Abstract
Diatomites are mineral resources formed from diatoms. They are widely used in sorption processes, medicine, cosmetology, and in protecting animals from parasites. Attempts are being made to incorporate them into concretes and construction binders to improve various performance properties. This paper presents the results of analyses (particle size analysis, XRD, and SEM) of a fine fraction of non-calcined and calcined diatomite as an additive to geopolymers made from fly ash from lignite combustion. The fly ash was also analyzed in the same way. Diatomite was introduced in its calcined and non-calcined form at 10%, 15%, and 30% by weight, replacing parts of the filler sand. The geopolymer mixtures were activated with 10 and 14 M aqueous sodium hydroxide solution with sodium water glass. As a result, it was found that it was possible to obtain geopolymers with diatomite additives with a compressive strength of about 34 MPa. In addition, after the strength tests, the microstructure of the obtained geopolymers was analyzed by scanning electron microscopy.
1. Introduction
The production of Portland cement contributes about 7% of the world’s CO2 emissions, instigating the search for alternatives to this type of material [1]. Alkali-activated binders or geopolymers are examples of such alternatives. These materials are formed by the polycondensation of aluminosilicates (containing silicates with alkali) and are characterized by an amorphous or semi-crystalline structure [2,3]. In the technology of cement and concrete, in addition to the already used and standardized mineral additives, attempts are being made to use undeveloped industrial waste [4,5]. Lach et al. investigated the possibility of immobilizing waste from municipal waste incinerators in geopolymers. The results of the study showed a high level of immobilization of compounds and elements such as sulfates, chlorides, fluorides, zinc, and barium [6]. Fly ash and slag have an advantage over other materials because, as finished waste from energy processes, they do not require additional reactivation during geopolymerization [7]. Moreover, their properties depend on their composition, the technology of production, or the raw material used in the combustion process. In addition, their use as a by-product of the energy industry is in line with the policy of a closed-loop economy [8]. Another group of potential mineral additives is power industry co-products, commonly known as calcium fly ash [9]. Calcium fly ash has a more complex composition than silica fly ash. The vitreous phase of calcium fly ash is characterized by a rich content of silica and aluminum. In their structure, there may also be glasses of the C-F-S structure (CaO-Fe2O3-SiO2) [10]. Among the main phase components of calcareous fly ash are gehlenite, anorthite, anhydrite, tricalcium aluminate, quartz, calcium sulfate-aluminate, and unbound calcium oxide (CaOx) [11].
Over recent times, diatomite material has become increasingly popular due to its unique properties and various applications. For example, the diatomite material is used in filtration processes and the production of insulating or sound-absorbing materials [12,13]. However, the most well-known example of the material’s applications is its ability to sorb various types of petroleum substances. Diatomite can also support the synthesis of zeolite structures [12]. Thus, it is possible to obtain zeolites from waste raw materials that have significant SiO2 content in their composition [14,15]. Researchers have also focused on the use of diatomite as an additive to cement binders or concrete [16]. Investigations have shown that the addition of diatomite lowers the consistency of fresh concrete mixes. The initial mechanical properties of mortars modified with diatomite additives were low, but after 28 days of seasoning, the strength increased [17].
This paper presents a comparison of the properties of geopolymers based on lime fly ash from an incinerator in Belchatow and sand, modified with different contents of diatomite dust—10%,15%, and 30% by weight. Alkali activation was carried out using two different activators: aqueous sodium glass solution and 10 and 14 mol sodium hydroxide. Diatomite dust was introduced into geopolymer mixtures in calcined and non-calcined forms. Investigations into the produced geopolymer materials showed great potential in using diatomite as one of the precursors in geopolymers. These investigations are important for providing new knowledge not only on the possibility of using local resources to produce construction materials by geopolymerization but also on the possibility of using industrial waste. Activities such as these are part of the environmentally friendly policy of a closed-loop economy.
2. Materials and Methods
2.1. Materials and Samples Preparation
Fly ash was obtained from the combustion of lignite coal from the Belchatow Power Plant (Belchatow, Poland). These ashes are characterized by relatively high calcium content. Other precursors used in the production of geopolymers were construction sand (Swietochlowice, Poland) and diatomite dust, obtained from an open-pit diatomite mine in Jawornik Ruski (Zohatin, Poland). The studies were conducted on non-calcined and calcined diatomite dust at 650 °C.
