction under physiological conditions, predicting its shelf life and degradation pathways.
- (ii)
- Reactivity and Mechanism Studies—Understanding a molecule’s electrochemical behavior allows researchers to evaluate its interaction with biological targets and predict possible side reactions that could affect its efficacy.
- (iii)
- Metabolic Pathway Prediction—Many drug metabolism processes involve oxidation or reduction, often mediated by enzymes like cytochrome P450. CV mimics these redox reactions and offers insights into the potential metabolic products and their biological activities.
- (iv)
- Structure-Activity Relationship (SAR) Analysis—Electrochemical data can be correlated with a drug’s pharmacological properties, aiding in the rational design of more effective and stable pharmaceutical compounds.
- (v)
- Drug Formulation and Delivery Optimization—By studying the electrochemical behavior, researchers can modify formulations to enhance solubility, bioavailability, and targeted drug release. Cyclic voltammetry and complementary electroanalytical techniques significantly enhance drug characterization, contributing to the development of safer and more effective therapeutics [74].
5.1.2. Screening of Drug Compounds Using Electroanalytical Methods
- (i)
- Rapid Identification of Active Compounds—Electrochemical techniques, such as CV and DPV, allow for the quick assessment of redox-active compounds, helping to narrow down promising drug candidates from large molecular libraries.
- (ii)
- Prediction of Pharmacokinetics and Metabolism—Many drugs undergo oxidation or reduction during metabolism. Screening for electrochemical activity helps predict how a compound will be processed in vivo, aiding drug design and optimization.
- (iii)
- Cost-Effective and High-Throughput Analysis—Compared to conventional biological assays, electroanalytical methods provide a faster and more economical approach for evaluating drug properties, making them ideal for early-stage screening.
- (iv)
- Assessment of Drug Stability—By analyzing oxidation-reduction potentials, researchers can determine a compound’s susceptibility to degradation, ensuring the selection of chemically stable candidates.
- (v)
- Environmental and Toxicity Profiling—Electrochemical screening can also help identify potentially toxic compounds or those prone to environmental persistence, contributing to safer drug development. By integrating electroanalytical methods into drug discovery workflows, pharmaceutical researchers can efficiently evaluate large compound libraries, thereby accelerating the development of effective and safe therapeutic agents [76].
5.2. Quality Control and Assurance
- (i)
- Determination of Active Pharmaceutical Ingredients (APIs)
- (ii)
- Detection of Impurities and Degradation Products
5.3. Pharmacokinetics and Drug Metabolism
- (i)
- Monitoring Drug Metabolism
- (ii)
- Bioavailability Studies
5.4. Therapeutic Drug Monitoring (TDM)
- (i)
- Real-Time Monitoring of Drug Levels
- (ii)
- Point-of-Care Testing (POCT)
5.5. Environmental Monitoring Using Electroanalytical Techniques
- (i)
- Detection of Pharmaceutical Residues in Environmental Samples
- (ii)
- Ensuring Compliance with Environmental Regulations
5.6. Counterfeit Drug Detection Using Electroanalytical Techniques
- (i)
- Authentication of Pharmaceuticals
- (ii)
- Rapid and Portable Screening for Law Enforcement and Pharmacies
5.7. Real-World Examples in Drug Analysis
5.7.1. Detection of Pharmaceutical Compounds
- Cyclic Voltammetry (CV): This technique is applied to detect and quantify pharmaceutical compounds, such as paracetamol (acetaminophen), in dosage forms and biological fluids. This technique provides high sensitivity and accuracy, allowing for effective monitoring of drug concentration.
- Differential Pulse Voltammetry (DPV): Employed for the analysis of drugs like propranolol, a beta-blocker used in treating hypertension. DPV was used to detect propranolol in pharmaceutical tablets. This method offers high selectivity and sensitivity, allowing the drug to be distinguished from other substances in a sample [90].
5.7.2. Analysis of Antibiotics
- Square Wave Voltammetry (SWV): This technique is used to determine antibiotic residues in food products and biological samples. SWV has been used to detect ciprofloxacin, an antibiotic, in milk samples. This technique provides rapid and accurate results, ensuring food safety and compliance with regulatory standards.
- Amperometric Detection: This method is used with liquid chromatography to determine antibiotics in pharmaceutical formulations and biological fluids. Amperometric detection was coupled with high-performance liquid chromatography (HPLC) to analyze amoxicillin in urine samples. This combined approach enhances the sensitivity and precision of the measurements [91].
5.7.3. Analysis of Illicit Drugs
- Electrochemical Sensors: Portable electrochemical sensors are used for the on-site detection of illicit drugs, such as cocaine and methamphetamine. An electrochemical sensor was developed to detect cocaine in banknotes. The sensor provides rapid, non-destructive testing, enabling law enforcement agencies to quickly identify contaminated currency.
- Potentiometric Methods: Used for the determination of drug metabolites in biological fluids. Potentiometric ion-selective electrodes were used to measure the concentrations of morphine metabolites in the urine samples. This technique offers high specificity and ease of use, making it suitable for both clinical and forensic applications [92].
5.7.4. Quality Control in Drug Manufacturing
- Electrochemical Impedance Spectroscopy (EIS): Used for the characterization of drug release profiles in pharmaceutical formulations. EIS was used to study the release of a drug from a polymer matrix in controlled-release tablets. This technique provides detailed information on the drug release mechanisms and kinetics.
- Chronoamperometry: Used to monitor the electrochemical stability and degradation of pharmaceutical compounds. Chronoamperometry was employed to investigate the stability of ascorbic acid (vitamin C) in a pharmaceutical formulation. This method allows for real-time monitoring of degradation processes, ensuring product quality and shelf life. Table 2 lists the methods and applications of electroanalysis in drug analysis.
5.8. Comparative Studies with Other Analytical Methods
- Cyclic Voltammetry (CV): CV is often employed in the initial stages of method development due to its ability to elucidate the redox behavior of paracetamol. While it offers valuable mechanistic insights, its sensitivity is moderate, making it less suitable for detecting low concentrations.
- Differential Pulse Voltammetry (DPV): DPV enhances sensitivity by applying a series of voltage pulses, allowing the detection of paracetamol at trace levels. Its high selectivity minimizes interference from other substances, making it ideal for use in complex matrices.
- Square Wave Voltammetry (SWV): SWV combines rapid analysis with high sensitivity, making it effective in environments with significant background noise. Its ability to discriminate capacitive currents enhances the detection of paracetamol in various samples.
- Amperometric Detection: This technique is advantageous for continuous monitoring applications, such as flow systems or biosensors. Its very high sensitivity enables the detection of paracetamol at very low concentrations. However, it may suffer from interference from other electroactive species present in the sample.
