Phenolic Compounds in Edible Tropaeolum majus L. Leaves and Its In Vitro Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Maceration and Ultrasonic-Assisted Extraction Techniques
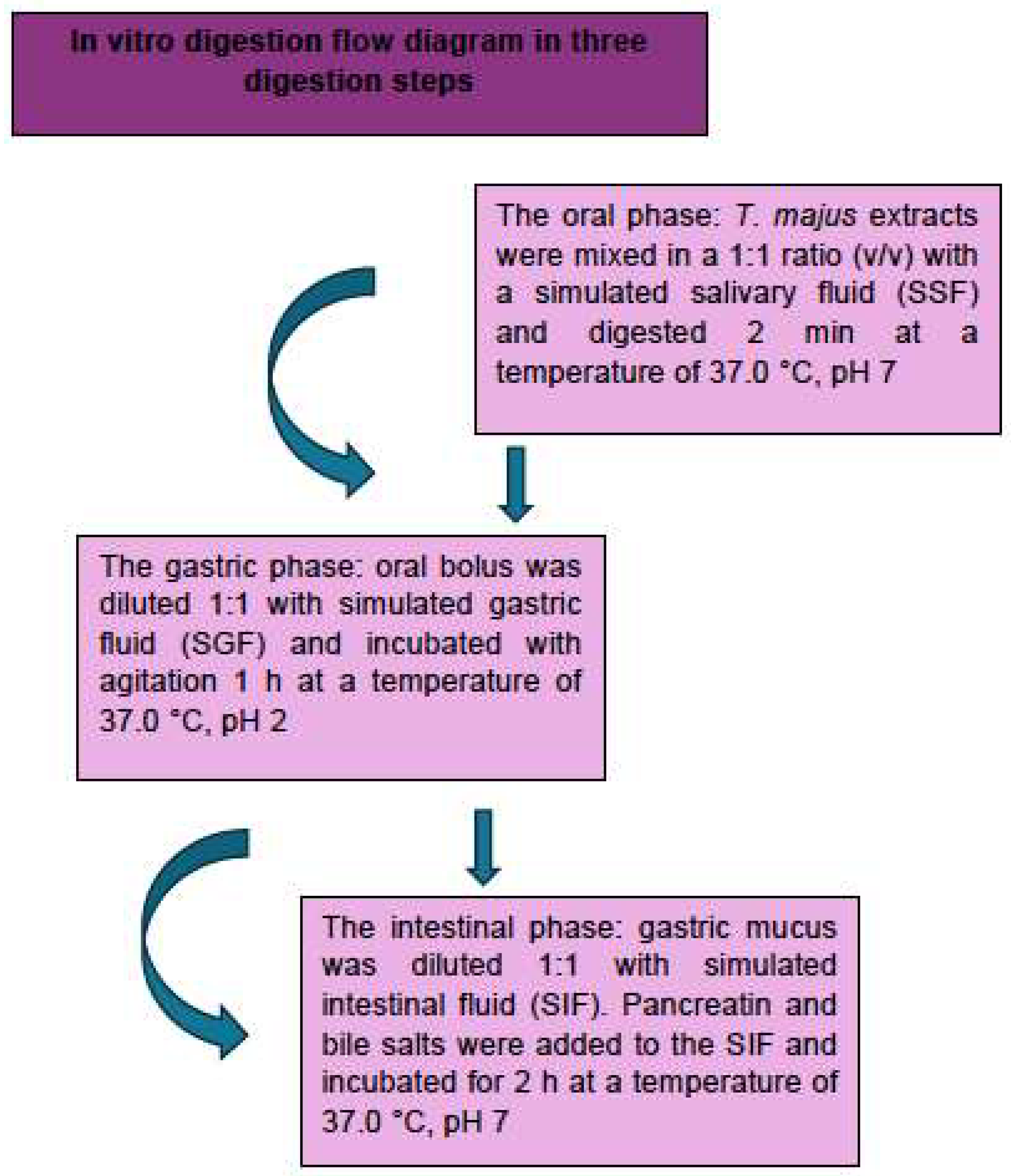
2.3. In Vitro Digestion
2.4. Reagents and Standards for LC/MS-MS Analysis
2.5. Chemical Characterization of Phenolic Compounds
2.6. Statistical Analyses
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vrca, I.; Ramić, D.; Fredotović, Ž.; Smole Možina, S.; Blažević, I.; Bilušić, T. Chemical Composition and Biological Activity of Essential Oil and Extract from the Seeds of Tropaeolum majus L. Var. Altum. Food Technol. Biotechnol. 2022, 60, 533–542. [Google Scholar] [CrossRef]
- Brondani, J.C.; Cuelho, C.H.F.; Marangoni, L.D.; de Lima, R.; Guex, C.G.; Bonilha, I.d.F.; Manfron, M.P. Traditional Usages, Botany, Phytochemistry, Biological Activity and Toxicology of Tropaeolum majus L.—A Review. Bol. Latinoam. Y Del Caribe Plantas Med. Y Aromat. 2016, 15, 264–273. [Google Scholar]
- Cobus, D.; Nunes, G.; Maior, L.d.O.; Lacerda, L.G.; Ito, V. Unconventional Food Plants (UFPs): An Approach to the Nutritional and Functional Properties of Nasturtium (Tropaeolum majus L.). Food Sci. Today 2023, 1, 1–6. [Google Scholar] [CrossRef]
- Bass, A.; Theodoro, H.; Minello, L.V.; Scur, L.; Pansera, M.R.; Sartori, V.C. Plantas Alimentícias Não Convencionais—PANC: Resgatando a Soberania Alimentar e Nutricional; Editora da Universidade de Caxias do Sul: Los Angeles, CA, USA, 2020; ISBN 978-85-7061-992-1. [Google Scholar]
- Garzón, G.A.; Manns, D.C.; Riedl, K.; Schwartz, S.J.; Padilla-Zakour, O. Identification of Phenolic Compounds in Petals of Nasturtium Flowers (Tropaeolum majus) by High-Performance Liquid Chromatography Coupled to Mass Spectrometry and Determination of Oxygen Radical Absorbance Capacity (ORAC). J. Agric. Food Chem. 2015, 63, 1803–1811. [Google Scholar] [CrossRef]
- Lorenzi, H.; Matos, F.J.d.A. Plantas Medicinais No Brasil: Nativas e Exóticas; Instituto Plantarum de Estudos da Flora: Nova Odessa, Brazil, 2002; ISBN 8586714186. [Google Scholar]
- Ferreira, R.B.G.; Vieira, M.C.; Zárete, N.A.H. Análise de Crescimento de Tropaeolum majus “Jewel” Em Função de Espaçamentos Entre Plantas. Rev. Bras. Plantas Med. 2005, 7, 57–66. [Google Scholar]
- Ferro, D. Fitoterapia: Conceitos Clínicos; Atheneu: São Paulo, Brazil, 2008; ISBN 978857379824. [Google Scholar]
- Bown, D. Encyclopedia of Herbs and Their Uses; DK Publishing: London, UK, 1995; ISBN 0888503342. [Google Scholar]
- Binet, L. A Biologist Physician in the Country. Biol. Med. 1964, 53, 5–28. [Google Scholar]