Table 1 shows the results of the particle size distribution for raw diatomite and fly ash dust. Investigations were carried out using a particle size analyzer (AntonPaar GmbH, Graz, Austria).

Table 1.
Particle size distribution.
The average particle size for diatomite dust is smaller than for fly ash from Belchatow and is approximately 12 µm. For the fly ash from Belchatow, it is approximately 21 µm. Figure 1 shows the particles of fly ash from Belchatow from the lignite combustion process and the particles of diatomite dust.
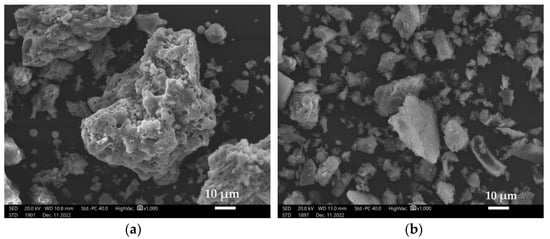
Figure 1.
Particle morphology: (a) Belchatow fly ash (1000× magnification), (b) diatomite dust (1000× magnification).
The alkaline activator was a 10 and 14 mol sodium hydroxide solution (PCC Rokita SA, Brzeg Dolny, Poland), and the water glass was sodium R-145 (STANLAB, Gliwice, Poland) with a molar modulus of 2.5 and a density of about 1.45 g/cm3; the Na/Al ratio was 1:2. To prepare the mass, the precursors were mixed with the activator for about 10 min and poured into molds. The molds were placed on a vibrating table to eliminate air bubbles. After preparing the masses, the samples were tightly covered with foil and then placed in a laboratory dryer (SLW 750 STD, Pol-Eko-Aparatura, Wodzislaw Slaski, Poland) for 24 h at 75 °C. After 24 h, the samples were unmolded and cured under laboratory conditions (temperature about 20 °C, relative humidity about 50%) for 28 days. Strength tests were carried out in the next stage. Table 2 shows the names of the samples to better systematize the mixing ratios.

Table 2.
Composition of geopolymer samples based on fly ash from Belchatow, sand, and diatomite dust (non-calcined (DN) and calcined (DK)).
The sand used in the produced geopolymers functioned as a filler. Furthermore, the amount of alkaline solution added increased with the percentage of diatomite dust in the mixture. Diatomite is commonly used as a sorbent [13]. A higher amount of alkaline solution was necessary to achieve the proper workability and consistency of the geopolymer mixture.
2.2. Research Methods
2.2.1. Phase Composition of Precursors
The PANalytical Aeris instrument (Malvern PANalytical, Lelyweg 1, Almelo, The Netherlands) was used to investigate the mineralogical composition of the geopolymer samples prepared. Quantitative analysis was carried out using the Rietveld method, which was implemented in HighScore Plus software (version: 4.8, Malvern PANalytical B.V., Almelo, The Netherlands). The International Centre for Diffraction Data (ICDD) PDF-4+ database was used during the analysis. Measurements were recorded in the range of 10–100°, with a step size of 0.003° (2θ) and a time per step of 340 s, using Cu Kα radiation.
2.2.2. Strength Tests
Compressive strength tests were carried out by EN 12390-3 (“Testing of hardened concrete. Compressive strength of specimens”) on cubic specimens (50 × 50 × 50 mm) using a Matest 3000 kN universal testing machine (Matest, Treviolo, Italy). The test speed was set at 0.05 MPa/s. The test was carried out using a load cell designed for lower loads—300 kN. Strength tests were conducted after 28 days of conditioning of the specimens. For the compressive strength tests, 10 samples of each type of geopolymer were used to calculate the average of the results.
2.2.3. Microstructure
The geopolymer materials were microscopically observed to characterize the shaped structure. The study was carried out using a JEOL JSN5510LV scanning electron microscope (JEOL Ltd., Tokyo, Japan). Samples were used for the study after mechanical property tests. Before the examination, the surface of the sample was coated with a conductive gold layer on a JOEL JEE-4X vacuum evaporator (JEOL Ltd., Tokyo, Japan).
3. Results and Discussion
3.1. Phase Composition of Precursors Results
Table 3 shows the identified phases for calcium fly ash from Belchatow.

Table 3.
The phase composition of fly ash from Belchatow.
