Abstract
The development of calcium phosphate cement (CaPC) has been regarded as a significant advance in the field of bone defect reconstruction. This patent analysis-based research concerns patent documents with an active legal status until 2022. The state of the art is reviewed by introducing what has been patented concerning CaPCs for bone repair. As a result, 740 active patent documents were found, and 51% of them were published during the last 7 years. According to the findings, the United States ranked as the first jurisdiction, with academic institutions from France and the United States leading the patenting way. The Cooperative Patent Classification reveals that most inventions are intended for materials or treatments for tissue regeneration, such as the reconstruction of bones with weight-bearing implants, as well as inorganic materials for grafts or prostheses, such as phosphorus-containing materials.
1. Introduction
The development of calcium phosphate cement (CaPC) has been regarded as a significant advance in the field of bone defect reconstruction [1]. This bioceramic-based biomaterial is injected within the osseous cavity due to its bioactivity and rheological properties, which results in a very quick response to bone attachment and simultaneous regeneration [2]. Furthermore, CaPC may be useful as a bone drug delivery system, encapsulating cells for bone regeneration while also being capable of impregnating drugs for long-term release [3,4].
Active research on CaPCs for bone repair is focused on the development of an injectable, macroporous, and resorbable formula with a high compressive strength [5,6]. To prove the innovation in this way, we need to see an increase in the number of patent applications filed each year in this area around the world [7].
The first granted patent in this field was filed in 1963 by “Biorex Laboratories LTD” (London, UK) and published in 1967 [8]. Through the claimed invention, Baxendale and Kirk proposed different dental fillers and bone cements. The compositions comprised collagen gel (doped with calcium and phosphate ions), calcium hydroxyapatite powder, an antibiotic, and an anti-inflammatory agent. In the broad aspect of the invention, the inventors proposed the developed formulation for filling teeth or cementing bone [8].
This research, in the form of a patent analysis, focuses on patent documents relating to the development of CaPCs for bone repair, which have an active legal status until 2022. It is established as a research planning tool to analyze what has been patented in this area in accordance with patent analysis standards [9,10,11]. Furthermore, we determine, through different patent databases, publication dates, applicants, owners, patent classifications, and patent jurisdictions. This type of research has already been demonstrated in various fields through several recent publications on hydrogel-based coatings [9], biocontrol agents [10], bioinks [11], energy [12,13], carbon [14], plant-based vaccines [15], and orthosis [16].
2. Resources and Methodology
Three patent databases were used in this study: Espacenet patent search [17], PatFT-AppFT databases [18], and Google Patents database [7]. Different terms and related keywords to CaPCs for bone repair were used, and the search was carried out on titles, abstracts, and claims. The search fields and queries used in this study were based on a previous study concerning the patent landscape [11]. The results were then filtered to include only patent documents (i.e., patent applications and granted patents) with publication dates until 2022 and which have an active legal status.
3. Results and Discussion
3.1. Patent Documents and Legal Status
During a search, 740 patent documents related to CaPCs for bone repair were found. It encompasses 394 patent applications and 346 granted patents. The legal status of the found patent documents is “active status”, indicating that all granted patents are still in force. Moreover, if a patent application has been granted, all published patent documents associated with the application have the status “active”.