- Goos, K.-H.; Albrecht, U.; Schneider, B. Wirksamkeit Und Verträglichkeit Eines Pflanzlichen Arzneimittels Mit Kapuzinerkressenkraut Und Meerrettich Bei Akuter Sinusitis, Akuter Bronchitis Und Akuter Blasenentzündung Im Vergleich Zu Anderen Therapien Unter Den Bedingungen Der Täglichen Praxis. Arzneimittelforschung 2011, 56, 249–257. [Google Scholar] [CrossRef]
- Barboza, L.N.; Prando, T.B.L.; Dalsenter, P.R.; Gasparotto, F.M.; Gasparotto, F.; Jacomassi, E.; Araújo, V.D.O.; Lourenço, E.L.B.; Gasparotto Junior, A. Prolonged Diuretic Activity and Calcium-Sparing Effect of Tropaeolum majus: Evidence in the Prevention of Osteoporosis. Evidence-Based Complement. Altern. Med. 2014, 2014, 958291. [Google Scholar] [CrossRef]
- Gasparotto Junior, A.; Gasparotto, F.M.; Boffo, M.A.; Lourenço, E.L.B.; Stefanello, M.É.A.; Salvador, M.J.; Da Silva-Santos, J.E.; Marques, M.C.A.; Kassuya, C.A.L. Diuretic and Potassium-Sparing Effect of Isoquercitrin—An Active Flavonoid of Tropaeolum majus L. J. Ethnopharmacol. 2011, 134, 210–215. [Google Scholar] [CrossRef]
- Vrca, I.; Burčul, F.; Blažević, I.; Bratanić, A.; Bilušić, T. Comparison of Gastrointestinal Stability of Isothiocyanates from Tropaeolum majus L. Altum Using in Vitro and Ex Vivo Digestion Methods. Croat. J. food Sci. Technol. 2021, 13, 160–166. [Google Scholar] [CrossRef]
- Garzón, G.A.; Wrolstad, R.E. Major Anthocyanins and Antioxidant Activity of Nasturtium Flowers (Tropaeolum majus). Food Chem. 2009, 114, 44–49. [Google Scholar] [CrossRef]
- Bazylko, A.; Parzonko, A.; Jeż, W.; Osińska, E.; Kiss, A.K. Inhibition of ROS Production, Photoprotection, and Total Phenolic, Flavonoids and Ascorbic Acid Content of Fresh Herb Juice and Extracts from the Leaves and Flowers of Tropaeolum majus. Ind. Crops Prod. 2014, 55, 19–24. [Google Scholar] [CrossRef]
- Das, M.; Biswas, S.K.; Zaman, S.; Mitra, A. Nasturtium (Tropaeolum majus) an Annual Herb Has Medicinal Property to Cure Throat Sore and It Has Antivirus Property. Int. J. Environ. Agric. Sci. 2019, 3, 16. [Google Scholar]
- Jakubczyk, K.; Janda, K.; Watychowicz, K.; Łukasiak, J.; Wolska, J. Garden Nasturtium (Tropaeolum majus L.)—a Source of Mineral Elements and Bioactive Compounds. Rocz. Panstw. Zakl. Hig. 2018, 69, 119–126. [Google Scholar]
- Navarro-González, I.; González-Barrio, R.; García-Valverde, V.; Bautista-Ortín, A.; Periago, M. Nutritional Composition and Antioxidant Capacity in Edible Flowers: Characterisation of Phenolic Compounds by HPLC-DAD-ESI/MSn. Int. J. Mol. Sci. 2014, 16, 805–822. [Google Scholar] [CrossRef]
- Niizu, P.Y.; Rodriguez-Amaya, D.B. Flowers and Leaves of Tropaeolum majus L. as Rich Sources of Lutein. J. Food Sci. 2006, 70, S605–S609. [Google Scholar] [CrossRef]
- Rop, O.; Mlcek, J.; Jurikova, T.; Neugebauerova, J.; Vabkova, J. Edible Flowers—A New Promising Source of Mineral Elements in Human Nutrition. Molecules 2012, 17, 6672–6683. [Google Scholar] [CrossRef]