- Electrochemical Impedance Spectroscopy (EIS): EIS is valuable for characterizing electrode surfaces and understanding interfacial phenomena. While not typically used for the direct quantification of paracetamol, it plays a crucial role in developing and optimizing electrochemical sensors for its detection. The choice of electroanalytical method for detecting paracetamol depends on the specific requirements of the analysis, including the desired sensitivity, selectivity, sample matrix, and available instrumentation. For instance, DPV and SWV are preferred for trace analysis due to their high sensitivity, whereas amperometric detection is suitable for real-time monitoring applications. Understanding the unique capabilities of each technique ensures the selection of the most appropriate method for the accurate and reliable detection of pharmaceuticals like paracetamol.
6. Advancements in Electroanalysis for Pharmaceuticals
6.1. Recent Technological Developments
6.2. Integration with Other Analytical Techniques
| S. No. | Analytical Technique | Electroanalysis Integration | Applications | Ref. |
|---|---|---|---|---|
| 1. | Spectroscopy (UV-Vis, IR, Raman, etc.) | Electrochemical methods can provide information about redox states, which complements spectroscopic data. | Identification of electroactive species, monitoring reaction kinetics, studying redox properties. | [70] |
| 2. | Mass Spectrometry (MS) | Coupling electroanalysis with MS allows real-time monitoring of electrochemical reactions and the identification of products. | Detection of reaction intermediates and products, studying reaction mechanisms. | |
| 3. | Chromatography (HPLC, GC) | Electrochemical detectors are often used after chromatographic separation to detect analytes that are electroactive. | Separation and quantification of trace analytes, detection of pollutants in environmental analysis. | [108] |
| 4. | Atomic Absorption Spectroscopy (AAS) | Electrochemical pretreatment can concentrate analytes for enhanced detection by AAS. | Trace metal analysis, improving detection limits in metal ion quantification. | |
| 5. | X-ray Diffraction (XRD) | Electrochemical processes can alter the structure of materials, which can then be studied using XRD to observe changes in crystallinity. | Characterization of electrode materials, study of phase changes during electrochemical cycling. | [109] |
| 6. | Thermogravimetric Analysis (TGA) | Electrochemical reactions can be studied with TGA to observe weight changes corresponding to redox reactions and material decomposition. | Studying degradation of battery materials, analysis of oxidation/reduction mechanisms. | [110] |
| 7. | Nuclear Magnetic Resonance (NMR) | Electroanalysis can alter the chemical environment, providing insight into changes that can be analyzed via NMR. | Studying the structure of electroactive molecules and understanding molecular interactions during redox reactions. | [111] |
| 8. | Microscopy (SEM, TEM, AFM) | Electrochemical techniques are combined with microscopy to visualize changes in surface morphology during reactions. | Monitoring electrode surface changes, studying corrosion, material science applications. | [112] |
| 9. | Capillary Electrophoresis (CE) | Electrochemical detectors can be coupled with CE for the detection of electroactive species after electrophoretic separation. | Separation and detection of biomolecules, drugs, and ions. | |
| 10. | Fluorescence Spectroscopy | Fluorescent molecules can be used as probes in electrochemical systems to monitor redox processes in real-time. | Biosensing applications, detection of specific ions and molecules, bioimaging of electroactive species. | |
| 11. | Spectroelectrochemistry (SEC) | Electrochemical techniques are coupled with spectroscopic methods (UV-Vis, IR, Raman) to monitor electrochemical reactions and their corresponding spectral changes simultaneously. | Identification of redox states, tracking reaction intermediates, real-time monitoring of charge transfer mechanisms in pharmaceuticals and biomolecules. | [113] |
| 12. | Voltabsorptometry (VA) | Correlates voltammetric data with optical absorption measurements to analyze changes in electronic states during redox reactions. | Characterization of electroactive compounds, study of oxidation-reduction processes, evaluation of molecular stability under electrochemical conditions. |
6.3. Innovations in Sensor Materials and Design
7. Challenges and Limitations
7.1. Sensitivity and Selectivity Issues
7.2. Regulatory and Standardization Challenges
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bard, A.J.; Faulkner, L.R. Electrochemical methods: Fundamentals and applications. Surf. Technol. 1983, 20, 91–92. [Google Scholar]
- Gupta, V.K.; Jain, R.; Agarwal, S.; Mishra, R.; Dwivedi, A. Electrochemical determination of antihypertensive drug irbesartan in pharmaceuticals. Anal. Biochem. 2011, 410, 266–271. [Google Scholar] [PubMed]
- Crow, D.R. Analytical Electrochemistry. In Principles and Applications of Electrochemistry, 3rd ed.; Wang, J., Ed.; Routledge: London, UK; Wiley-VCH: Hoboken, NJ, USA, 1994. [Google Scholar]
- Bounegru, A.V.; Dinu Iacob, A.; Iticescu, C.; Georgescu, P.L. Electrochemical Sensors and Biosensors for the Detection of Pharmaceutical Contaminants in Natural Waters—A Comprehensive Review. Chemosensors 2025, 13, 65. [Google Scholar] [CrossRef]
- Khonina, S.N.; Kazanskiy, N.L. Trends and Advances in Wearable Plasmonic Sensors Utilizing Surface-Enhanced Raman Spectroscopy (SERS): A Comprehensive Review. Sensors 2025, 25, 1367. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.; Gurevich, L.; Magnusson, N.E. Aspects of Electrochemical Biosensors Using Affinity Assays. Biosensors 2025, 15, 166. [Google Scholar] [CrossRef]
- Tonanon, P. Electrochemistry for Environmental Applications: Water Disinfection, Pharmaceutical Analysis, and CO2 Reduction. Ph.D. Thesis, Nanyang Technological University, Singapore, 2025. [Google Scholar]