3.2. Publication Dates
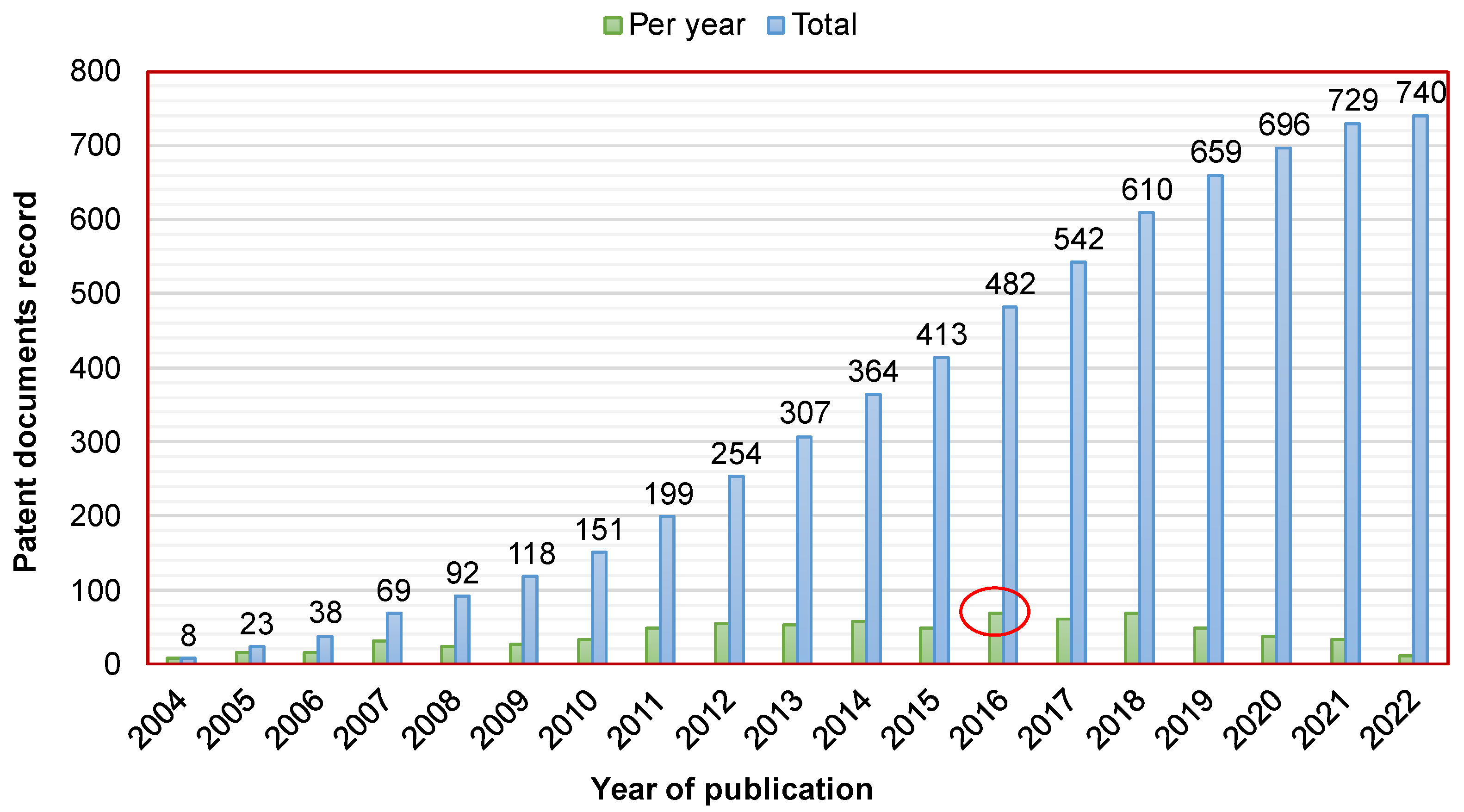
Figure 1 shows the patent document record as a function of the publication dates. These publications concern only the active patent documents until 2022. Overall, 51% of them were published during the last 7 years. Furthermore, the maximum numbers of patent applications and granted patents were recorded in 2016 and 2018, with 35 and 38, respectively; however, the maximum number of patent documents was recorded in 2016 with 69.
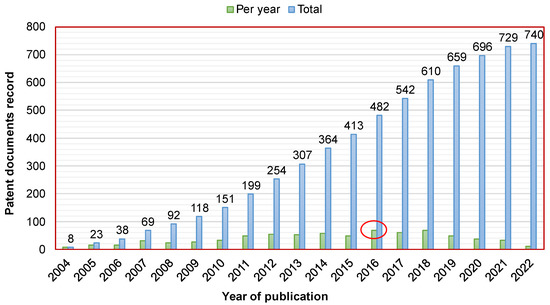
Figure 1.
Publication dates of the active patent documents related to the development of CaPCs for bone repair. The red circle in the figure represents the maximum number of patent documents that was recorded in 2016 with 69.