- Salem, O.; Szwajkowska-Michałek, L.; Przybylska-Balcerek, A.; Szablewski, T.; Cegielska-Radziejewska, R.; Świerk, D.; Stuper-Szablewska, K. New Insights into Bioactive Compounds of Wild-Growing Medicinal Plants. Appl. Sci. 2023, 13, 13196. [Google Scholar] [CrossRef]
- Mathews, A.; Arbal, A.V.; Kaarunya, A.; Jha, P.K.; Le-Bail, A.; Rawson, A. Conventional vs. Modern Extraction Techniques in the Food Industry. In Extraction Processes in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2024; pp. 97–146. ISBN 978-0-12-819516-1. [Google Scholar]
- Đurović, S.; Domínguez, R.; Pateiro, M.; Teslić, N.; Lorenzo, J.M.; Pavlić, B. Industrial Hemp Nutraceutical Processing and Technology. In Industrial Hemp; Elsevier: Amsterdam, The Netherlands, 2022; pp. 191–218. ISBN 978-0-323-90910-5. [Google Scholar]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound Assisted Extraction of Food and Natural Products. Mechanisms, Techniques, Combinations, Protocols and Applications. A Review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A Comprehensive Review of Ultrasonic Assisted Extraction (UAE) for Bioactive Components: Principles, Advantages, Equipment, and Combined Technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Abubakar, A.R.; Haque, M. Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bajkacz, S.; Baranowska, I.; Buszewski, B.; Kowalski, B.; Ligor, M. Determination of Flavonoids and Phenolic Acids in Plant Materials Using SLE-SPE-UHPLC-MS/MS Method. Food Anal. Methods 2018, 11, 3563–3575. [Google Scholar] [CrossRef]
- Yilmaz, M.A. Simultaneous Quantitative Screening of 53 Phytochemicals in 33 Species of Medicinal and Aromatic Plants: A Detailed, Robust and Comprehensive LC–MS/MS Method Validation. Ind. Crops Prod. 2020, 149, 112347. [Google Scholar] [CrossRef]
- Ozkan, G.; Sakarya, F.B.; Tas, D.; Yurt, B.; Ercisli, S.; Capanoglu, E. Effect of In Vitro Digestion on the Phenolic Content of Herbs Collected from Eastern Anatolia. ACS Omega 2023, 8, 12730–12738. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Vrca, I.; Fredotović, Ž.; Jug, B.; Nazlić, M.; Dunkić, V.; Jug, D.; Radić, J.; Možina, S.S.; Restović, I. Chemical Profile of Kumquat (Citrus japonica Var. Margarita) Essential Oil, In Vitro Digestion, and Biological Activity. Foods 2024, 13, 3545. [Google Scholar] [CrossRef]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds from Fruits and Vegetables: A Review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of Bioactive Food Compounds: A Challenging Journey to Bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- Žitek, T.; Kučuk, N.; Postružnik, V.; Leitgeb, M.; Knez, Ž.; Primožič, M.; Marevci, M.K. Synergistic Effect of Supercritical and Ultrasound-Assisted Ginger (Zingiber Officinale Roscoe) Extracts. Plants 2022, 11, 2872. [Google Scholar] [CrossRef]