- El Hamdouni, Y.; Labjar, N.; Laasri, S.; Dalimi, M.; Labjar, H.; El Hajjaji, S. Applications and Commercialization Challenges of Voltammetry in Biosensing Applications. In Advancements in Voltammetry for Biosensing Applications; Springer: Singapore, 2025; pp. 461–482. [Google Scholar]
- Adamson, H.; Bond, A.M.; Parkin, A. Probing biological redox chemistry with large amplitude Fourier transformed ac voltammetry. Chem. Commun. 2017, 53, 9519–9533. [Google Scholar]
- Alyamni, N.; Abot, J.L.; Zestos, A.G. Perspective—Advances in voltammetric methods for the measurement of biomolecules. ECS Sens. Plus 2024, 3, 027001. [Google Scholar]
- Rodeberg, N.T.; Sandberg, S.G.; Johnson, J.A.; Phillips, P.E.; Wightman, R.M. Hitchhiker’s guide to voltammetry: Acute and chronic electrodes for in vivo fast-scan cyclic voltammetry. ACS Chem. Neurosci. 2017, 8, 221–234. [Google Scholar] [CrossRef]
- Bakirhan, N.K.; Topal, B.D.; Ozcelikay, G.; Karadurmus, L.; Ozkan, S.A. Current advances in electrochemical biosensors and nanobiosensors. Crit. Rev. Anal. Chem. 2022, 52, 519–534. [Google Scholar]
- Mahajan, P.G.; Yadav, A.; Tiwari, P.; Chandrashekharappa, S. Brief Overview of Voltammetry for Biosensing Applications. In Advancements in Voltammetry for Biosensing Applications; Springer: Singapore, 2025; pp. 45–62. [Google Scholar]
- Crapnell, R.D.; Ferrari, A.G.; Dempsey, N.C.; Banks, C.E. Electroanalytical overview: Screen-printed electrochemical sensing platforms for the detection of vital cardiac, cancer and inflammatory biomarkers. Sens. Diagn. 2022, 1, 405–428. [Google Scholar]
- Mincu, N.B.; Lazar, V.; Stan, D.; Mihailescu, C.M.; Iosub, R.; Mateescu, A.L. Screen-Printed Electrodes (SPE) for in vitro diagnostic purpose. Diagnostics 2020, 10, 517. [Google Scholar] [CrossRef] [PubMed]
- Couto, R.A.; Lima, J.L.; Quinaz, M.B. Recent developments, characteristics and potential applications of screen-printed electrodes in pharmaceutical and biological analysis. Talanta 2016, 146, 801–814. [Google Scholar]
- Sousa, C.P.; Ribeiro, F.W.; Oliveira, T.M.; Salazar-Banda, G.R.; de Lima-Neto, P.; Morais, S.; Correia, A.N. Electroanalysis of pharmaceuticals on boron-doped diamond electrodes: A review. ChemElectroChem 2019, 6, 2350–2378. [Google Scholar] [CrossRef]
- Janssen, L.J.; Koene, L. The role of electrochemistry and electrochemical technology in environmental protection. Chem. Eng. J. 2002, 85, 137–146. [Google Scholar]
- Lalei, M.; Zarei, K. One-Step Electrochemical Modification of Pencil Graphite Electrode with Poly (DPASA-co-VP)-RuO2NPs and its Application for the Trace Analysis of Sumatriptan. J. Electron. Mater. 2025, 54, 251–261. [Google Scholar]
- Vural, K.; Dilgin, D.G.; Dilgin, Y. An application of a disposable electrode for the sensitive, selective, and cost-effective voltammetric determination of antiparkinsonism drug entacapone based on both its oxidation and reduction. J. Solid State Electrochem. 2025, 1–11. [Google Scholar] [CrossRef]
- Rathore, S.S.; Jenita, J.J. A Comprehensive Review of Analytical Techniques for Quantifying Cyclin-dependent Kinase 4 and 6 Inhibitors in Biological Samples, Bulk, and Pharmaceutical Samples. Sep. Sci. Plus 2025, 8, e202400211. [Google Scholar]
- Karakaya, S.; Dilgin, Y. Disposable and sensitive electrochemical determination of molnupiravir at a pencil graphite electrode (PGE) by differential pulse voltammetry (DPV). Anal. Lett. 2024, 57, 783–796. [Google Scholar]
- Malode, S.J.; Ali Alshehri, M.; Shetti, N.P. Nanomaterial-Based Electrochemical Sensors for the Detection of Pharmaceutical Drugs. Chemosensors 2024, 12, 234. [Google Scholar] [CrossRef]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical methods for the analysis of clinically relevant biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef] [PubMed]
- Bard, A.J.; Zoski, C.G. Electroanalytical Chemistry: A Series of Advances; CRC Press: Boca Raton, FL, USA, 2010; Volume 23. [Google Scholar]
- Wang, J. Voltammetry following nonelectrolytic preconcentration. In Electroanalytical Chemistry; CRC Press: Boca Raton, FL, USA, 2021; pp. 1–88. [Google Scholar]
- Kissinger, P.; Heineman, W.R. (Eds.) Laboratory Techniques in Electroanalytical Chemistry, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Kauffmann, J.M.; Guilbault, G.G. Biosensors. In Analysis of Addictive and Misused Drugs; CRC Press: Boca Raton, FL, USA, 2021; pp. 21–40. [Google Scholar]
- Bakker, E.; Telting-Diaz, M. Electrochemical sensors. Anal. Chem. 2002, 74, 2781–2800. [Google Scholar] [CrossRef] [PubMed]
- Desagani, D.; Ben-Yoav, H. Chemometrics meets electrochemical sensors for intelligent in vivo bioanalysis. TrAC Trends Anal. Chem. 2023, 164, 117089. [Google Scholar] [CrossRef]
- Gumustas, M.; Ozkan, S.A. Electrochemical evaluation and determination of antiretroviral drug fosamprenavir using boron-doped diamond and glassy carbon electrodes. Anal. Bioanal. Chem. 2010, 397, 189–203. [Google Scholar] [CrossRef]
- Buffa, A.; Erel, Y.; Mandler, D. Carbon nanotube based flow-through electrochemical cell for electroanalysis. Anal. Chem. 2016, 88, 11007–11015. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Ali, M.A. Nanomaterials in biosensors: Fundamentals and applications. Nanomater. Biosens. 2018, 1–74. [Google Scholar] [CrossRef]
- Nesakumar, N.; Kesavan, S.; Li, C.Z.; Alwarappan, S. Microfluidic electrochemical devices for biosensing. J. Anal. Test. 2019, 3, 3–18. [Google Scholar] [CrossRef]
- Baum, Z.J.; Yu, X.; Ayala, P.Y.; Zhao, Y.; Watkins, S.P.; Zhou, Q. Artificial intelligence in chemistry: Current trends and future directions. J. Chem. Inf. Model. 2021, 61, 3197–3212. [Google Scholar] [CrossRef]
- Liu, Y.L.; Liu, R.; Qin, Y.; Qiu, Q.F.; Chen, Z.; Cheng, S.B.; Huang, W.H. Flexible electrochemical urea sensor based on surface molecularly imprinted nanotubes for detection of human sweat. Anal. Chem. 2018, 90, 13081–13087. [Google Scholar] [CrossRef]
- Mohan, A.V.; Rajendran, V.; Mishra, R.K.; Jayaraman, M. Recent advances and perspectives in sweat based wearable electrochemical sensors. TrAC Trends Anal. Chem. 2020, 131, 116024. [Google Scholar] [CrossRef]
- Patil, R.; Jain, V. Andrographolide: A review of analytical methods. J. Chromatogr. Sci. 2021, 59, 191–203. [Google Scholar] [PubMed]