3.3. Applicants and Owners
An applicant for a patent is a natural person or legal entity that seeks to obtain a patent from a patent office for a claimed invention [19].
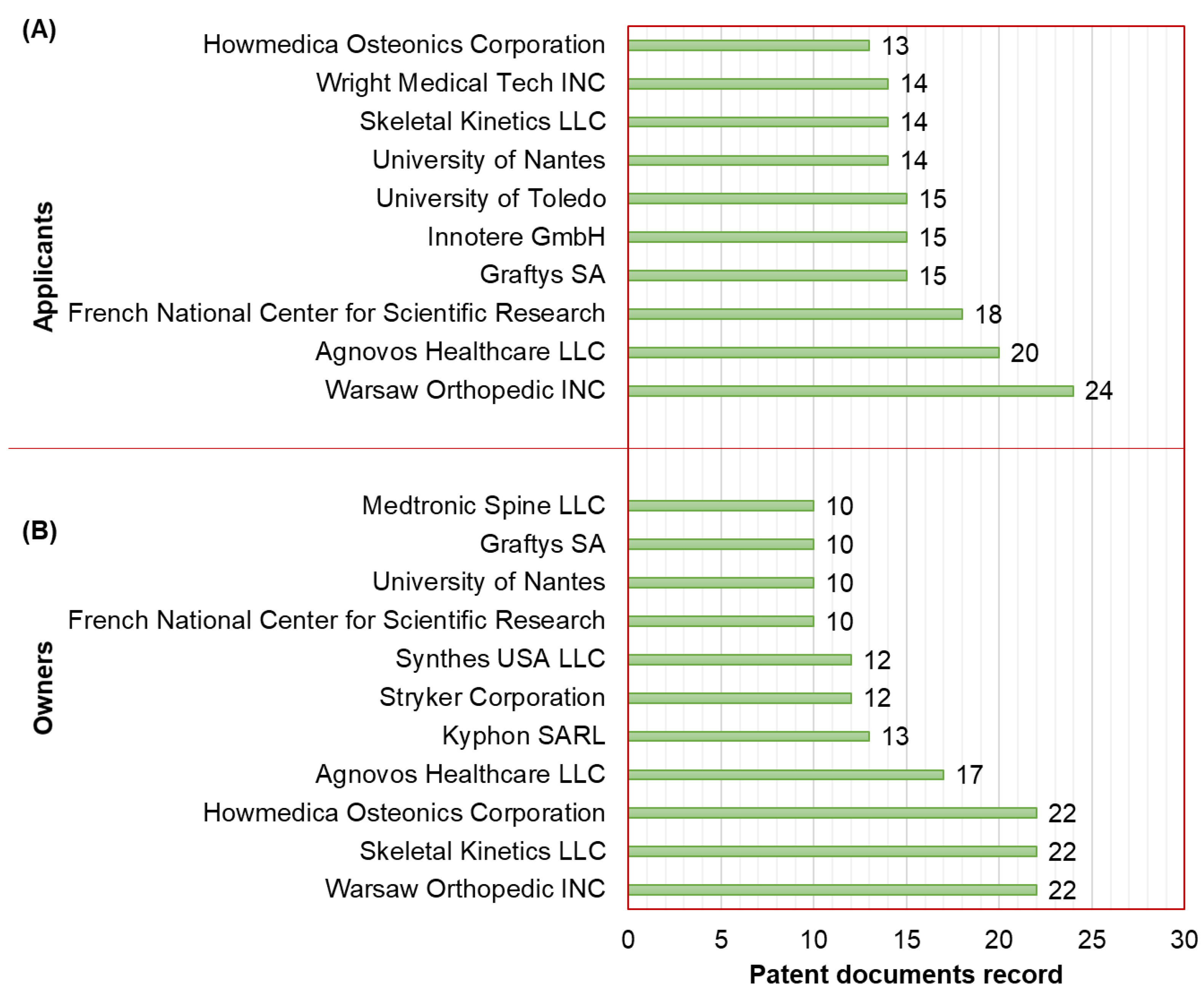
The top 11 applicants of active patent documents related to the development of CaPCs for bone repair are displayed in Figure 2A. In the first instance, we note that these top 11 applicants include only legal entities from the United States, France, and Germany, which are considered companies, universities, or research institutions. “Warsaw Orthopedic INC” (Warsaw, IN, USA) is ranked as the first applicant, having recorded 24 patent documents. In second place, the applicant “Agnovos Healthcare LLC” (Derwood, MD, USA) has recorded 20 patent documents. Thirdly, the “French National Center for Scientific Research” (Paris, France), as a research institution, has recorded 18 patent documents.