- Vrca, I.; Orhanović, S.; Pezelj, I.; Sušić, K.; Dunkić, V.; Kremer, D.; Nazlić, M. Identification of Phenolic Compounds Present in Three Speedwell (Veronica L.) Species and Their Antioxidant Potential. Antioxidants 2024, 13, 738. [Google Scholar] [CrossRef]
- Živković, J.; Barreira, J.C.M.; Stojković, D.; Ćebović, T.; Santos-Buelga, C.; Maksimović, Z.; Ferreira, I.C.F.R. Phenolic Profile, Antibacterial, Antimutagenic and Antitumour Evaluation of Veronica Urticifolia Jacq. J. Funct. Foods 2014, 9, 192–201. [Google Scholar] [CrossRef]
- Gadjalova, A.V.; Mihaylova, D.S. Ultrasound-Assisted Extraction of Medicinal Plants and Evaluation of Their Biological Activity. Food Res. 2019, 3, 530–536. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The Effects of Ultrasound Assisted Extraction on Yield, Antioxidant, Anticancer and Antimicrobial Activity of Polyphenol Extracts: A Review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Mnayer, D.; Fabiano-Tixier, A.-S.; Petitcolas, E.; Ruiz, K.; Hamieh, T.; Chemat, F. Extraction of Green Absolute from Thyme Using Ultrasound and Sunflower Oil. Resour. Technol. 2017, 3, 12–21. [Google Scholar] [CrossRef]
- Bazylko, A.; Granica, S.; Filipek, A.; Piwowarski, J.; Stefańska, J.; Osińska, E.; Kiss, A.K. Comparison of Antioxidant, Anti-Inflammatory, Antimicrobial Activity and Chemical Composition of Aqueous and Hydroethanolic Extracts of the Herb of Tropaeolum majus L. Ind. Crops Prod. 2013, 50, 88–94. [Google Scholar] [CrossRef]
- Ercan, L.; Doğru, M. Determination of Phenolic Compounds in Nasturtium Officinale by LC-MS / MS Using Different Extraction Methods and Different Solvents. Int. J. Chem. Technol. 2023, 7, 124–130. [Google Scholar] [CrossRef]
- Boo, Y.C. P-Coumaric Acid as An Active Ingredient in Cosmetics: A Review Focusing on Its Antimelanogenic Effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef]
- Musolino, V.; Marrelli, M.; Perri, M.R.; Palermo, M.; Gliozzi, M.; Mollace, V.; Conforti, F. Centranthus ruber (L.) DC. and Tropaeolum majus L.: Phytochemical Profile, In Vitro Anti-Denaturation Effects and Lipase Inhibitory Activity of Two Ornamental Plants Traditionally Used as Herbal Remedies. Molecules 2022, 28, 32. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of Phenolic Compounds towards Free Radicals under in Vitro Conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef]
