Effect of Low-Quality Calcined Clay on the Suppression of the Alkali–Silica Reaction †
Abstract
:1. Introduction
2. Materials
3. Methods
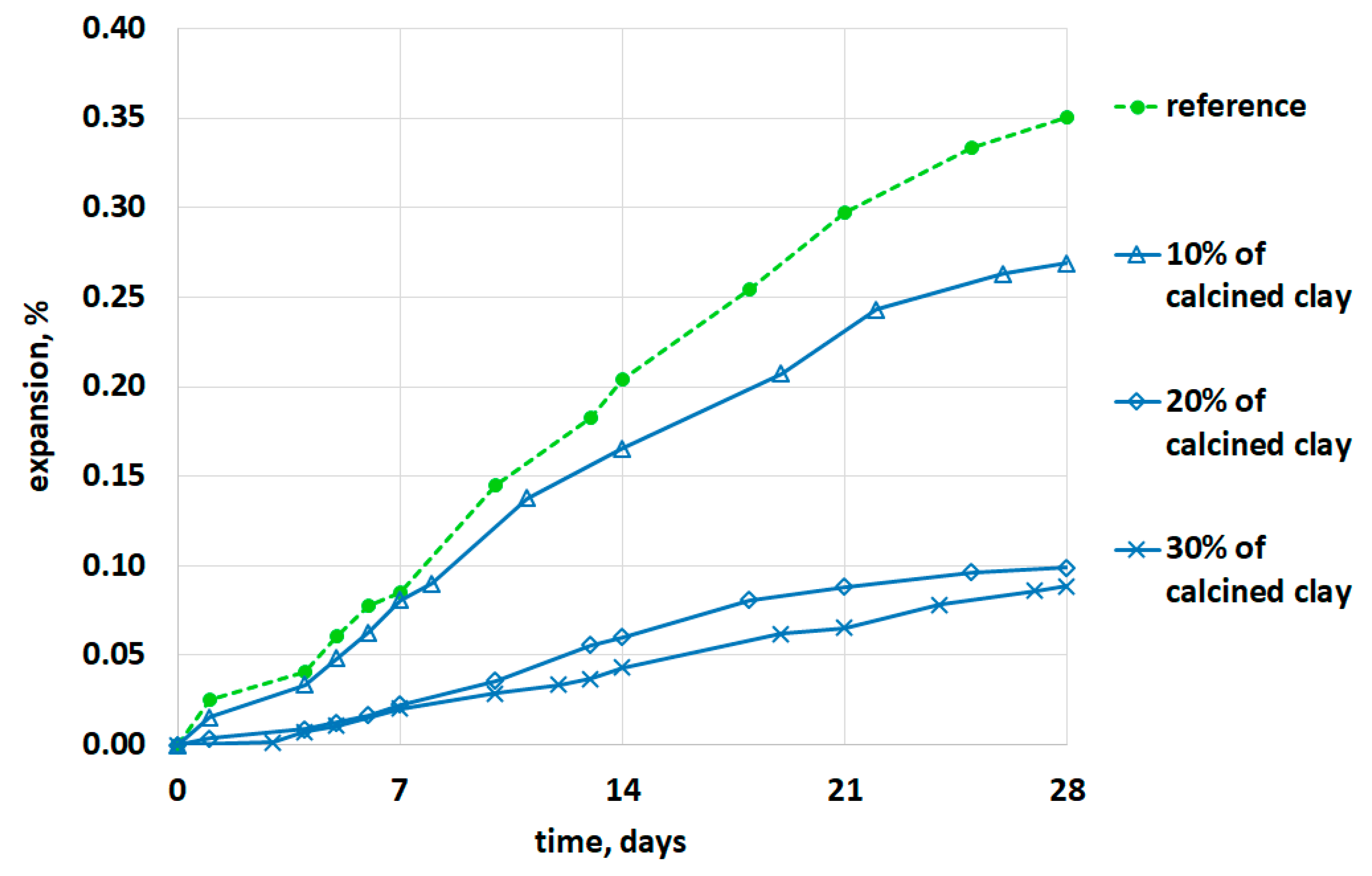
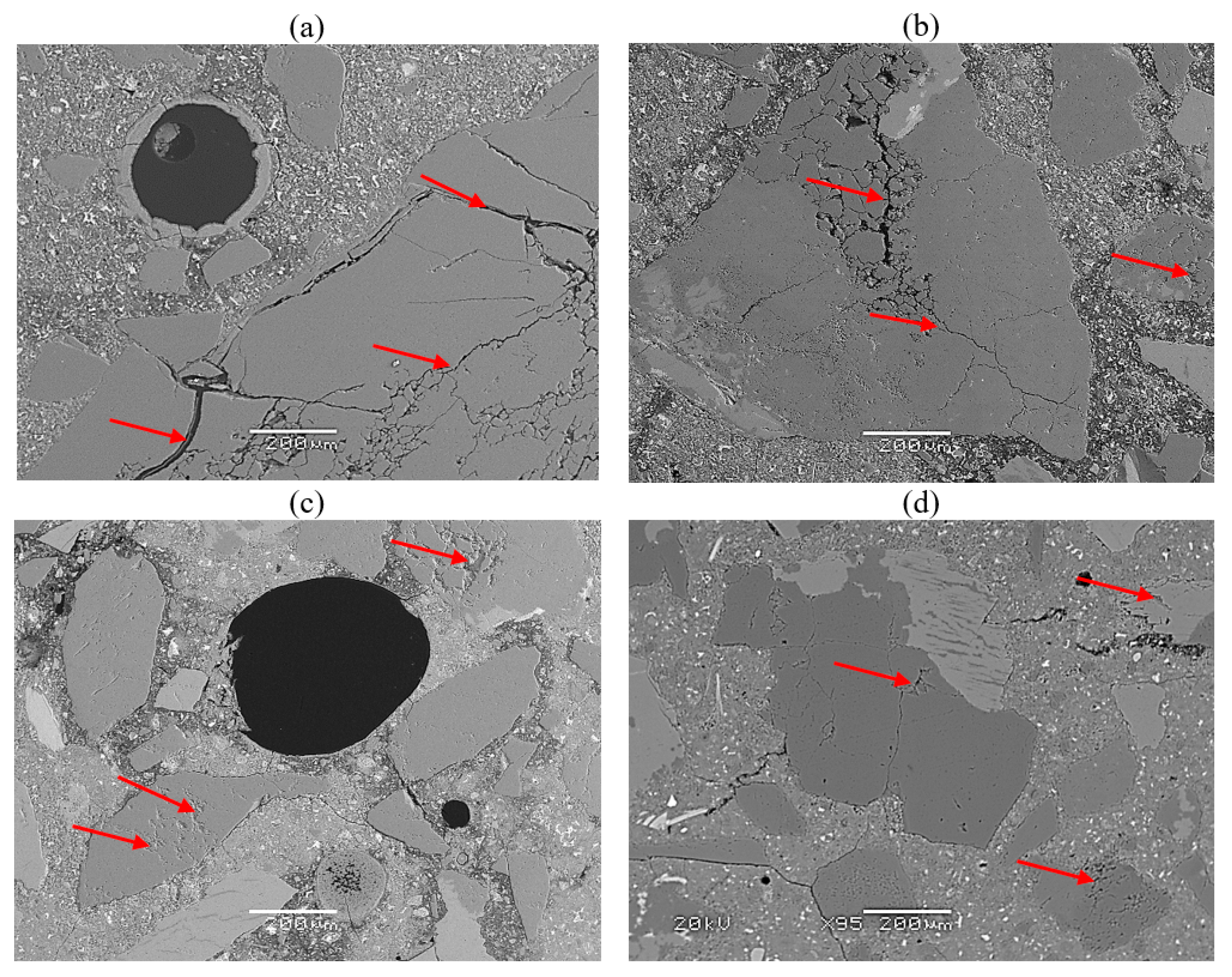