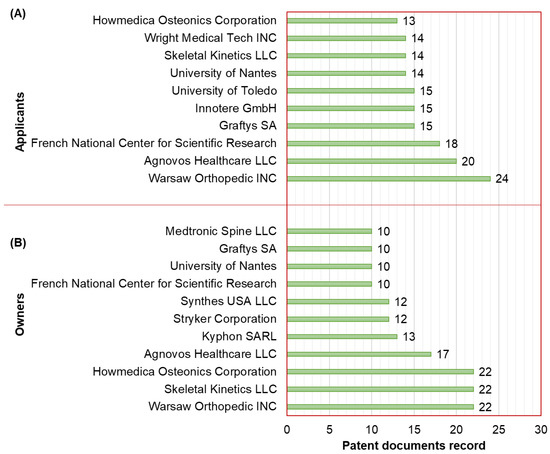
Figure 2.
(A) Applicants (top 11) and (B) owners (top 10) of active patent documents related to the development of CaPCs for bone repair.
An owner of a patent is a natural person or legal entity that holds the legal rights to the patent. This may be the inventor(s) and/or applicant(s) of the patent, or it may be an organization to which the inventor(s) and/or applicant(s) have assigned the rights to the patent [19].
The top 10 owners of active patent documents related to the development of CaPCs for bone repair are displayed in Figure 2B. In the first instance, we note that these top 10 owners include seven owners who are already classified as applicants. As owner, the parent organization Medtronic leads the patenting way with 45 patent documents through its branches or divisions: “Warsaw Orthopedic INC” (Warsaw, IN, USA), “Kyphon SARL” (Neuchâtel, Switzerland), and “Medtronic Spine LLC” (Sunnyvale, CA, USA). Stryker is another parent organization that is ranked as the second owner with 34 patent documents through its branches or divisions: “Howmedica Osteonics Corporation” (Mahwah, NJ, USA) and “Stryker Corporation” (Kalamazoo, MI, USA). Lastly, the company “Skeletal Kinetics LLC” (Beach, FL, USA) is ranked as the third owner, having recorded 22 patent documents.
Furthermore, academic institutions from France and the United States are leading the patenting way on the development of CaPCs for bone repair as applicants and/or owners. These institutions are the “University of Nantes” (Nantes, France) and the “University of Toledo” (Toledo, OH, USA).
3.4. Patent Classifications
Patent classifications are used to organize and classify patents according to the subject matter of the invention or technology described in the patent. There are several different patent classification systems in use around the world. Hereinafter, we use the Cooperative Patent Classification (CPC) system that is used by the European Patent Office (EPO) and the United States Patent and Trademark Office (USPTO). It consists of a hierarchical structure of classes, subclasses, and groups, with each level of the hierarchy providing increasing levels of detail [19].