- Rees, N.V.; Compton, R.G. Electrochemical Applications of Power Ultrasound. In Ultrasound in Chemistry: Analytical Applications; Wiley-VCH: Hoboken, NJ, USA, 2009; Volume 81. [Google Scholar]
- March, G.; Nguyen, T.D.; Piro, B. Modified electrodes used for electrochemical detection of metal ions in environmental analysis. Biosensors 2015, 5, 241–275. [Google Scholar] [CrossRef] [PubMed]
- Uslu, B.; Ozkan, S.A. Solid Electrodes in Electroanalytical Chemistry: Present Applications and Prospects for High Throughput Screening of Drug Compounds. Comb. Chem. High Throughput Screen. 2007, 10, 495–513. [Google Scholar] [PubMed]
- Smith, K. The Removal of Selected Pharmaceuticals from a Municipal Membrane Bioreactor Secondary Effluent with an Electrochemical Oxidation Process. Ph.D. Thesis, Cape Peninsula University of Technology, Cape Town, South Africa, 2023. [Google Scholar]
- Thomas, J.D.R.; Janata, J.; Guilbault, G.G.; Briggs, R.; Christian, G.D. International Symposium on Electroanalysis in Clinical, Environmental and Pharmaceutical Chemistry. Anal. Proc. 1982, 19, 60b–79. [Google Scholar]
- Arvand, M.; Fallahi, P. Voltammetric determination of rivastigmine in pharmaceutical and biological samples using molecularly imprinted polymer modified carbon paste electrode. Sens. Actuators B Chem. 2013, 188, 797–805. [Google Scholar]
- Brown, M.D.; Schoenfisch, M.H. Electrochemical nitric oxide sensors: Principles of design and characterization. Chem. Rev. 2019, 119, 11551–11575. [Google Scholar]
- Freitas, L.; Lima, K.; Silva, S.; Leite, F.; Fernandes, R.; Santos, W.; Damos, F.; Luz, R. Amperometric electrochemical platform for hydrazine determination exploiting reduced graphene oxide, co (salophen) and DNA: Application in pharmaceutical formulations samples. J. Braz. Chem. Soc. 2018, 29, 2096–2103. [Google Scholar]
- Ghoneim, M.M.; El-Desoky, H.S.; Abdel-Galeil, M.M. Voltammetry of naltrexone in commercial formulation and human body fluids: Quantification and pharmacokinetic studies. Bioelectrochemistry 2011, 81, 65–73. [Google Scholar]
- Ağın, F. A Review of Voltammetric Methods for Determination of Dopamine Agonists. Curr. Anal. Chem. 2021, 17, 1104–1113. [Google Scholar]
- Siddiqui, M.R.; AlOthman, Z.A.; Rahman, N. Analytical techniques in pharmaceutical analysis: A review. Arab. J. Chem. 2017, 10, S1409–S1421. [Google Scholar]
- da Silveira, G.D.; Di Turo, F.; Dias, D.; da Silva, J.A.F. Electrochemical analysis of organic compounds in solid-state: Applications of voltammetry of immobilized microparticles in bioanalysis and cultural heritage science. J. Solid State Electrochem. 2020, 24, 2633–2652. [Google Scholar] [CrossRef]
- Murphy, E. Electroanalysis of Small Molecule Therapeutics at Nanostructured Electrode Surfaces. Ph.D. Thesis, National University of Ireland Maynooth, Maynooth, Ireland, 2023. [Google Scholar]
- Di, L.; Kerns, E.H.; Carter, G.T. Drug-like property concepts in pharmaceutical design. Curr. Pharm. Des. 2009, 15, 2184–2194. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Banks, C.E. Electroanalytical overview: The electroanalytical detection of theophylline. Talanta Open 2021, 3, 100037. [Google Scholar] [CrossRef]
- Zheng, X.; Wojcik, R.; Zhang, X.; Ibrahim, Y.M.; Burnum-Johnson, K.E.; Orton, D.J.; Monroe, M.E.; Moore, R.J.; Smith, R.D.; Baker, E.S. Coupling front-end separations, ion mobility spectrometry, and mass spectrometry for enhanced multidimensional biological and environmental analyses. Annu. Rev. Anal. Chem. 2017, 10, 71–92. [Google Scholar]
- Azimi, S.; Docoslis, A. Recent advances in the use of surface-enhanced Raman scattering for illicit drug detection. Sensors 2022, 22, 3877. [Google Scholar] [CrossRef]
- Wirzal, M.D.H.B. Determination of Nifedipine, Ampicillin and Penicillin G and Their Electro-oxidation Products by Voltammetric Techniques. Ph.D. Thesis, Universiti Teknologi Malaysia, Johor Bahru, Malaysia, 2016. [Google Scholar]
- Blaha, C.D.; Phillips, A.G. A critical assessment of electrochemical procedures applied to the measurement of dopamine and its metabolites during drug-induced and species-typical behaviours. Behav. Pharmacol. 1996, 7, 675–708. [Google Scholar]
- Anojčić, J.; Guzsvány, V.; Kónya, Z.; Mikov, M. Rapid, trace-level direct cathodic voltammetric determination of dopamine by oxidized multiwalled carbon nanotube–modified carbon paste electrode in selected samples of pharmaceutical importance. Ionics 2019, 25, 6093–6106. [Google Scholar]
- Martin, R.S.; Root, P.D.; Spence, D.M. Microfluidic technologies as platforms for performing quantitative cellular analyses in an in vitro environment. Analyst 2006, 131, 1197–1206. [Google Scholar]
- Bonet-San-Emeterio, M.; Felipe Montiel, N.; Del Valle, M. Graphene for the building of electroanalytical enzyme-based biosensors. Appl. Inhib. Detect. Emerg. Pollut. Nanomater. 2021, 11, 2094. [Google Scholar]
- Merkoçi, A.; Pumera, M.; Llopis, X.; Pérez, B.; Del Valle, M.; Alegret, S. New materials for electrochemical sensing VI: Carbon nanotubes. TrAC Trends Anal. Chem. 2005, 24, 826–838. [Google Scholar]
- Fernando, P.A.I.; Glasscott, M.W.; Pokrzywinski, K.; Fernando, B.M.; Kosgei, G.K.; Moores, L.C. Analytical methods incorporating molecularly imprinted polymers (MIPs) for the quantification of microcystins: A mini-review. Crit. Rev. Anal. Chem. 2022, 52, 1244–1258. [Google Scholar] [CrossRef] [PubMed]
- Ozer, T.; Henry, C.S. Recent Trends in Nanomaterial Based Electrochemical Sensors for Drug Detection: Considering Green Assessment. Curr. Top. Med. Chem. 2024, 24, 952–972. [Google Scholar] [CrossRef]
- Radzik, D.M.; Lunte, S.M. Application of liquid chromatography/electrochemistry in pharmaceutical and biochemical analysis: A critical review. Crit. Rev. Anal. Chem. 1989, 20, 317–358. [Google Scholar] [CrossRef]
- Lin, S. Ubiquitous Non- and Minimally-Invasive Biosensing Technologies for Personalized and Precision Medicine. Ph.D. Thesis, University of California, Los Angeles, CA, USA, 2022. [Google Scholar]