Calcium fly ash from Belchatow Power Plant is a product of lignite coal combustion in pulverized coal furnaces. Due to the coal’s mineral part composition, the ash has an aluminous–silicon–calcium character [18]. The material has a high gehlenite content (around 30%). Anhydrite, anorthite, and mullite oscillate within 14–16%. Hematite occurs within 10%. About 3% of the phase composition consists of chlormayenite and lime. The smallest percentage of the composition is occupied by quartz—about 1%. Similar phases have been shown by other researchers [11].
Table 4 shows the phase composition of non-calcined and calcined diatomite dust.

Table 4.
Phase composition of non-calcined and calcined diatomite dust.
The analyzed diatomite dust was characterized by the presence of phases such as silicon oxide, kaolinite, albite, and aluminum oxide. Similar phases were obtained in the investigations by Ediz et al. and Ren et al. [19,20]. As a result of the calcination of diatomite, the percentage of albite increased to about 30%, while the percentage of kaolinite decreased to about 31%. In addition, in the calcined diatomite dust, the percentage of the silicon oxide phase increased to almost 38%. The non-calcined diatomite dust had a higher percentage of phases, such as kaolinite (about 49%) and aluminum oxide (0.5%), in its phase composition, compared to calcined diatomite dust.
3.2. Mechanical Properties
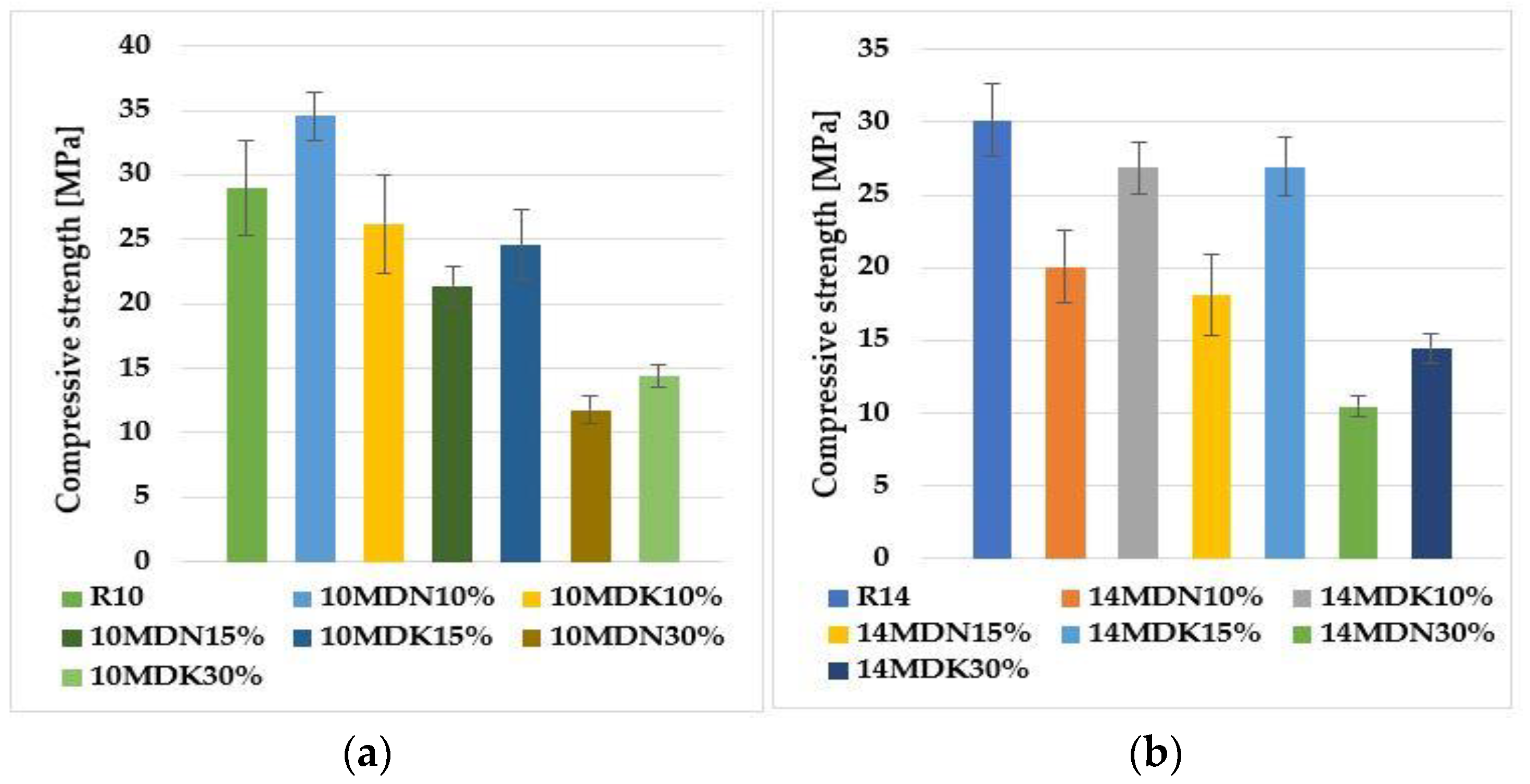
Compressive strength is one of the basic methods for evaluating the proper course of the geopolymerization process. The strength results depend on several factors, such as the structure, the presence of a crystalline phase, and the distribution and hardness of insoluble Al-Si particles. In addition, the type of alkali used and the %CaO and %K2O also affect the mechanical properties of the geopolymer composite [21,22]. Figure 2 shows a summary of the average results of compressive strength investigations performed for each type of geopolymer material.