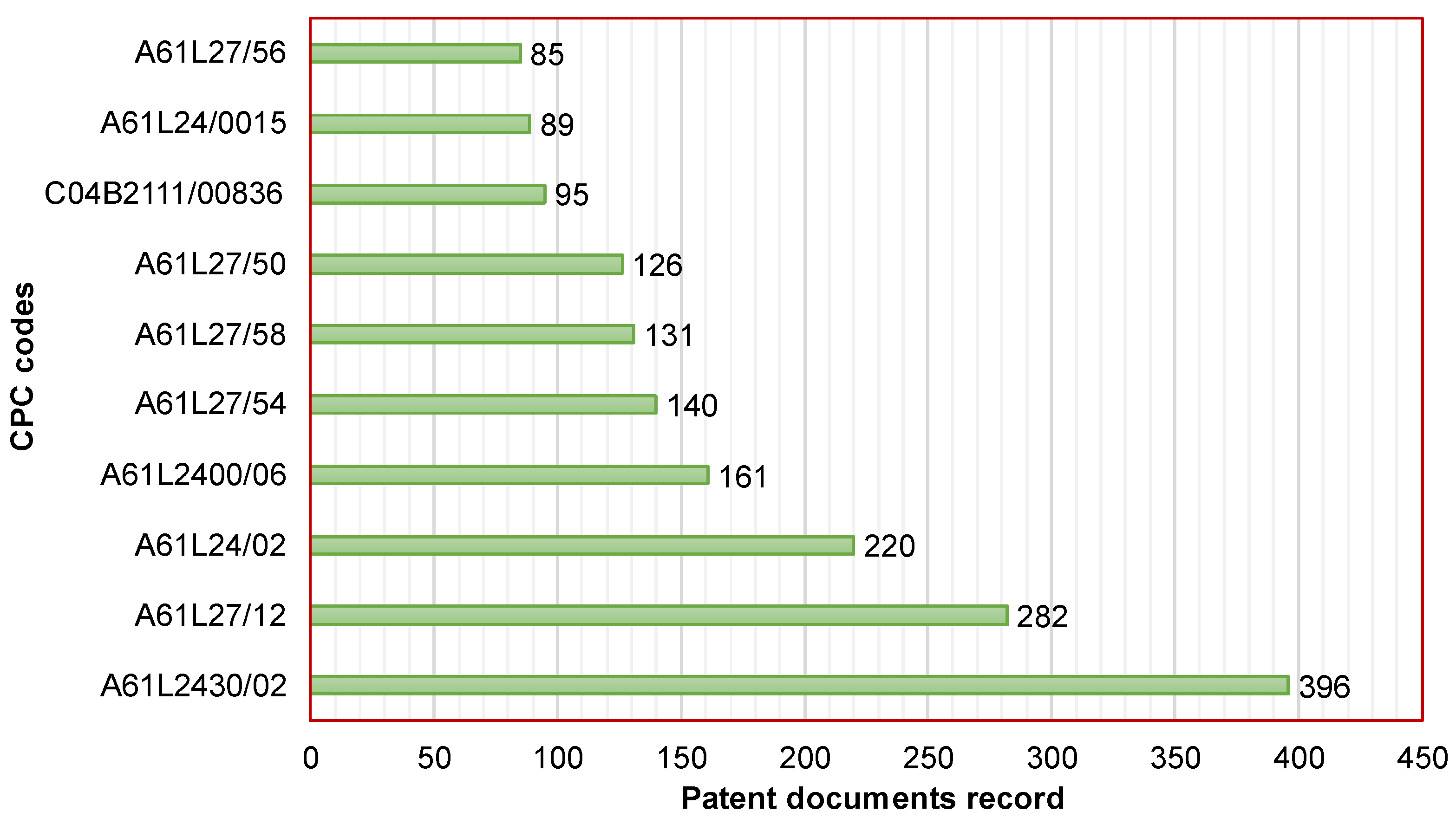
The top 10 CPC codes of active patent documents related to the development of CaPCs for bone repair are displayed in Figure 3. The most common code corresponds to A61L2430/02. It is a characteristic of materials or treatments for tissue regeneration, such as the reconstruction of bones with weight-bearing implants. This code was found in 396 patent documents (i.e., 53.5% of total patent documents). Secondly, the code A61L27/12 was found in 282 patent documents (i.e., 38.1% of total patent documents). It concerns inorganic materials for grafts or prostheses, such as phosphorus-containing materials. Thirdly, the code A61L24/02 was found in 220 patent documents (i.e., 29.7% of total patent documents). It concerns surgical adhesives or cement adhesives for colostomy devices containing inorganic materials. For the purpose of searching patent applications and granted patents related to the development of CaPCs for bone repair, Table 1 presents details concerning the description of the top 10 CPC codes.