- Goud, K.Y.; Mahato, K.; Teymourian, H.; Longardner, K.; Litvan, I.; Wang, J. Wearable electrochemical microneedle sensing platform for real-time continuous interstitial fluid monitoring of apomorphine: Toward Parkinson management. Sens. Actuators B Chem. 2022, 354, 131234. [Google Scholar] [CrossRef]
- Eltzov, E.; Marks, R.S. Whole-cell aquatic biosensors. Anal. Bioanal. Chem. 2011, 400, 895–913. [Google Scholar] [CrossRef] [PubMed]
- David, I.G.; Buleandra, M.; Popa, D.E.; Cheregi, M.C.; David, V.; Iorgulescu, E.E.; Tartareanu, G.O. Recent developments in voltammetric analysis of pharmaceuticals using disposable pencil graphite electrodes. Processes 2022, 10, 472. [Google Scholar] [CrossRef]
- Tasić, Ž.Z.; Mihajlović, M.B.; Simonović, A.T.; Radovanović, M.B.; Antonijević, M.M. Review of applied surface modifications of pencil graphite electrodes for paracetamol sensing. Results Phys. 2021, 22, 103911. [Google Scholar] [CrossRef]
- David, I.G.; Oancea, A.G.; Buleandră, M.; Popa, D.E.; Iorgulescu, E.E.; Ciobanu, A.M. Disposable pencil graphite electrode for diosmin voltammetric analysis. Micromachines 2021, 12, 351. [Google Scholar] [CrossRef]
- David, I.G.; Numan, N.; Buleandră, M.; Popa, D.E.; Lițescu, S.C.; Riga, S.; Ciobanu, A.M. Rapid voltammetric screening method for the assessment of bioflavonoid content using the disposable bare pencil graphite electrode. Chemosensors 2021, 9, 323. [Google Scholar] [CrossRef]
- Buleandra, M.; Popa, D.E.; David, I.G.; Bacalum, E.; David, V.; Ciucu, A.A. Electrochemical behavior study of some selected phenylurea herbicides at activated pencil graphite electrode. Electrooxidation of linuron and monolinuron. Microchem. J. 2019, 147, 1109–1116. [Google Scholar] [CrossRef]
- Pham, T.H.; Mai, T.T.; Nguyen, H.A.; Chu, T.T.; Vu, T.T.; Le, Q.H. Voltammetric determination of amoxicillin using a reduced graphite oxide nanosheet electrode. J. Anal. Methods Chem. 2021, 2021, 8823452. [Google Scholar]
- Gorylewski, D.; Tyszczuk-Rotko, K.; Wójciak, M.; Sowa, I. Fast, Simple, and Sensitive Voltammetric Measurements of Acyclovir in Real Samples via Boron-Doped Diamond Electrode. Materials 2024, 17, 4480. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.F.; Foster, J.R. A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br. J. Biomed. Sci. 2012, 69, 83–93. [Google Scholar]
- Wong, A.; Santos, A.M.; da Fonseca Alves, R.; Vicentini, F.C.; Fatibello-Filho, O.; Sotomayor, M.D. Simultaneous determination of direct yellow 50, tryptophan, carbendazim, and caffeine in environmental and biological fluid samples using graphite pencil electrode modified with palladium nanoparticles. Talanta 2021, 222, 121539. [Google Scholar] [PubMed]
- El-Laboudi, A.; Oliver, N.S.; Cass, A.; Johnston, D. Use of microneedle array devices for continuous glucose monitoring: A review. Diabetes Technol. Ther. 2013, 15, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.J.; Nguyen, T.; Chu, M.; Pegan, J.D.; Khine, M. Highly stretchable wrinkled gold thin film wires. Appl. Phys. Lett. 2016, 108, 061901. [Google Scholar] [CrossRef]
- Yamada, T.; Hayamizu, Y.; Yamamoto, Y.; Yomogida, Y.; Izadi-Najafabadi, A.; Futaba, D.N.; Hata, K. A stretchable carbon nanotube strain sensor for human-motion detection. Nat. Nanotechnol. 2011, 6, 296–301. [Google Scholar]
- Wang, Y.; Wang, L.; Yang, T.; Li, X.; Zang, X.; Zhu, M.; Wang, K.; Wu, D.; Zhu, H. Wearable and highly sensitive graphene strain sensors for human motion monitoring. Adv. Funct. Mater. 2014, 24, 4666–4670. [Google Scholar]
- Pang, Y.; Tian, H.; Tao, L.; Li, Y.; Wang, X.; Deng, N.; Yang, Y.; Ren, T.-L. Flexible, highly sensitive, and wearable pressure and strain sensors with graphene porous network structure. ACS Appl. Mater. Interfaces 2016, 8, 26458–26462. [Google Scholar] [CrossRef]
- Abawajy, J. Comprehensive analysis of big data variety landscape. Int. J. Parallel Emergent Distrib. Syst. 2015, 30, 5–14. [Google Scholar]
- Phillips-Wren, G.; Hoskisson, A. An analytical journey towards big data. J. Decis. Syst. 2015, 24, 87–102. [Google Scholar]
- Sivarajah, U.; Kamal, M.M.; Irani, Z.; Weerakkody, V. Critical analysis of Big Data challenges and analytical methods. J. Bus. Res. 2017, 70, 263–286. [Google Scholar]
- Annu Sharma, S.; Jain, R.; Raja, A.N. Pencil graphite electrode: An emerging sensing material. J. Electrochem. Soc. 2020, 167, 037501. [Google Scholar]
- David, I.G.; Popa, D.E.; Buleandra, M. Pencil graphite electrodes: A versatile tool in electroanalysis. J. Anal. Methods Chem. 2017, 2017, 1905968. [Google Scholar] [PubMed]
- Buledi, J.A.; Shah, Z.U.; Mallah, A.; Solangi, A.R. Current perspective and developments in electrochemical sensors modified with nanomaterials for environmental and pharmaceutical analysis. Curr. Anal. Chem. 2022, 18, 102–115. [Google Scholar] [CrossRef]
- Matrouf, M.; Loudiki, A.; Azriouil, M.; Laghrib, F.; Farahi, A.; Bakasse, M.; Saqrane, S.; Lahrich, S.; El Mhammedi, M.A. Recent Advancements in Electrochemical Sensors for 4-Aminoquinoline Drugs Determination in Biological and Environmental Samples. J. Electrochem. Soc. 2022, 169, 067503. [Google Scholar]
- Safaei, M.; Shishehbore, M.R. A review on analytical methods with special reference to electroanalytical methods for the determination of some anticancer drugs in pharmaceutical and biological samples. Talanta 2021, 229, 122247. [Google Scholar] [PubMed]
- Altunkaynak, Y.; Yavuz, Ö.; Levent, A. Firstly electrochemical examination of vildagliptin at disposable graphite sensor: Sensitive determination in drugs and human urine by square-wave voltammetry. Microchem. J. 2021, 170, 106653. [Google Scholar]
- Kouchakinejad, S.; Babaee, S.; Roshani, F.; Kouchakinejad, R.; Kaki, S. The performance of the new modified pencil graphite electrode in quantifying of insulin. Chem. Phys. Lett. 2020, 759, 137987. [Google Scholar] [CrossRef]
- David, I.G.; Panait, A.L.; Buleandra, M.; Popa, D.E.; Cheregi, M.C. Simple voltammetric analysis of sulfamethoxazole at a disposable pencil graphite electrode. Rev. Roum. Chim. 2022, 67, 177–183. [Google Scholar]