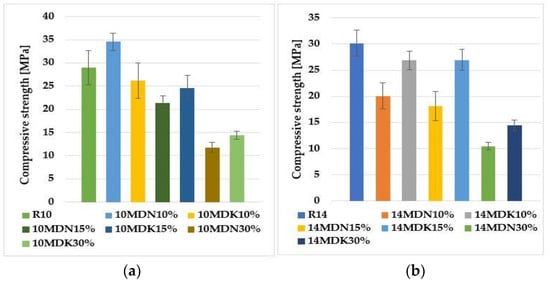
Figure 2.
Compressive strength results for geopolymers based on calcium fly ash, sand, and calcined and non-calcined activated diatomite: (a) 10 mol alkali solution, (b) 14 mol alkali solution.
For geopolymers activated with a 10-mole alkali solution, the highest compressive strength value was obtained for the 10MDN10% sample—almost 35 MPa. The lowest value was obtained for the 10MDN30% material—about 12 MPa. The values of the average compressive strength for samples 10MDK10% and 10MDK15% oscillate at a similar level—about 25 MPa. The addition of 30% calcined diatomite (10MDK30%) caused a decrease in compressive strength by almost 50% compared to the reference sample (R10). The largest mean standard deviation was recorded for the reference material (R10) and 10MDK10%. However, the lowest values were for 10MDN30% and 10MDK30%.
For geopolymers activated with 14 molar alkali solution, the highest compressive strength value was obtained for sample R14 (reference material)—almost 30 MPa. The lowest value was obtained for the 14MDN30% material—about 11 MPa. The values of the average compressive strength for samples 14MDN10% and 14MDN15% oscillate at a similar level—about 20 MPa. The addition of 30% calcined diatomite (14MDK30%) caused a decrease in compressive strength by almost 50% compared to the reference sample (R14). The highest mean standard deviation, which remained similar, was recorded for the reference material (R14) and the 14MDN15% material. On the other hand, the smallest was for 14MDN30% and 14MDK30%.
3.3. Microscopic Observations
Scanning electron microscopy (SEM) allows a visual examination of the material to obtain morphological information and allows the evaluation of structures that cannot be revealed by other examination methods [23].