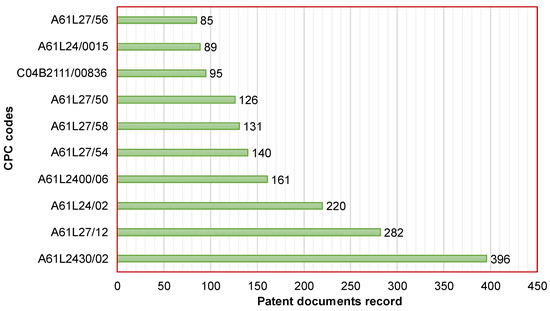
Figure 3.
Cooperative Patent Classification (Top 10) of active patent documents related to the development of CaPCs for bone repair.

Table 1.
Details of the top 10 CPC codes of active patent documents related to the development of CaPCs for bone repair [17,18].
Lastly, it should be noted that applicants may use multiple CPC codes to further classify their patents, more specifically if the patent document is related to interlinked fields or multidisciplinary sectors.
3.5. Patent Jurisdictions
Patent jurisdictions are the countries (i.e., national patent jurisdictions) or regions (i.e., regional patent jurisdictions) in which a patent is recognized and enforceable. However, there is an international system, administered by the World Intellectual Property Organization (WIPO), that allows inventors to seek protection for their inventions in multiple countries worldwide by filing a single application under the Patent Cooperation Treaty (PCT) [19,20].
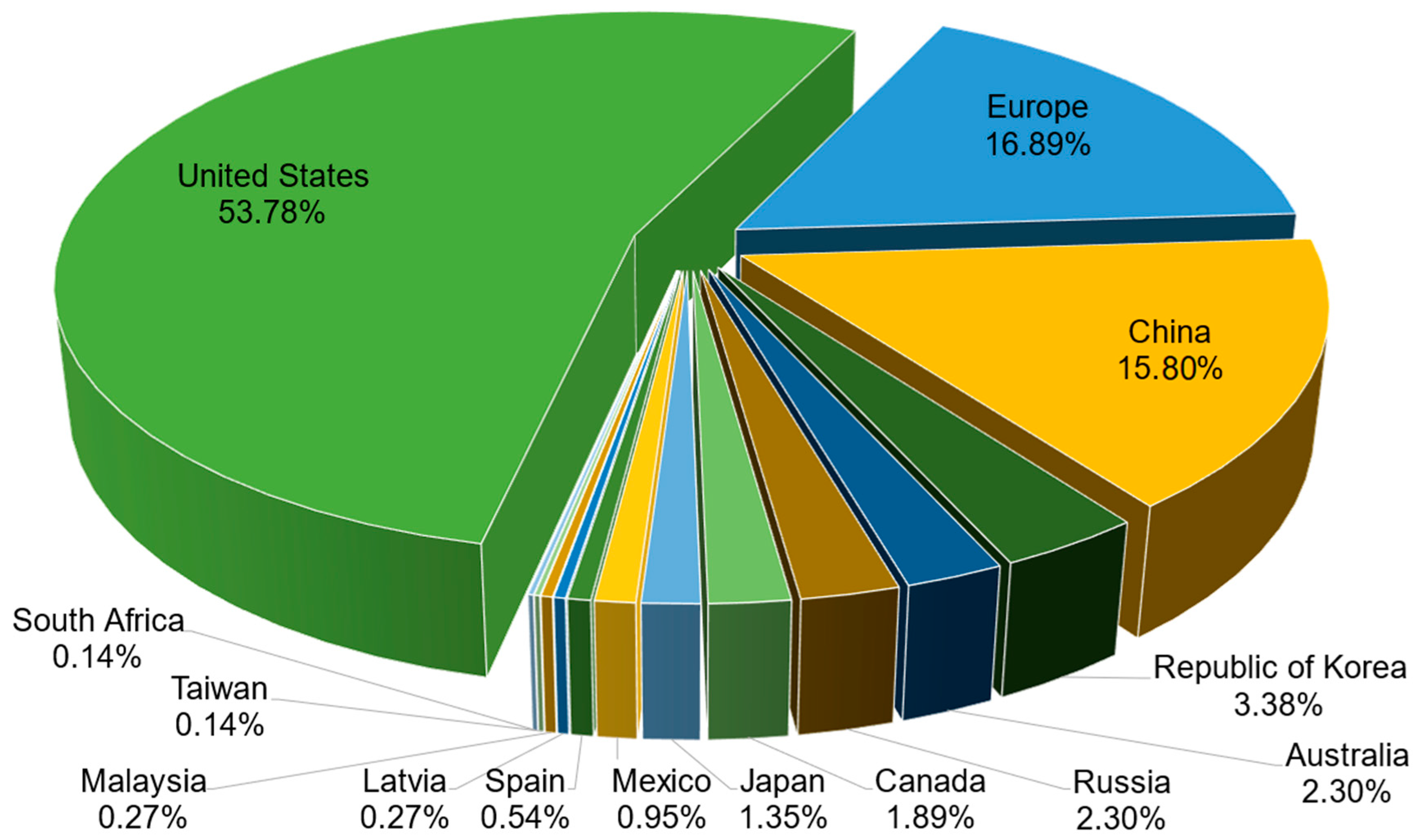
The jurisdictions of active patent documents related to the development of CaPCs for bone repair are displayed in Figure 4. The list includes 14 jurisdictions, one of which is a regional patent jurisdiction, Europe. The most patent-related jurisdictions correspond to the United States, Europe, and China. Firstly, the United States, through the USPTO, encapsulates 398 patent documents (i.e., 53.78% of total patent documents). Secondly, the Europe through the EPO encapsulates 125 patent documents (i.e., 16.89% of total patent documents). Thirdly, China, through the CNIPA (i.e., China National Intellectual Property Administration), encapsulates 117 patent documents (i.e., 15.81% of total patent documents).
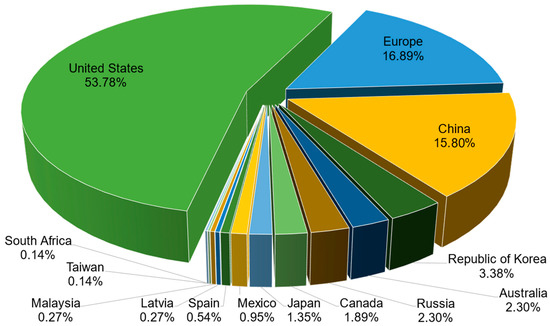
Figure 4.
Jurisdictions of active patent documents related to the development of CaPCs for bone repair.