- Pan, M.; Guo, P.; Liu, H.; Lu, J.; Xie, Q. Graphene oxide modified screen-printed electrode for highly sensitive and selective electrochemical detection of ciprofloxacin residues in milk. J. Anal. Sci. Technol. 2021, 12, 55. [Google Scholar] [CrossRef]
- Laghlimi, C.; Moutcine, A.; Chtaini, A.; Isaad, J.; Soufi, A.; Ziat, Y.; Amhamdi, H.; Belkhanchi, H. Recent advances in electrochemical sensors and biosensors for monitoring drugs and metabolites in pharmaceutical and biological samples. ADMET DMPK 2023, 11, 151–173. [Google Scholar] [CrossRef] [PubMed]
- Mosquera-Ortega, M.; de Sousa, L.R.; Susmel, S.; Cortón, E.; Figueredo, F. When microplastics meet electroanalysis: Future analytical trends for an emerging threat. Anal. Methods 2023, 15, 5978–5999. [Google Scholar] [CrossRef]
- Liu, G.; Luo, D.; Wang, L.; Wang, C.; Cao, Y.; Singh, L.; Ahmadzadeh, S.; He, Z. Current status and future perspective in electro-Fenton techniques for wastewater treatment: A bibliometric review. Appl. Nanosci. 2023, 13, 5885–5902. [Google Scholar] [CrossRef]
- Karadurmus, L.; Ozcelikay, G.; Vural, S.; Ozkan, S.A. An overview on quantum dot-based nanocomposites for electrochemical sensing on pharmaceutical assay. Iran. J. Pharm. Res. IJPR 2021, 20, 187. [Google Scholar]
- Dogan-Topal, B.; Ozkan, S.A.; Uslu, B. The analytical applications of square wave voltammetry on pharmaceutical analysis. Open Chem. Biomed. Methods J. 2010, 3, 56–73. [Google Scholar]
- Mei, C.J.; Ahmad, S.A. A review on the determination heavy metals ions using calixarene-based electrochemical sensors. Arab. J. Chem. 2021, 14, 103303. [Google Scholar]
- Li, S.; Zhang, H.; Zhu, M.; Kuang, Z.; Li, X.; Xu, F.; Miao, S.; Zhang, Z.; Lou, X.; Li, H.; et al. Electrochemical biosensors for whole blood analysis: Recent progress, challenges, and future perspectives. Chem. Rev. 2023, 123, 7953–8039. [Google Scholar] [CrossRef]
- de Oliveira, R.S.; Azevedo, A.L.; Pacheco, W.F.; Semaan, F.S.; Ponzio, E.A. Practical Electroanalysis: Overcoming Drawbacks and Going Further. In Theory, Types and Applications; Nova Science Publishers: Hauppauge, NY, USA, 2014; p. 171. [Google Scholar]
- Geisler, J.; Thompson, T. Choosing the Best Detection Method: Absorbance vs. Fluorescence; Biocompare: San Francisco, CA, USA, 2015. [Google Scholar]
- Liu, Y.; Li, J.; Xiao, S.; Liu, Y.; Bai, M.; Gong, L.; Zhao, J.; Chen, D. Revolutionizing precision medicine: Exploring wearable sensors for therapeutic drug monitoring and personalized therapy. Biosensors 2023, 13, 726. [Google Scholar] [CrossRef]
- Campuzano, S.; Barderas, R.; Moreno-Casbas, M.T.; Almeida, Á.; Pingarrón, J.M. Pursuing precision in medicine and nutrition: The rise of electrochemical biosensing at the molecular level. Anal. Bioanal. Chem. 2024, 416, 2151–2172. [Google Scholar] [CrossRef]
- Gupta, A.K.; Dubey, R.S.; Malik, J.K. Application of modern electroanalytical techniques: Recent trend in pharmaceutical and drug analysis. Int. J. Pharm. Sci. Res. 2013, 4, 2450. [Google Scholar]
- Brooks, M.A.; Tsai, E.W. Electrochemistry in pharmaceutical analysis. In Laboratory Techniques in Electroanalytical Chemistry, Revised and Expanded; CRC Press: Boca Raton, FL, USA, 2018; pp. 769–812. [Google Scholar]
- Wang, C.; Xu, J.; Zhou, G.; Qu, Q.; Yang, G.; Hu, X. Electrochemical detection coupled with high-performance liquid chromatography in pharmaceutical and biomedical analysis: A mini review. Comb. Chem. High Throughput Screen. 2007, 10, 547–554. [Google Scholar] [CrossRef]
- Oeracher, H.; Pitterl, F.; Chervet, J.P. “Omics” applications of electrochemistry coupled to mass spectrometry—A review. LCGC Eur. 2015, 28, 138–150. [Google Scholar]
- Göldner, V.; Fangmeyer, J.; Karst, U. Mass Spectrometric Methods for the Analysis of Electrochemical Transformation Products. TrAC Trends Anal. Chem. 2025, 185, 118178. [Google Scholar]
- de Matos Morawski, F.; Ferreira, L.M.; Jost, C.L.; Bergamini, M.F.; Marcolino-Junior, L.H. Silica graphite as an ink additive for stencil printed electrodes: A novel approach for electroanalytical determination of sulfanilamide. J. Electroanal. Chem. 2024, 967, 118458. [Google Scholar] [CrossRef]
- Macedo, A.A.; Pimentel, D.M.; de Souza, A.N.; Mundim, H.M.; Lião, L.M.; Costa, L.M.; Verly, R.M.; dos Santos, W.T. Voltammetric detection with a comprehensive electrochemistry study of minoxidil using nuclear magnetic resonance and infrared analyses: Applications in the forensic and pharmaceutical fields. Electrochim. Acta 2025, 510, 145362. [Google Scholar]
- Ahmad, R.; Abdullah Rehman, M.T.; AlAjmi, M.F.; Alam, S.; Bhat, K.S.; Mishra, P.; Lee, B.I. An Electroanalytical Enzymeless α-Fe2O3-ZnO Hybrid Nanostructure-Based Sensor for Sensitive Quantification of Nitrite Ions. Nanomaterials 2024, 14, 706. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, E.C.; Hosseini, S.; Petro, A.G.; Rudman, K.K.; Gerroll, B.H.; Mubarak, M.S.; Baker, L.A.; Little, R.D. Versatile tools for understanding electrosynthetic mechanisms. Chem. Rev. 2021, 122, 3292–3335. [Google Scholar]
- Figueiredo-Filho, L.C.; Sartori, E.R.; Fatibello-Filho, O. Electroanalytical determination of the linuron herbicide using a cathodically pretreated boron-doped diamond electrode: Comparison with a boron-doped diamond electrode modified with platinum nanoparticles. Anal. Methods 2015, 7, 643–649. [Google Scholar]
- Arduini, F.; Cinti, S.; Scognamiglio, V.; Moscone, D.; Palleschi, G. How cutting-edge technologies impact the design of electrochemical (bio) sensors for environmental analysis. A review. Anal. Chim. Acta 2017, 959, 15–42. [Google Scholar] [CrossRef] [PubMed]
- Ozcelikay, G.; Karadurmus, L.; Kaya, S.I.; Bakirhan, N.K.; Ozkan, S.A. A review: New trends in electrode systems for sensitive drug and biomolecule analysis. Crit. Rev. Anal. Chem. 2020, 50, 212–225. [Google Scholar]
- Norouzi, P.; Haji-Hashemi, H.; Larijani, B.; Aghazadeh, M.; Pourbasheer, E.; Ganjali, M.R. Application of new advanced electrochemical methods combine with nano-based materials sensor in drugs analysis. Curr. Anal. Chem. 2017, 13, 70–80. [Google Scholar]
- Cai, M.; Wang, W. Advances in Electrochemical Methods for the Determination of Ephedrine: Current Status and Future Trends. Int. J. Electrochem. Sci. 2024, 20, 100903. [Google Scholar] [CrossRef]