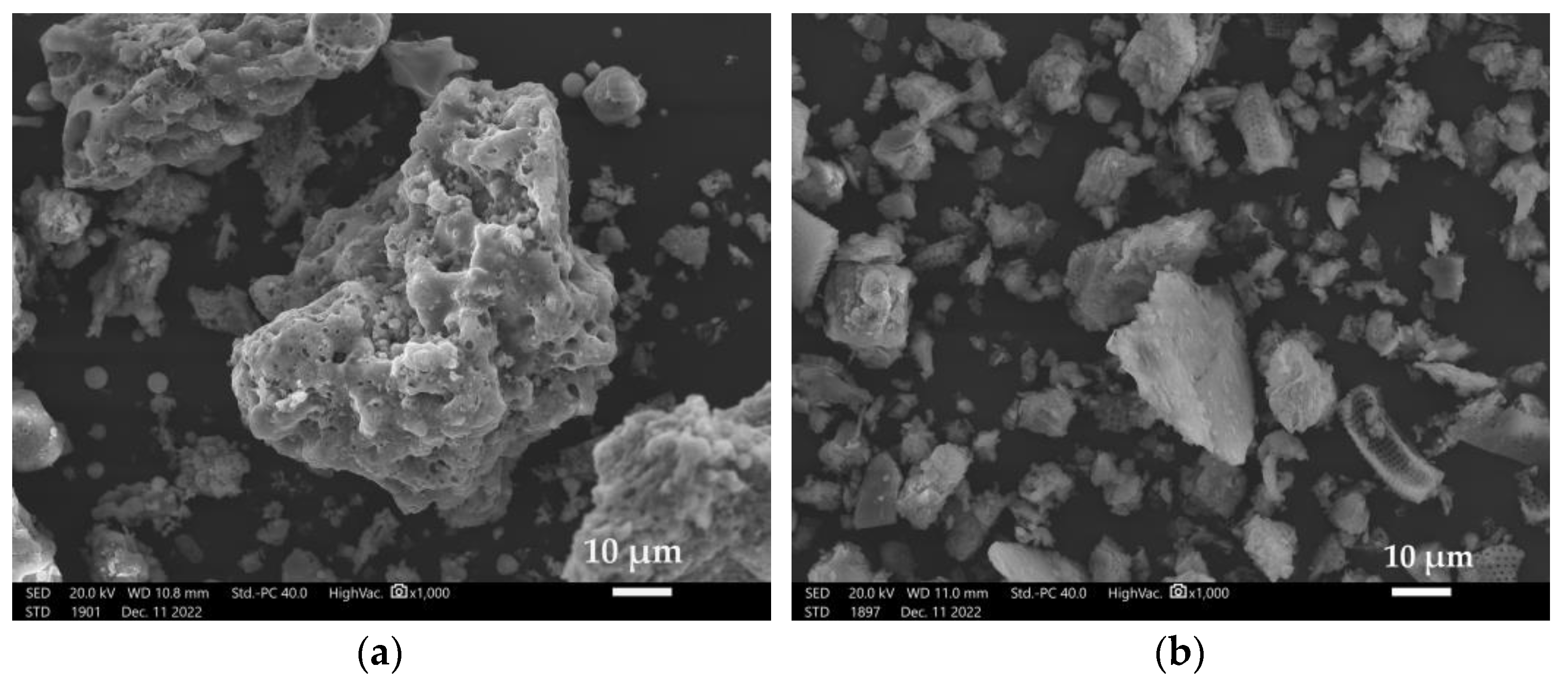
Figure 3 shows the microstructure of geopolymers based on limestone fly ash and sand activated with a 10 mol alkali solution (Figure 3a) and a 14 mol alkali solution (Figure 3b).

Figure 3.
Microstructure of reference geopolymers in magnifications 1000×: (a) 10R, (b) 14R.
In Figure 3a, the inconsistent structure of the porous material can be observed.
These are grains of unreacted limestone fly ash from Belchatow. However, as opposed to silica ash, limestone fly ash grains are characterized by very large particles of unburned carbon, porous and poorly sintered [11]. Furthermore, in addition to the geopolymer matrix, we can observe C-S-H. A similar structure was analyzed by Zhang et al. in their work [24].
Figure 3b shows the microstructure of a geopolymer based on lime fly ash from Belchatow and sand, activated with a 14 mol alkali solution. In this case, a much smaller amount of unreacted fly ash can be observed. The structure is more compact than that of the 10R material. Alehyen et al., in their work, focused on studying the microstructures of fly-ash-based geopolymer mortars. They described them as porous heterogeneous mixtures in which some of the ash grains did not react or had reacted partially. In addition, they indicated the possible presence of residual alkaline deposits and geopolymer gel [25].