- Sensoy, I. A Review on the Food Digestion in the Digestive Tract and the Used in Vitro Models. Curr. Res. Food Sci. 2021, 4, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Ebrahimi, F.; Agar, O.T.; Dunshea, F.R.; Barrow, C.J.; Suleria, H.A.R. In Vitro Digestion and Colonic Fermentation of Phenolic Compounds and Their Antioxidant Potential in Australian Beach-Cast Seaweeds. Sci. Rep. 2024, 14, 4335. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Z.; Lu, P.; Barrow, C.; Dunshea, F.R.; Suleria, H.A.R. Bioaccessibility and Bioactivities of Phenolic Compounds from Roasted Coffee Beans during in Vitro Digestion and Colonic Fermentation. Food Chem. 2022, 386, 132794. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, G.; Franco, P.; De Marco, I.; Capanoglu, E.; Esatbeyoglu, T. Investigating the Effects of Supercritical Antisolvent Process and Food Models on Antioxidant Capacity, Bioaccessibility and Transepithelial Transport of Quercetin and Rutin. Food Funct. 2022, 13, 4469–4477. [Google Scholar] [CrossRef]
- de Paulo Farias, D.; de Araújo, F.F.; Neri-Numa, I.A.; Dias-Audibert, F.L.; Delafiori, J.; Catharino, R.R.; Pastore, G.M. Effect of in Vitro Digestion on the Bioaccessibility and Bioactivity of Phenolic Compounds in Fractions of Eugenia Pyriformis Fruit. Food Res. Int. 2021, 150, 110767. [Google Scholar] [CrossRef]
- Garzón, A.G.; Van de Velde, F.; Drago, S.R. Gastrointestinal and Colonic in Vitro Bioaccessibility of γ-Aminobutiric Acid (GABA) and Phenolic Compounds from Novel Fermented Sorghum Food. LWT 2020, 130, 109664. [Google Scholar] [CrossRef]
- Sęczyk, Ł.; Sugier, D.; Świeca, M.; Gawlik-Dziki, U. The Effect of in Vitro Digestion, Food Matrix, and Hydrothermal Treatment on the Potential Bioaccessibility of Selected Phenolic Compounds. Food Chem. 2021, 344, 128581. [Google Scholar] [CrossRef]

| Compound | Precursor m/z | Fragment m/z | tr (min) |
|---|---|---|---|
| p-hydroxybenzoic acid | 137.02 | 93.03 | 3.48 |
| protocatechuic acid | 153.02 | 109.03 | 2.21 |
| Gentisic acid | 153.02 | 109.03 | 3.21 |
| Vanillic acid | 167.03 | 152.01 | 4.66 |
| Gallic acid | 169.01 | 125.02 | 1.33 |
| Syringic acid | 197.04 | 123.01 | 5.59 |
| p-coumaric acid | 163.04 | 119.05 | 7.18 |
| o-coumaric acid | 163.04 | 119.05 | 9.16 |
| Caffeic acid | 179.03 | 135.04 | 4.9 |
| Ferulic acid | 193.05 | 134.03 | 8.19 |
| Chlorogenic acid | 353.08 | 179,03 | 4.64 |
| Quinic acid | 191.05 | 85.03 | 4.64 |
| Sinapic acid | 223.06 | 193.01 | 8.49 |
| Rosmarinic acid | 359.08 | 161.02 | 9.88 |
| Cinnamic acid | 147.05 | 103.06 | 11.17 |
| Epicatechin | 289.07 | 109.03 | 6.25 |
| Catechin | 289.07 | 109.03 | 4.17 |
| Resveratrol | 227.07 | 143.05 | 10.42 |
| Astringin | 405.12 | 243.06 | 7.50 |
| EGCG (Epigallocatechin gallate) | 457.08 | 169.01 | 6.95 |
| Hesperetin | 301.07 | 164.01 | 12.61 |
| Quercetin | 301.03 | 151.00 | 11.36 |
| Myricetin | 317.03 | 151.00 | 10.03 |
| Apigenin | 269.04 | 117.03 | 12.46 |
| Naringenin | 271.06 | 151.00 | 12.14 |
| Rutin | 609.15 | 300.03 | 8.86 |
| Extracts | T. majus Extract After Maceration | T. majus Extract After UAE |