- Singh, R.; Gupta, R.; Bansal, D.; Bhateria, R.; Sharma, M. A review on recent trends and future developments in electrochemical sensing. ACS Omega 2024, 9, 7336–7356. [Google Scholar] [CrossRef]
- Kagilev, A.A.; Gafurov, Z.N.; Kantyukov, A.O.; Mikhailov, I.K.; Yakhvarov, D.G. The power of in situ spectroelectrochemistry for redox study of organometallic and coordination compounds. J. Solid State Electrochem. 2024, 28, 897–910. [Google Scholar]
- Mirica, K.A. Unlocking the potential of wearable sensors in healthcare and beyond. ACS Sens. 2024, 9, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.I.; Karabulut, T.C.; Kurbanoglu, S.; Ozkan, S.A. Chemically modified electrodes in electrochemical drug analysis. Curr. Pharm. Anal. 2020, 16, 641–660. [Google Scholar] [CrossRef]
- Wang, Q.; Bian, Y.; Zhang, Y.; Sun, D.M.; Wang, W.L.; Zhou, Y.; Liu, Z.F.; Feng, X.S.; He, Z.W. Development of sampling, pretreatment and detection methods for ephedrine and related substances in complex samples. Microchem. J. 2023, 189, 108538. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Elsabahy, M.; Mohamed Elbadry, A.M.; Abdelazim, E.B.; Mohsen, A.A.; AAleem, M.; Gao, H.; Eissa, N.G.; Elghamry, I.; Salim, S.A. Recent Progress of Polymer-Based Biosensors for Cancer Diagnostic Applications: Natural versus Synthetic Polymers. ACS Omega 2025, 10, 8816–8831. [Google Scholar] [CrossRef]
| S. No. | Electroanalysis Methods | About | Used For | Ref. |
|---|---|---|---|---|
| 1. | Electrochemical Detection of Antibiotics (Amoxicillin Detection using Modified Electrodes) | A glassy carbon electrode modified with multi-walled carbon nanotubes (MWCNTs) was used to detect amoxicillin in pharmaceutical formulations. | This technique provides exceptional sensitivity, selectivity, and a low detection limit for the quantification of amoxicillin in pharmaceutical formulations. | [60] |
| 2. | Electrochemical Quantification of Antiviral Drugs (Acyclovir Detection in Pharmaceutical Tablets) | A voltammetric method using a boron-doped diamond electrode was developed to detect and quantify acyclovir, an antiviral drug. | This method offers high sensitivity, selectivity, and a low recognition parameter for the quantification of amoxicillin in pharmaceutical drugs. | |
| 3. | Electrochemical Analysis of Anticancer Drugs (Cisplatin Detection using DNA-modified Electrodes) | A DNA-modified electrode was used to detect the interaction of cisplatin, a chemotherapy drug, with DNA. The change in electrochemical signal was used to study the drug’s binding to DNA. | This method helps understand drug-DNA interactions, crucial for assessing the efficacy and side effects of anticancer drugs. | |
| 4. | Electrochemical Biosensor for Monitoring Drug Metabolites (Paracetamol (Acetaminophen) Metabolite Detection) | An electrochemical biosensor based on graphene-modified electrodes was developed to detect paracetamol and its metabolites in biological fluids. | The sensor was used for real-time monitoring of drug metabolism in patients. | [93] |
| 5. | Electrochemical Detection of Psychoactive Drugs (Cocaine Detection using a Disposable Electrochemical Sensor) | A disposable screen-printed electrode modified with gold nanoparticles was used for the rapid and sensitive detection of cocaine in biological samples. | This method is used in forensic analysis and roadside testing to detect cocaine use. | |
| 6. | Electrochemical Analysis of Cardiovascular Drugs (Atenolol Detection using a Modified Carbon Electrode) | Differential pulse voltammetry (DPV) with a carbon electrode modified with carbon black was applied to detect atenolol in pharmaceutical formulations. | It allowed the quantification of atenolol, a common beta-blocker, with high sensitivity and specificity. | |
| 7. | Electrochemical Detection of Anti-Inflammatory Drugs (Ibuprofen Detection in Pharmaceutical Products) | Square wave voltammetry (SWV) with a modified gold electrode was used to detect ibuprofen in drug formulations. | This technique provided a quick and delicate method for analyzing the content of ibuprofen in commercial drug products. | [94] |
| 8. | Electrochemical Sensing of Antidepressant Drugs (Sertraline Detection using a Graphene-Modified Electrode) | A graphene-modified electrode was developed to detect sertraline, an antidepressant drug, in pharmaceutical formulations using differential pulse voltammetry (DPV). | The method was applied to quantify sertraline in commercial tablets. | |
| 9. | Electrochemical Detection of Antimalarial Drugs (Chloroquine and Hydroxychloroquine Detection) | An electrochemical method using a glassy carbon electrode was developed to detect chloroquine and hydroxychloroquine in pharmaceutical preparations. | This method was useful during the COVID-19 pandemic when these drugs were tested for their potential antiviral activity. | |
| 10. | Electrochemical Sensors for Antibiotic Residue Detection in Food (Electrochemical Detection of Tetracycline Residue in Milk) | A sensor using a carbon-based electrode was developed to detect tetracycline residues in milk samples. | This method provided a quick, sensitive, and cost-effective way to ensure food safety and compliance with drug residue regulations. |
| S. No. | Method | Detection Limit | Sensitivity | Selectivity | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|
| 1. | Cyclic Voltammetry (CV) | Moderate | Moderate | Moderate | Provides information on redox processes and reaction mechanisms | Less sensitive compared to pulse techniques | [97] |
| 2. | Differential Pulse Voltammetry (DPV) | Low | High | High | Enhanced sensitivity and resolution; suitable for trace analysis | Requires more complex instrumentation | |
| 3. | Square Wave Voltammetry (SWV) | Low | High | High | Rapid analysis with high sensitivity; effective for noisy environments | It may require extensive optimization | [98] |
| 4. | Amperometric Detection | Very Low | Very High | Moderate | Suitable for continuous monitoring; simple instrumentation | Potential interference from other electroactive species | |