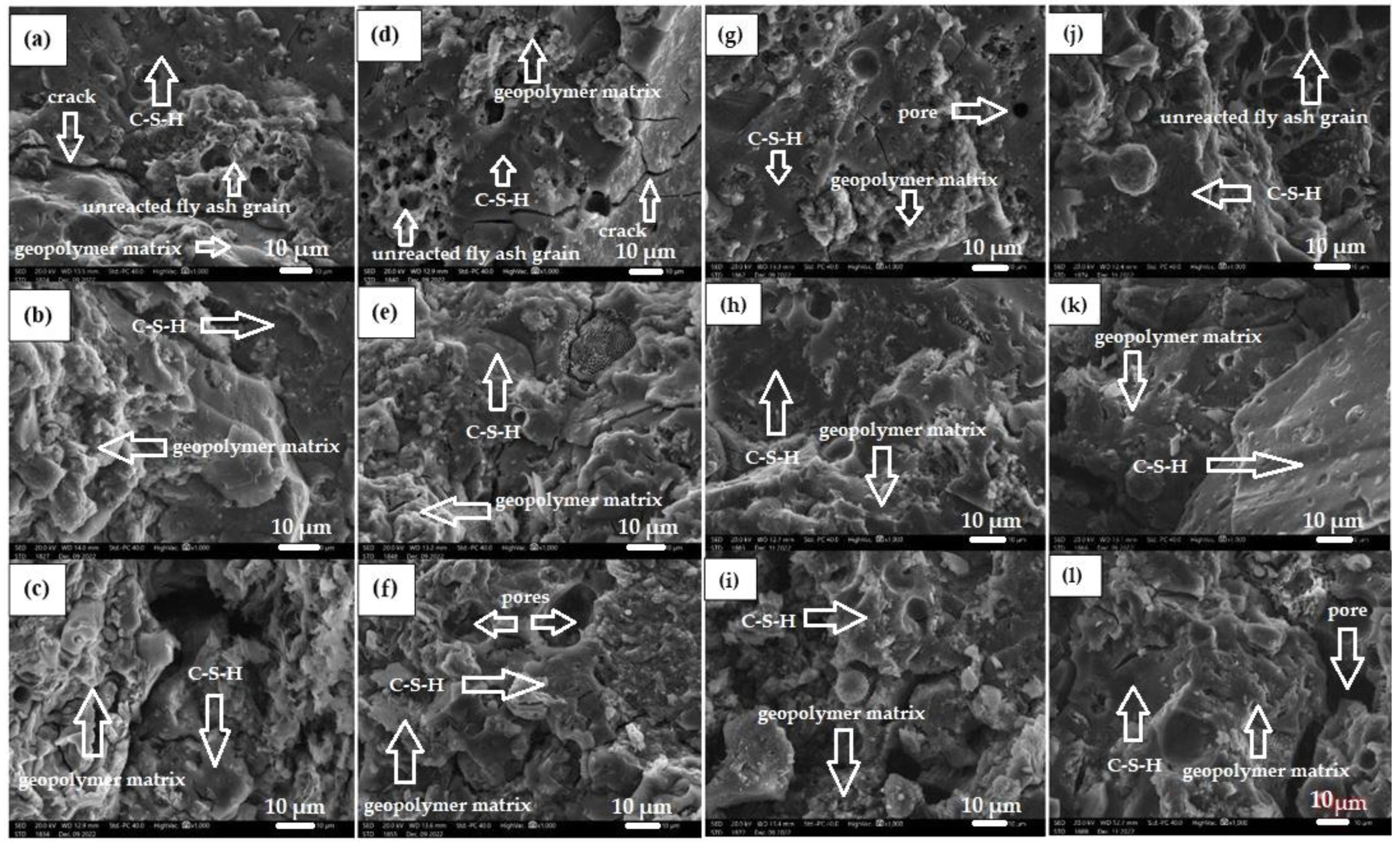
Figure 4 shows the morphology of all geopolymer materials based on fly ash, sand, and diatomite dust (calcined and non-calcined).
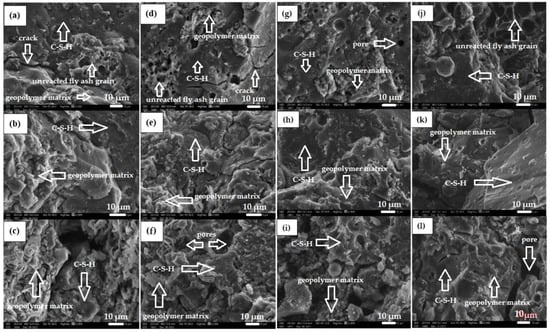
Figure 4.
Microstructure of geopolymers in magnification 1000x: (a) 10MDN10%, (b) 10MDK10%, (c) 10MDN15%, (d) 10MDK15%, (e) 10MDN30%, (f) 10MDK30%, (g) 14MDN10%, (h) 14MDK10%, (i) 14MDN15%, (j) 14MDK15%, (k)14MDN30%, (l)14MDK30%.
As a result of the SEM analysis, it can be observed that materials based on calcium fly ash, sand, and diatomite dust (calcined and non-calcined), activated with a 10 mol alkali solution (Figure 4a–f), have more unreacted lignite particles (clusters of porous structures) in their structure. Compounds of geopolymers activated with 14 molar alkali solution show significantly fewer particles of unreacted ash (Figure 4g–l). According to the presented microstructure images, the fly ash-based materials are characterized by an amorphous structure and contain undecomposed fly ash particles. It was also noted that there is a changeable pore content in the microstructure of the materials.