|---|---|---|
| Extraction Yield (mg/0.5 g of fresh material) | ||
| 80% EtOH extract | 25.97 ± 2.66 a | 32.63 ± 2.28 b |
| Water extract | 21.00 ± 3.26 a | 25.9 ± 3.84 a,b |
| T. majus Extract After Maceration | T. majus Extract After UAE | |||||||
|---|---|---|---|---|---|---|---|---|
| Compound | 80% EtOH Extract UD | 80% EtOH Extract D | Water Extract UD | Water Extract D | 80% EtOH Extract UD | 80% EtOH Extract D | Water Extract UD | Water Extract D |
| (µg/g of FM) | ||||||||
| p-hydroxybenzoic acid | 0.0091 ± 0.00009 a | 0.0088 ± 0.00002 a | 0.00773 ± 0.00005 a | 0.0073 ± 0.00011 a | 0.0110 ± 0.00004 a | 0.0074 ± 0.00627 a | 0.0096 ± 0.00005 a | 0.0089 ± 0.00003 a |
| Protocatechuic acid | 0.0091 ± 0.00005 a | n.d.a | 0.00838 ± 0.00011 a | 0.0072 ± 0.00001 a | 0.0113 ± 0.00005 a | n.d. a | 0.0089 ± 0.00002 a | n.d. b |
| Vanillic acid | n.d. | n.d. | 0.0133 ± 0.00035 a | n.d. b | n.d. | n.d. | n.d. | n.d. |
| Syringic acid | n.d. | n.d. | 0.0101 ± 0.00004 a | n.d. b | n.d. | n.d. | n.d. | n.d. |
| p-coumaric acid | 0.0195 ± 0.00003 a | 0.01306 ± 0.00082 a | 0.0616 ± 0.00251 a | 0.0218 ± 0.00011 b | 0.0242 ± 0.00003 a | 0.0209 ± 0.01766 a | 0.0211 ± 0.00023 a | 0.0210 ± 0.00058 a |
| o-coumaric acid | n.d. | n.d. | 0.0089 ± 0.00012 a | 0.0089 ± 0.00002 a | n.d. | n.d. | n.d. | n.d. |
| Caffeic acid | 0.0125 ± 0.00009 a | n.d. b | 0.0354 ± 0.00076 a | n.d. b | 0.0154 ± 0.00001 a | n.d. a | n.d. | n.d. |
| Ferulic acid | n.d. | n.d. | 0.0114 ± 0.00014 a | 0.0104 ± 0.00029 a | n.d. | n.d. | n.d. | n.d. |
| Chlorogenic acid | 0.6369 ± 0.00444 a | 0.01439 ± 0.00029 b | 0.0332 ± 0.00016 a | 0.0116 ± 0.00024 b | 0.5625 ± 0.06324 a | 0.0119 ± 0.01009 b | 0.0153 ± 0.00158 a | 0.0140 ± 0.00006 b |
| Quinic acid | 0.5696 ± 0.01557 a | n.d. b | n.d. | n.d. | 0.4599 ± 0.03130 a | n.d. b | n.d. | n.d. |
| Quercetin | 0.0220 ± 0.00094 a | n.d. b | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vrca, I.; Jukić, D.; Radić, J.; Anđelić, I. Phenolic Compounds in Edible Tropaeolum majus L. Leaves and Its In Vitro Digestion. Analytica 2025, 6, 14. https://doi.org/10.3390/analytica6020014
Vrca I, Jukić D, Radić J, Anđelić I. Phenolic Compounds in Edible Tropaeolum majus L. Leaves and Its In Vitro Digestion. Analytica. 2025; 6(2):14. https://doi.org/10.3390/analytica6020014
Chicago/Turabian StyleVrca, Ivana, Dora Jukić, Josip Radić, and Ivana Anđelić. 2025. "Phenolic Compounds in Edible Tropaeolum majus L. Leaves and Its In Vitro Digestion" Analytica 6, no. 2: 14. https://doi.org/10.3390/analytica6020014
APA StyleVrca, I., Jukić, D., Radić, J., & Anđelić, I. (2025). Phenolic Compounds in Edible Tropaeolum majus L. Leaves and Its In Vitro Digestion. Analytica, 6(2), 14. https://doi.org/10.3390/analytica6020014