| 5. | Electrochemical Impedance Spectroscopy (EIS) | Variable | Variable | High | Provides information on interfacial properties; useful for sensor development | Complex data analysis; not typically used for direct quantification | [99] |
| S. No. | Variable | Electroanalysis | Spectrophotometry | Chromatography | Mass Spectrometry | Fluorescence Spectroscopy | Ref. |
|---|---|---|---|---|---|---|---|
| 1. | Advantage | High sensitivity, ability to detect low concentrations, simple sample preparation, real-time monitoring, and low cost. | Simple and quick analysis, good for quantifying compounds that absorb light at specific wavelengths, high throughput. | Excellent separation of complex mixtures, high sensitivity, and specificity. | Provides structural information, extremely high sensitivity and specificity, capable of analyzing complex mixtures. | Direct analysis, low cost, minimal sample preparation. | [100] |
| 2. | Limitation | Selectivity can be influenced by interfering substances, requires electrode maintenance | Limited sensitivity for compounds that do not absorb light strongly, interference from other absorbing species, requires clear solutions. | Time-consuming, requires expensive instrumentation and consumables, extensive sample preparation. | Very expensive, requires skilled operation and maintenance, complex sample preparation. | High sensitivity, capable of detecting very low concentrations, useful for biological samples. | [101] |
| 3. | Comparative study | Using DPV, detection at very low concentrations with high sensitivity and minimal interference. | For paracetamol UV-Vis spectrophotometry required a derivatization step to improve sensitivity and faced interference from other absorbing substances in the sample. | Analysis of ciprofloxacin HPLC offered high separation efficiency and sensitivity, but the analysis time was longer and required more extensive sample preparation. | LC-MS offered detailed structural information and extremely high sensitivity, ideal for comprehensive metabolic profiling but at a higher cost and complexity | Provided high sensitivity and specificity, but required derivatization of propranolol with a fluorescent marker, adding to the complexity and cost. | [102] |
| S. No. | Challenges | Description | Impact | Reasoning | Ref. |
|---|---|---|---|---|---|
| 1. | Electrode Fouling | Over time, electrodes can become coated with reaction products or contaminants, reducing their effectiveness. | Leads to decreased sensitivity, reproducibility, and accuracy. | Regular cleaning or use of disposable electrodes. | [116] |
| 2. | Interference from Other Species | Other electroactive species in the sample can interfere with the analyte signal. | Causes overlapping signals and inaccurate measurements. | Use of selective electrodes, chemical modification of electrodes, or sample pretreatment. | |
| 3. | Complex Sample Matrices | Biological, environmental, and industrial samples often contain complex mixtures. | Difficult to isolate and measure specific analytes without interference. | Advanced separation techniques (e.g., chromatography), selective electrodes, or signal processing methods. | |
| 4. | Reproducibility and Stability | Variability in electrode preparation and environmental conditions can affect results. | Difficult to achieve consistent and reliable measurements. | Standardization of electrode preparation procedures and use of stable reference electrodes. | [117] |
| 5. | Detection Limits | Limited by noise and background currents, particularly for low-concentration analytes. | Difficult to detect trace levels of substances. | Enhancement techniques such as pre-concentration, use of nanostructured materials, or signal amplification. | |
| 6. | Kinetic and Mass Transport Limitations | Reaction kinetics and mass transport to the electrode surface can limit the rate of electrochemical reactions. | Affects the speed and efficiency of the analysis. | Optimizing electrode design and experimental conditions to enhance mass transport. | |
| 7. | Selectivity | Difficulty in achieving high selectivity for a particular analyte in the presence of similar species. | Inaccurate quantification and identification. | Use of selective binding agents (e.g., enzymes, antibodies) or electrode modification. | [118] |
| 8. | Instrumentation and Cost | Advanced electroanalytical techniques require sophisticated and often expensive equipment. | Limits accessibility for routine analysis or in resource-limited settings. | Development of low-cost, portable devices. | [119] |
| 9. | Environmental Factors | Temperature, pH, and ionic strength can affect electrochemical measurements. | Variability in results and reduced reproducibility. | Control of environmental conditions or use of robust sensor designs. | |
| 10. | Surface Characterization | Difficulty in characterizing and controlling the surface properties of electrodes. | Affects reproducibility and sensitivity. | Advanced surface characterization techniques (e.g., microscopy, spectroscopy) and consistent electrode preparation. | [120] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, R.; Salwan, S.; Kumar, P.; Bansal, N.; Kumar, B. Electroanalysis Advances in Pharmaceutical Sciences: Applications and Challenges Ahead. Analytica 2025, 6, 12. https://doi.org/10.3390/analytica6020012
Kumar R, Salwan S, Kumar P, Bansal N, Kumar B. Electroanalysis Advances in Pharmaceutical Sciences: Applications and Challenges Ahead. Analytica. 2025; 6(2):12. https://doi.org/10.3390/analytica6020012
Chicago/Turabian StyleKumar, Ram, Sushant Salwan, Pawan Kumar, Nisha Bansal, and Bhupinder Kumar. 2025. "Electroanalysis Advances in Pharmaceutical Sciences: Applications and Challenges Ahead" Analytica 6, no. 2: 12. https://doi.org/10.3390/analytica6020012
APA StyleKumar, R., Salwan, S., Kumar, P., Bansal, N., & Kumar, B. (2025). Electroanalysis Advances in Pharmaceutical Sciences: Applications and Challenges Ahead. Analytica, 6(2), 12. https://doi.org/10.3390/analytica6020012