Relevant remarks coming from this jurisdiction’s part should be highlighted hereinafter. They concern the absence of patent documents recognized and enforceable under the PCT international system. Furthermore, France is not presented above, although three leading organizations from this national patent jurisdiction (i.e., “French National Center for Scientific Research”, “Graftys SA”, and “University of Nantes”) are presented among applicants and owners. The majority of their patent documents are, in fact, recognized and enforceable in the United States and Europe (in member states of the European Patent Convention). The same remark also concerns the national patent jurisdictions of Germany and Switzerland. All patent documents of the companies “Innotere GmbH” and “Kyphon SARL” are, in fact, recognized and enforceable in the United States and Europe.
4. Conclusions
This study focused on patent documents for the development of CaPCs for bone repair that have an active legal status until 2022. The maximum number of patent documents recorded in 2016 was 69. On the other hand, 53.78% of the 740 active patent documents found were from the United States. This justifies the fact that the United States shows great interest in developing bone substitutes. An overview of relevant patent documents related to the development of CaPCs for bone repair reveals that these common bioceramic material cements are used for a variety of purposes, including filling and sealing defects in bone, repairing damaged teeth, reinforcing bone tissue, and producing artificial joints, as well as other medical devices. They are generally mixed with solutions to form a paste that can be molded and shaped into the desired form. They are then hardened by a chemical reaction with the solution, which results in the formation of a hard and stable material. According to findings in major patent documents, one of the main advantages of CaPCs is that they are injectable, self-hardening, biocompatible, and osteoconductive.