4. Conclusions
The conducted strength and structural studies were aimed at evaluating the possibility of using potential precursors in the form of diatomite dust (calcined and non-calcined) as one of the components of geopolymer materials. The presented investigations allow us to conclude the following:
- The phase composition of calcined and non-calcined diatomite dust differs only in the percentage of phases—the same phases are present in both cases but in different percentage ratios.
- Mechanical investigations have shown that the addition of diatomite dust can positively affect the strength properties of the geopolymer.
- In addition to the percentage addition of diatomite dust, the mechanical properties of the tested geopolymer materials were influenced by the concentration of the alkali activator used.
Author Contributions
Conceptualization, K.P. and M.Ł.; methodology, K.P., A.B. and M.H.-K.; formal analysis, K.P., and M.Ł.; investigation, K.P. and M.H.-K.; resources, M.Ł. and M.H.-K.; writing—original draft preparation, K.P. and A.B.; writing—review and editing, M.Ł.; supervision, M.Ł. and M.H.-K. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been financed by the National Centre for Research and Development in Poland under the grant: “Development and demonstration of technologies for the production of highly effective diatomite-based sorbents and diatomite fillers”; Project No.: POIR.04.01.04-00-0032/20.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The publication cost of this paper was covered with funds from the Polish National Agency for Academic Exchange (NAWA): “MATBUD’2023—Developing international scientific cooperation in the field of building materials engineering” BPI/WTP/2021/1/00002, MATBUD’2023.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Singh, N.B.; Middendorf, B. Geopolymers as an Alternative to Portland Cement: An Overview. Constr. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- Petrus, H.T.B.M.; Olvianas, M.; Shafiyurrahman, M.F.; Pratama, I.G.A.A.N.; Jenie, S.N.A.; Astuti, W.; Nurpratama, M.I.; Ekaputri, J.J.; Anggara, F. Circular Economy of Coal Fly Ash and Silica Geothermal for Green Geopolymer: Characteristic and Kinetic Study. Gels 2022, 8, 233. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.V.; Le, V.S.; Louda, P.; Szczypiński, M.M.; Ercoli, R.; Vojtěch, R.; Łoś, P.; Prałat, K.; Plaskota, P.; Pacyniak, T.; et al. Low-Density Geopolymer Composites for the Construction Industry. Polymers 2022, 14, 304. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A.; Habert, G.; Myers, R.J.; Harvey, J.T. Achieving Net Zero Greenhouse Gas Emissions in the Cement Industry via Value Chain Mitigation Strategies. One Earth 2021, 4, 1398–1411. [Google Scholar] [CrossRef]
- Plawecka, K.; Figiela, B.; Grela, A.; Buczkowska, K.E. Geopolymers Based on Plasma Incineration Waste as a Material for Circular Economy. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Wrocław, Poland, 23–25 June 2021; Volume 942. [Google Scholar]
- Łach, M.; Mierzwiński, D.; Korniejenko, K.; Mikuła, J.; Hebda, M. Geopolymers as a Material Suitable for Immobilization of Fly Ash from Municipal Waste Incineration Plants. J. Air Waste Manag. Assoc. 2018, 68, 1190–1197. [Google Scholar] [CrossRef]
- Pławecka, K.; Bazan, P.; Lin, W.-T.; Korniejenko, K.; Sitarz, M.; Nykiel, M. Development of Geopolymers Based on Fly Ashes from Different Combustion Processes. Polymers 2022, 14, 1954. [Google Scholar] [CrossRef]
- John, S.K.; Nadir, Y.; Girija, K. Effect of Source Materials, Additives on the Mechanical Properties and Durability of Fly Ash and Fly Ash-Slag Geopolymer Mortar: A Review. Constr. Build. Mater. 2021, 280, 122443. [Google Scholar] [CrossRef]