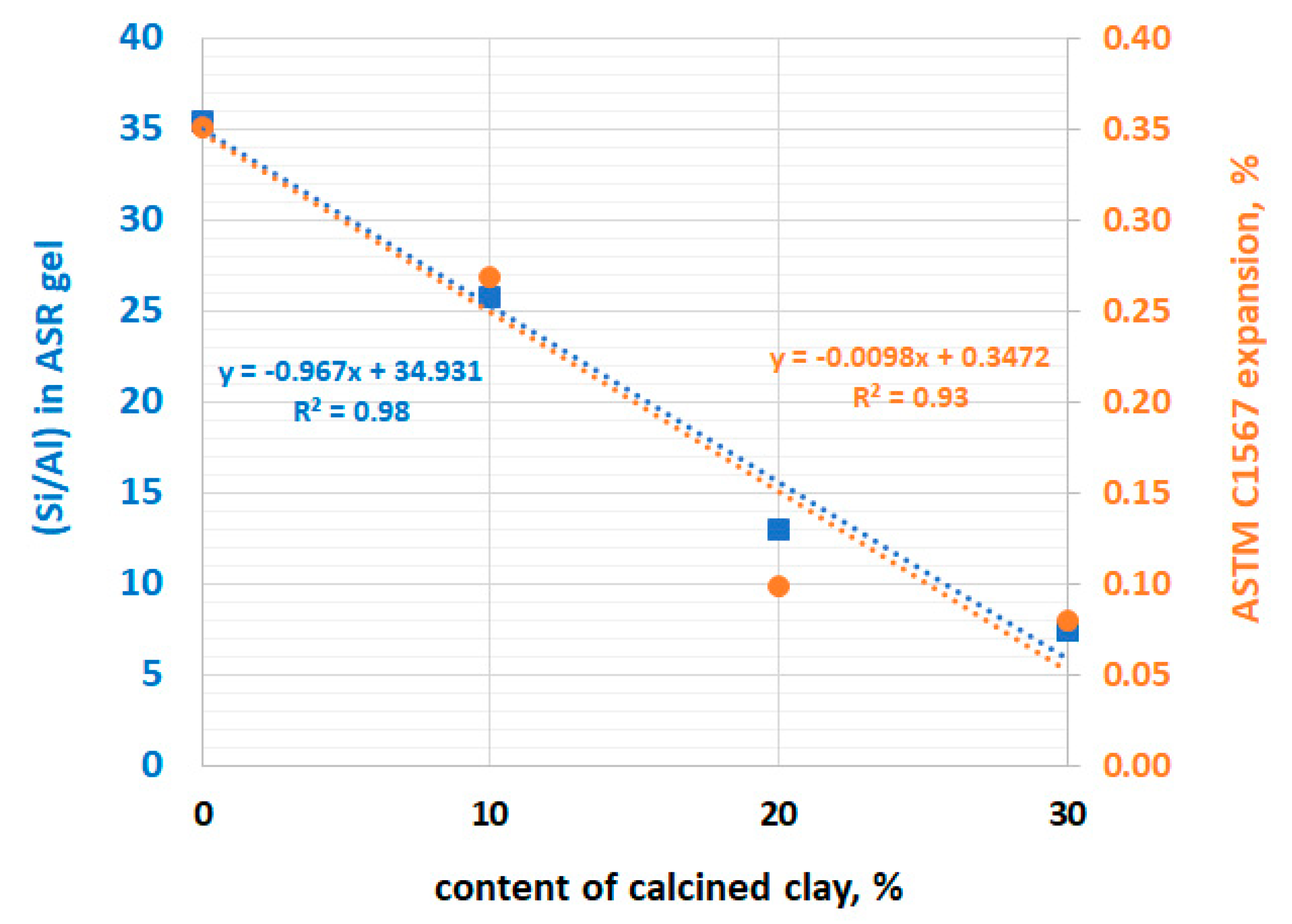
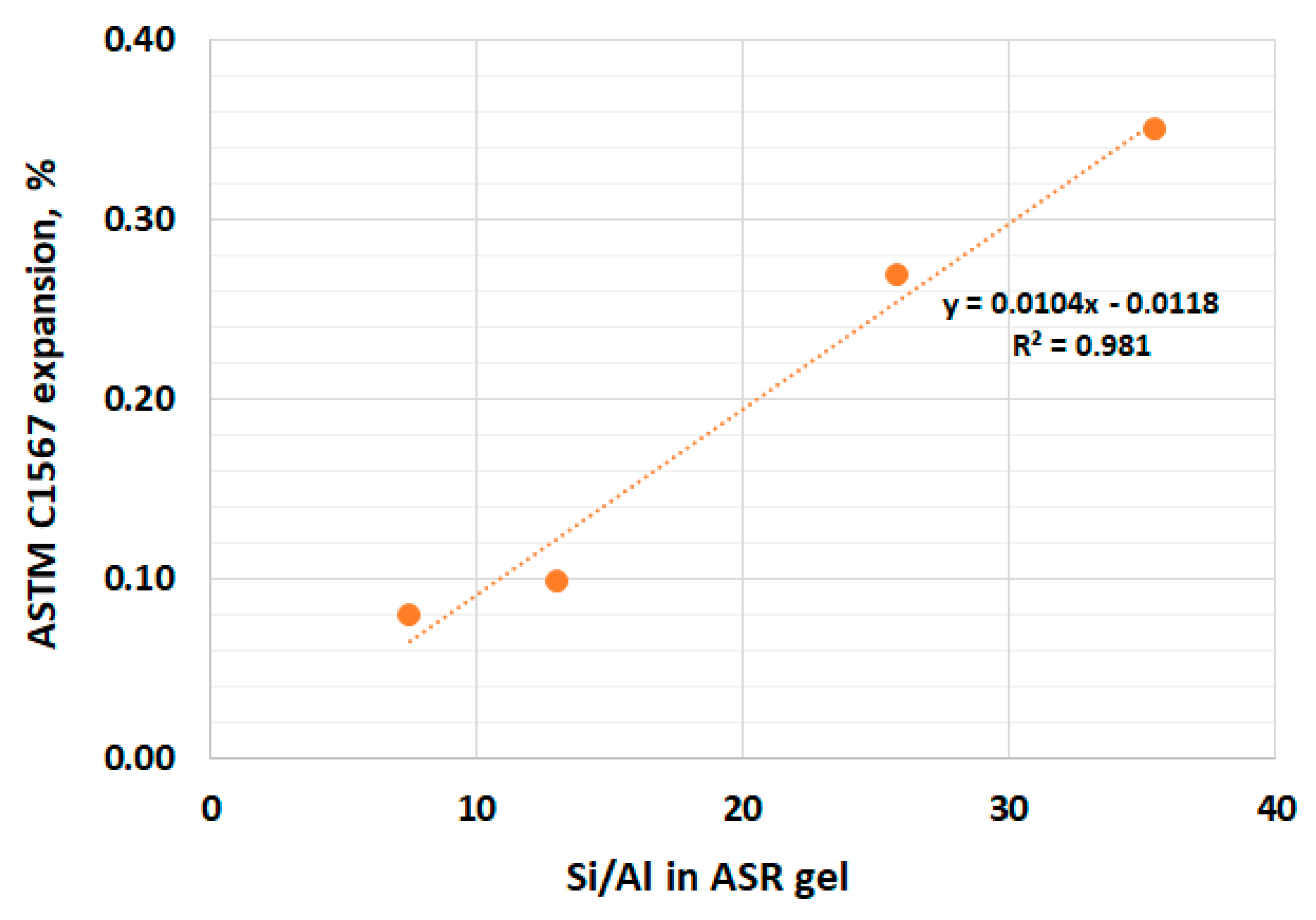
4. Results and Discussion
5. Conclusions
- The effect of low-grade calcined clay on the ASR expansion was found.
