1. Introduction
Acid mine drainage constitutes a major consequence of opencast mining, causing devastating effects on the environment; overburden dumps and voids result in degraded aesthetics, while erosion and pollution impose severe environmental concerns for the surrounding areas. Mine soils water is highly acidic carrying a pollution overload, i.e., toxic metal ions associated with mining activities. In particular, surface cracks are formed altering the appearance of the area and triggering moisture and nutrients drainage, thus, provoking soil degradation. Acid water, according to lithology [
1], enters the aquifer and negatively impacts the groundwater resources [
2,
3]. This may last for decades or even hundreds of years after mine closure [
4,
5].
Over the years, the mining and extractive metallurgy sector centered in the Lavrion area has constituted one of the most important and dynamic segments of the Greek industry; these mines, abandoned since 1977, have produced mostly silver, lead, and zinc, as well as brightly colored specimens of supergene minerals such as smithsonite, azurite, malachite, cerussite, adamite, goethite, jarosite, serpierite, gypsum and more.
Reclamation of these abandoned mine areas is an integral part of an environmental management plan aimed at the remediation of both acidity and heavy metal content. Among the mine soil remediation methods, adsorption appears as a reliable solution [
6,
7,
8,
9,
10].
Fly ash, the finest of coal ash particles, consisting of the noncombustible matter in coal, has been recognized over the years as a soil ameliorator offering nutrient supplementation and pH correction thanks to its unique physicochemical properties and useful micro- and macronutrients [
11,
12].
Clinoptilolite and mordenite are two species of natural zeolites collected from Samos Island, Greece. Clinoptilolite, with a Si/Al ratio and thermal stability different from heulandites [
13], is a common zeolite easily extracted from open pits, widely used as an adsorbent [
14,
15,
16]. Mordenite, on the other hand, is a high-silica zeolite, constitutes an alteration product of volcanoclastic material, and is sometimes found within veins, fissures or amygdales of some igneous rocks [
16,
17].
In this study, two natural zeolites (clinoptilolite and mordenite) and a synthetic zeolite derived from fly ash were employed for Lavrion abandoned mine soil rehabilitation in order to restore the dynamic balance between key ecological factors, such as soil acidity and metal content to support vegetation in this area. Greece is a dynamic producer of both zeolites and coal (lignite) and this fact represents an economic asset to this plan.
2. Experimental Part
2.1. Materials
Mine soil collected from the Lavrion mining area in Greece was homogenized, dried at 383 K for 96 h, pulverized and sieved (<250 μm).
The natural zeolite tuffs, i.e., clinoptilolite (CSA) and mordenite (MSA), originated from Karlovassi Basin, Samos Island, Greece. Both zeolite tuff materials were dried at 383 K, pulverized and sieved (<250 μm).
Coal fly ash (MLF) samples were received from the electrostatic precipitators of the Meliti Lignite-fired Power Station (Florina, Greece).
Na-P1 synthetic zeolite (ZML) was prepared from Meliti fly ash using a low-temperature alkaline hydrothermal treatment [
7,
8].
2.2. Mine Soil Amendment
Contaminated soil (2 kg) from the Lavrion abandoned mines (L) was mixed with each zeolitic amendment (ZML, CSA and MSA) at a rate of 10% w/w (200 g) to produce L-ZML, L-CSA and L-MSA, respectively. The mixtures were first moistened to 40% of their water holding capacity, and then left to equilibrate for 1 week. Control samples without any amendment were also set up. All experiments were replicated 3 times.
This experiment was carried out under natural environmental conditions and lasted ten weeks. Moisture was maintained by irrigation twice a week. At the end of the experiment, a compliance test for the leaching of heavy metals was conducted.
The percolation method was applied for the measurement of the water holding capacity of soil substrates. For this purpose, 25 g of a substrate was evenly distributed onto a filtering paper, placed in a glass funnel and set on a volumetric cylinder. Then, water was added (50 mL), and the mixture was left until the dripping in the cylinder stopped. The water still held by the soil was the difference between the original volume of water added and that in the cylinder.
2.3. Physicochemical Analyses
X-ray diffraction patterns of zeolites were acquired on fine powdered specimens using a Siemens D 5005 diffractometer (Ni-filtered Cu Ka, graphite monochromatographe), at 40 kV and 40 mA (0.02° (2θ) s−1 between 3° and 65°). The mineralogical content was identified using DIFFRACplus EVA 10.0 software.
Scanning electron micrographs were obtained with a Jeol JSM-5600 apparatus equipped with an OXFORD ISIS 300 EDS microanalyzer. Prior to examination, the samples were coated with a 10 nm thick graphite film using a B7341 Agar Automatic Sputter.
Acidity measurements were carried out using a PHS-3D (Beijing Jia Hua Zhong Xin Technology Co., Ltd., Beijing, China) pH-meter, as described in the SW-846 Test Method 9045D for soil and waste pH (EPA, United States) [
18]; 20 g of soil and 20 mL of distilled water were mixed, covered, stirred for 5 min, and then left to stand for 1 h to allow most of the dispersed clay to settle out from the suspension.