Author Contributions
Conceptualization, A.L. and A.F.; methodology, K.K., A.L. and A.F.; validation, A.F.; formal analysis, A.F.; investigation, K.K., A.L. and A.F.; data curation, A.F.; writing—original draft preparation, K.K.; writing—review and editing, A.L. and A.F.; visualization, A.F.; supervision, A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within this manuscript.
Acknowledgments
The authors acknowledge the EPO, the UPSTO, and Google Patents for the databases and the search service used in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xu, H.H.K.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2018, 3, 278–314. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, C.; Wang, P.; Wang, L.; Bao, C.; Weir, M.D.; Reynolds, M.A.; Ren, K.; Zhao, L.; Xu, H.H.K. Engineering bone regeneration with novel cell-laden hydrogel microfiber-injectable calcium phosphate scaffold. Mater. Sci. Eng. C 2017, 75, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Verron, E.; Khairoun, I.; Guicheux, J.; Bouler, J.-M. Calcium phosphate biomaterials as bone drug delivery systems: A review. Drug Discov. Today 2010, 15, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium Orthophosphate Cements and Concretes. Materials 2009, 2, 221–291. [Google Scholar] [CrossRef]
- Julien, M.; Khairoun, I.; LeGeros, R.Z.; Delplace, S.; Pilet, P.; Weiss, P.; Daculsi, G.; Bouler, J.M.; Guicheux, J. Physico-chemical–mechanical and in vitro biological properties of calcium phosphate cements with doped amorphous calcium phosphates. Biomaterials 2007, 28, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Google. Google Patents. Available online: https://patents.google.com (accessed on 5 January 2023).
- Baxendale, L.; Kirk, E.E.J. Dental Fillers and Bone Cements Comprising Collagen. Granted Patent GB1068587A, 10 May 1967. [Google Scholar]
- Hachimi Alaoui, C.; Fatimi, A. A 20-year patent review and innovation trends on hydrogel-based coatings used for medical device biofabrication. J. Biomater. Sci. Polym. Ed. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Fatimi, A. A patent data analysis of the innovation trends in biological control agent formulations. Recent Adv. Food Nutr. Agric. 2022, 13, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Fatimi, A. Exploring the patent landscape and innovation of hydrogel-based bioinks used for 3D bioprinting. Recent Adv. Drug Deliv. Formul. 2022, 16, 145–163. [Google Scholar] [CrossRef] [PubMed]
- Song, C.H. Examining the Patent Landscape of E-Fuel Technology. Energies 2023, 16, 2139. [Google Scholar] [CrossRef]
- Barbosa, N.B.; Nunes, D.D.G.; Santos, A.Á.B.; Machado, B.A.S. Technological Advances on Fault Diagnosis in Wind Turbines: A Patent Analysis. Appl. Sci. 2023, 13, 1721. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Zhou, B.; Hu, X.; Xie, Y. A Patent Bibliometric Analysis of Carbon Capture, Utilization, and Storage (CCUS) Technology. Sustainability 2023, 15, 3484. [Google Scholar] [CrossRef]
- Frisio, D.G.; Ventura, V. Global Innovation Trends for Plant-Based Vaccines Production: A Patent Analysis. Plants 2021, 10, 2558. [Google Scholar] [CrossRef] [PubMed]
- Francis, N.; Ong, A.; Suhaimi, H.; Abas, P.E. Patent Landscape Review on Ankle Sprain Prevention Method: Technology Updates. Inventions 2023, 8, 53. [Google Scholar] [CrossRef]
- European Patent Office. Espacenet Patent Search. Available online: https://worldwide.espacenet.com (accessed on 25 January 2023).
- United States Patent and Trademark Office. USPTO Database (PatFT-AppFT). Available online: https://uspto.gov/patents/search (accessed on 5 January 2023).
- European Patent Office. Espacenet Glossary. Available online: https://worldwide.espacenet.com/patent/help/espacenet-glossary (accessed on 25 January 2023).
- World Intellectual Property Organization. Summary of the Patent Cooperation Treaty (PCT). Available online: www.wipo.int/treaties/en/registration/pct/summary_pct.html (accessed on 25 January 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).