- The results indicated a reduced alkali silica expansion in mortar containing low-grade calcined clay regardless of the percentage substitution with cement.
- At a lower percentage (10%) of calcined clay, the expansion results did not satisfy ASTM standard limits.
- Replacing 20% of the cement with calcined clay appeared to be sufficient to mitigate the alkali–silica reaction.
- The morphology and chemical composition, as well as the width of the ASR gel in air voids, was different depending on the calcined clay content.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okashah, A.M.; Zainal, F.F.; Hayazi, N.F.; Nordin, M.N.; Abdullah, A. Pozzolanic properties of calcined clay in geopolymer concrete: A review. AIP Conf. Proc. 2021, 2339, 020234. [Google Scholar] [CrossRef]
- Jaskulski, R.; Jóźwiak-Niedźwiedzka, D.; Yakymechko, Y. Calcined clay as supplementary cementitious material. Materials 2020, 13, 4734. [Google Scholar] [CrossRef] [PubMed]
- Yanguatin, H.; Tobón, J.; Ramírez, J. Pozzolanic reactivity of kaolin clays, a review. Rev. Ing. Constr. 2017, 32, 13–24. [Google Scholar] [CrossRef]
- Zunino, F.; Boehm-Courjault, E.; Scrivener, K. The impact of calcite impurities in clays containing kaolinite on their reactivity in cement after calcination. Mater. Struct. 2020, 53, 44. [Google Scholar] [CrossRef]
- Li, S.C.; Ideker, J.H.; Drimalas, T.; Adams, M.P. Review on using calcined clays to mitigate alkali-silica reaction. In Proceedings of the 15th International Conference on Alkali-Aggregate Reaction in Concrete (ICAAR), São Paulo, Brazil, 3–7 July 2016. [Google Scholar]
- Li, C.; Ideker, J.H.; Drimalas, T. The Efficacy of Calcined Clays on Mitigating Alakli-Silica Reaction (ASR) in Mortar and Its Influence on Microstructure. In Calcined Clays for Sustainable Concrete; Scrivener, K., Favier, A., Eds.; RILEM Bookseries; Springer: Dordrecht, The Netherlands, 2015; Volume 10. [Google Scholar] [CrossRef]
- Walters, G.V.; Jones, T.R. Effect of metakaolin on alkali-silica reaction (ASR) in concrete manufactured with reactive aggregate, durability of concrete. In Proceedings of the 2nd International Conference, Montreal, QC, Canada, 4–9 August 1991; Volume II, pp. 941–953. [Google Scholar]
- Ramlochan, T.; Thomas, M.; Gruber, K.A. The effect of metakaolin on alkali-silica reaction in concrete. Cem. Concr. Res. 2000, 30, 339–344. [Google Scholar] [CrossRef]
- Gruber, K.; Ramlochan, T.; Boddy, A.; Hooton, R.; Thomas, M. Increasing concrete durability with high-reactivity metakaolin. Cem. Concr. Compos. 2001, 23, 479–484. [Google Scholar] [CrossRef]
- ASTM C1567-21; Standard Test Method for Determining the Potential Alkali-Silica Reactivity of Combinations of Cementitious Materials and Aggregate (Accelerated Mortar-Bar Method). Book of Standards. ASTM International: West Conshohocken, PA, USA, 2021; Volume 04.02, p. 6. [CrossRef]
- Alujas, A.; Fernandez, R.; Quintana, R.; Scrivener, K.; Martirena, F. Pozzolanic reactivity of low grade kaolinitic clays: Influence of calcination temperature and impact of calcination products on OPC hydration. Appl. Clay Sci. 2015, 108, 94–101. [Google Scholar] [CrossRef]
- Ferreiro, S.; Canut, M.M.C.; Lund, J.; Herfort, D. Influence of fineness of raw clay and calcination temperature on the performance of calcined clay-limestone blended cements. Appl. Clay Sci. 2019, 169, 81–90. [Google Scholar] [CrossRef]
- AASHTO R80-17; Standard Practice for Determining the Reactivity of Concrete Aggregates and Selecting Appropriate Measures for Preventing Deleterious Expansion in New Concrete Construction. American Association of State and Highway Transportation Officials: Washington, DC, USA, 2017; p. 25.