To determine the cation exchange capacity (CEC) of soils and zeolites, a CH
3COONH
4 solution was added to the original material; the mixture was left for 24 h and then centrifuged. The same process was repeated five times [
19,
20]. The samples were then mixed with ethanol and centrifuged (five times). The solids were air dried, and finally, NaCl solution was added and the mixtures were centrifuged. Na
+ and NH
4+ concentrations in the supernatant were determined using flame (JENWAY PFP 7) and UV-Vis (HACH DR4000) spectrophotometers, respectively.
The determination of metal concentrations in soils was performed as follows [
21]: 1 mL distilled water, 3 mL concentrated HNO
3, 1 mL concentrated HCl, 5 mL HF, and 4 mL HClO
4 were added to 0.2 g soil and placed in a Teflon vessel. The mixture was left for 30 min and then heated to dryness. The solid obtained was dissolved in HCl 0.1 M solution in a 100 mL flask.
Metal leaching was performed according to the European Standard 12457-2 [
22]; the solid matter and distilled water were mixed at a solid to liquid ratio of 1/10 (
w/
v). The mixture was agitated for 24 h and then left to settle for 15 min. Finally, the eluant was separated via filtration to acquire the “leachates”, and the metal concentrations therein were determined with the use of a Perkin Elmer 1100b atomic absorption spectrophotometer (AAS).
3. Results and Discussion
3.1. Characterization of the Polluted Soil and Aluminosilicates
The major elements’ concentrations in all materials used (FML, ZML, CSA and MSA) and Lavrion soil are presented in
Table 1. Regarding the mineralogical composition, Meliti fly ash mostly contains quartz and to a lesser extent, anorthite, albite, hematite and lime. The synthetic zeolite prepared from FML additionally contains large amounts of Na-P1 zeolite [
7,
8]. CSA largely contains clinoptilolite and small amounts of mordenite and sanidine, while MSA, besides mordenite, contains clinoptilolite, sanidine and quartz. In Lavrion soil quartz, calcite and clay minerals were mostly detected; other minerals that remained from the mineral activities, e.g., gypsum, were identified in small quantities.
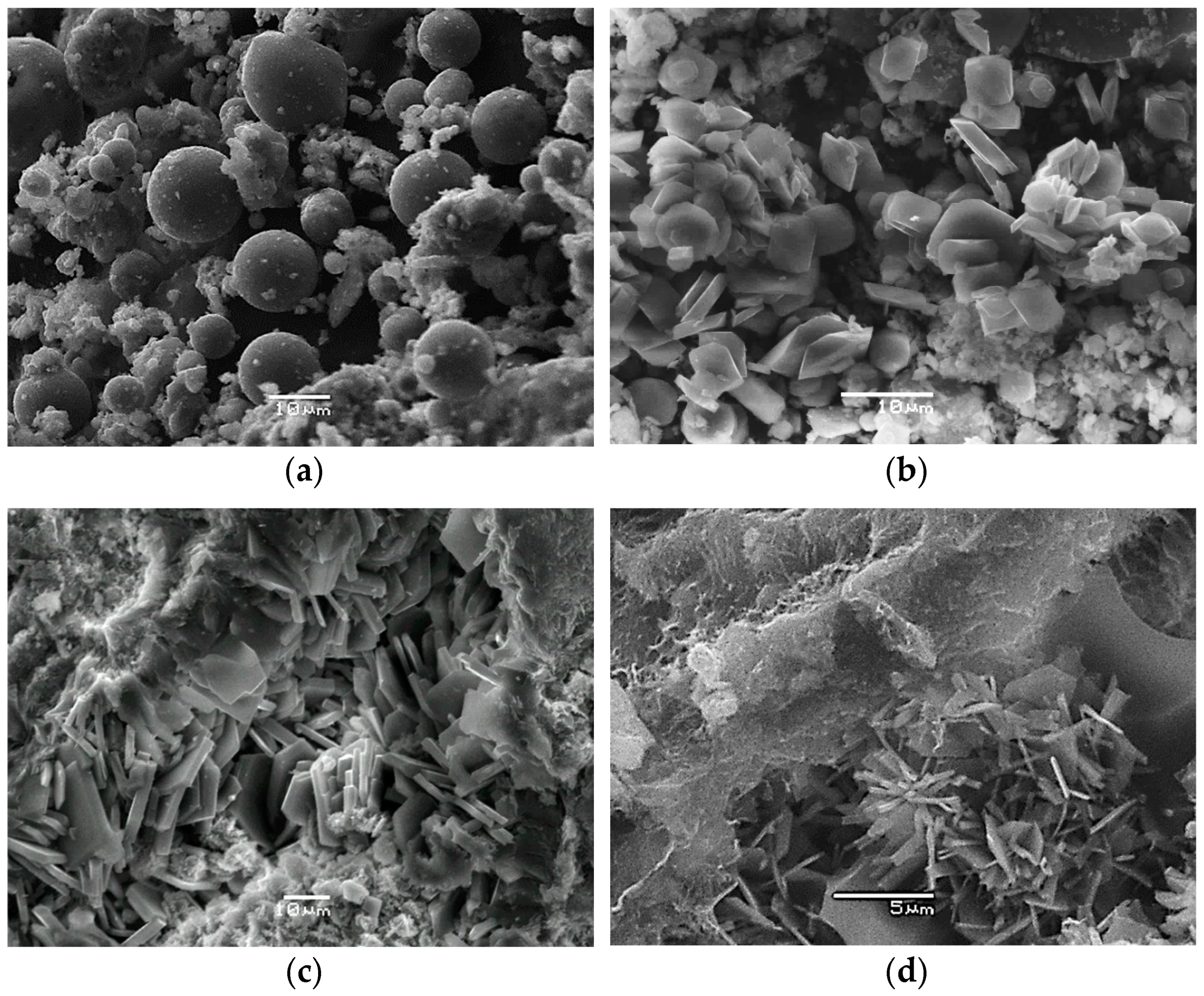
Figure 1 presents typical SEM micrographs of all materials studied.