- Nuaklong, P.; Jongvivatsakul, P.; Pothisiri, T.; Sata, V.; Chindaprasirt, P. Influence of Rice Husk Ash on Mechanical Properties and Fire Resistance of Recycled Aggregate High-Calcium Fly Ash Geopolymer Concrete. J. Clean. Prod. 2020, 252, 119797. [Google Scholar] [CrossRef]
- Nuaklong, P.; Wongsa, A.; Sata, V.; Boonserm, K.; Sanjayan, J.; Chindaprasirt, P. Properties of High-Calcium and Low-Calcium Fly Ash Combination Geopolymer Mortar Containing Recycled Aggregate. Heliyon 2019, 5, e02513. [Google Scholar] [CrossRef]
- Dąbrowski, M. Effect of Addition of Lime Fly Ash on Microstructure and Frost Resistance of Composites with Cementitious Matrices; Institute of Fundamental Technological Research, Polish Academy of Sciences, Laboratory of Strain Fields: Warsaw, Poland, 2016. [Google Scholar]
- Ersoy, O.; Rençberoğlu, M.; Karapınar Güler, D.; Özkaya, Ö.F. A Novel Flux That Determines the Physico-Chemical Properties of Calcined Diatomite in Its Industrial Use as a Filler and Filter Aid: Thenardite (Na2SO4). Crystals 2022, 12, 503. [Google Scholar] [CrossRef]
- Łach, M.; Pławecka, K.; Marczyk, J.; Ziejewska, C.; Hebdowska-Krupa, M.; Nykiel, M.; Hebda, M.; Miernik, K.; Mierzwiński, D.; Korniejenko, K.; et al. Use of Diatomite from Polish Fields in Sustainable Development as a Sorbent for Petroleum Substances. J. Clean. Prod. 2023, 389, 136100. [Google Scholar] [CrossRef]
- Łach, M.; Grela, A.; Pławecka, K.; Guigou, M.D.; Mikuła, J.; Komar, N.; Bajda, T.; Korniejenko, K. Surface Modification of Synthetic Zeolites with Ca and HDTMA Compounds with Determination of Their Phytoavailability and Comparison of CEC and AEC Parameters. Materials 2022, 15, 4083. [Google Scholar] [CrossRef] [PubMed]
- Łach, M.; Grela, A.; Bajda, T.; Mierzwiński, D.; Komar, N.; Mikuła, J. Production of Zeolite Sorbents from Burning and Co-Burning Biomass with Coal. E3S Web Conf. 2018, 44, 00097. [Google Scholar] [CrossRef]
- Lv, Z.; Jiang, A.; Liang, B. Development of Eco-Efficiency Concrete Containing Diatomite and Iron Ore Tailings: Mechanical Properties and Strength Prediction Using Deep Learning. Constr. Build. Mater. 2022, 327, 126930. [Google Scholar] [CrossRef]
- Kapeluszna, E.; Szudek, W.; Wolka, P.; Zieliński, A. Implementation of Alternative Mineral Additives in Low-Emission Sustainable Cement Composites. Materials 2021, 14, 6423. [Google Scholar] [CrossRef]
- Formela, M.; Stryczek, S. Fly Ashes from the Combustion of Lignite as Additive to the Cement Slurry Used in the Work of Filling Voids in the Rock Mass. Sci. J. Inst. Miner. Energy Econ. Pol. Acad. Sci. 2017, 97, 117–134. [Google Scholar]
- Ediz, N.; Bentli, I.; Tatar, I. Improvement in Filtration Characteristics of Diatomite by Calcination. Int. J. Miner. Process. 2010, 94, 129–134. [Google Scholar] [CrossRef]
- Ren, Z.; Gao, H.; Zhang, H.; Liu, X. Effects of Fluxes on the Structure and Filtration Properties of Diatomite Filter Aids. Int. J. Miner. Process. 2014, 130, 28–33. [Google Scholar] [CrossRef]
- Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. Do Geopolymers Actually Contain Nanocrystalline Zeolites? A Reexamination of Existing Results. Chem. Mater. 2005, 17, 3075–3085. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer Technology: The Current State of the Art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Relucenti, M.; Familiari, G.; Donfrancesco, O.; Taurino, M.; Li, X.; Chen, R.; Artini, M.; Papa, R.; Selan, L. Microscopy Methods for Biofilm Imaging: Focus on SEM and VP-SEM Pros and Cons. Biology 2021, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; Li, Z.; Zhang, Q.; Li, H.; Du, J.; Qi, Y. Properties of Fresh and Hardened Geopolymer-Based Grouts. Ceramics Silikaty 2019, 63, 164–173. [Google Scholar] [CrossRef]
- Alehyen, S.; Achouri, M.E.; Taibi, M. Characterization, Microstructure and Properties of Fly Ash-Based Geopolymer. J. Mater. Environ. Sci. 2017, 8, 1783–1796. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).