- Garbacik, A.; Glinicki, M.A.; Jóźwiak-Niedźwiedzka, D.; Adamski, G.; Gibas, K. Technical Guidelines for the Classification of Domestic Aggregates and Prevention of the Alkali-Aggregate Reaction in Concrete Used in Road Pavements and Road Engineering Facilities, ICiMB and IPPT PAN, Kraków-Warszawa, 2019. Available online: https://www.gddkia.gov.pl/pl/1118/dokumenty-techniczne (accessed on 12 September 2022). (In Polish)
- Glinicki, M.A.; Jóźwiak-Niedźwiedzka, D.; Antolik, A.; Dziedzic, K.; Gibas, K. Susceptibility of selected aggregates from sedimentary rocks to alkali-aggregate reaction. Roads Bridges Drog. Mosty 2019, 18, 5–24. [Google Scholar] [CrossRef]
- Antolik, A.; Jóźwiak-Niedźwiedzka, D. Assessment of the alkali-silica reactivity potential in granitic rocks. Constr. Build. Mater. 2021, 295, 123690. [Google Scholar] [CrossRef]


| Constituent | Raw Clay | CEM I |
|---|---|---|
| Content % | ||
| SiO2 | 58.23 | 19.43 |
| TiO2 | 2.04 | 0.27 |
| Al2O3 | 26.18 | 4.84 |
| Fe2O3 | 1.53 | 3.18 |
| Mn2O3 | 0.03 | 0.06 |
| MgO | 0.27 | 2.56 |
| CaO | 0.22 | 61.81 |
| Na2O | 0.13 | 0.41 |
| K2O | 1.86 | 1.08 |
| P2O5 | 0.06 | 0.11 |
| SO3 | <0.01 | 3.93 |
| Cl | 0.01 | 0.03 |
| F | 0.03 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jóźwiak-Niedźwiedzka, D.; Jaskulski, R.; Dziedzic, K.; Antolik, A. Effect of Low-Quality Calcined Clay on the Suppression of the Alkali–Silica Reaction. Mater. Proc. 2023, 13, 15. https://doi.org/10.3390/materproc2023013015
Jóźwiak-Niedźwiedzka D, Jaskulski R, Dziedzic K, Antolik A. Effect of Low-Quality Calcined Clay on the Suppression of the Alkali–Silica Reaction. Materials Proceedings. 2023; 13(1):15. https://doi.org/10.3390/materproc2023013015
Chicago/Turabian StyleJóźwiak-Niedźwiedzka, Daria, Roman Jaskulski, Kinga Dziedzic, and Aneta Antolik. 2023. "Effect of Low-Quality Calcined Clay on the Suppression of the Alkali–Silica Reaction" Materials Proceedings 13, no. 1: 15. https://doi.org/10.3390/materproc2023013015
APA StyleJóźwiak-Niedźwiedzka, D., Jaskulski, R., Dziedzic, K., & Antolik, A. (2023). Effect of Low-Quality Calcined Clay on the Suppression of the Alkali–Silica Reaction. Materials Proceedings, 13(1), 15. https://doi.org/10.3390/materproc2023013015