Figure 1a,b, in particular, displays the changes in microscale observed during the transformation of the glassy fly ash to crystalline Na-P1 synthetic zeolite [
7,
8]. Clinoptilolite crystals have tabular texture (
Figure 1c) while the mordenite structure is mostly fibrous with needle-like crystals.
3.2. Soil Amelioration
The introduction of the zeolitic amendments to Lavrion soil positively affected its basic physicochemical characteristics, i.e., acidity and cation exchange capacity (CEC). The pH of the untreated, highly acidic Lavrion soil was found to be 3.7. Soil pH constitutes one of the key chemical indicators of good health, being extremely important for plant growth and ion availability in solution. It affects essential nutrients’ bioavailability, plants’ water uptake, crop performance, soil microorganisms’ activity and metal ion circulation. In low soil pHs (<4) heavy metals become soluble and are readily absorbed by plants; thus, plants are more susceptible to heavy-metal toxicity [
23,
24]. All zeolite materials selected for Lavrion soil rehabilitation are alkaline, and thus suitable for soil pH correction; ZML, CSA and MSA possess pHs 12.1, 8.2 and 7.6, respectively. In fact, after treatment, soil acidification was alleviated, and L soil pH raised to 5.7, 5,2 and 5.0, respectively. It has been reported that soil pH rise positively correlates with an increase in crop yield [
25,
26].
Cation exchange capacity (CEC) a parameter also associated with soil pH, was amended with zeolites; after ZML, CSA and MSA addition to Lavrion soil, the L cation exchange capacity increased from 21.2 to 34.1, 32.7 and 41.0, respectively, representing a 50 to 100% increase. The CEC values of the zeolite amendments were found 148, 79.9 and 81.1 meq/100 g, respectively.
The origin of soil’s CEC has been attributed to both the presence of clay and organic materials. Soil’s negative charges arise from isomorphous substitutions in clays and acid dissociation in organic matter, and they are capable of withholding positively charged compounds; thus, regulation of nutrients and metal ions bioavailability is achieved. The organic acids’ functionality implies the underlining correlation between soil pH and CEC. Soils with low CEC are expected to demonstrate deficiencies, infertility and reduced water-holding capacity; in general, low CEC in soils has been negatively correlated with crop yield [
25,
26,
27].
3.3. Metal Ions Leaching
Besides amelioration of Lavrion soil parameters, the addition of the environment-friendly zeolitic amendments also contributed to heavy metal leaching abatement (
Table 2). Six, possibly toxic, metals were investigated, i.e., Pb, Cd, Zn, Cu, Mn and Fe. Heavy metals in mine soils do not degrade; rather, they accumulate in both aquatic and terrestrial ecosystems and enter the food chain, triggering plant growth disorders and toxicity to humans [
28,
29,
30].
The results demonstrate that Lavrion soil amended with the synthetic Na-P1 zeolite produced from Meliti fly ash (followed by Samos Mordenite) exhibited the best performance in preventing metal leaching, i.e., 38%, 72%, 61%, 67%, 77% and 33% for Pb, Cd, Zn, Cu, Mn and Fe, respectively. The better performance of the synthetic zeolite can be attributed to both its higher alkalinity and its increased cation exchange capacity compared with natural zeolites.
4. Conclusions
Lavrion mines (abandoned since 1977) have been long used for silver, lead, and zinc extraction. Acid mine drainage stems from both active and abandoned mines; it lowers pH in both surface and ground waters, which, in turn, mobilizes toxic chemicals, mostly heavy metals, percolating into the environment. In addition, acid mine drainage degrades soil quality and negatively impacts both terrestrial and aquatic ecosystems.
Two natural zeolites originating from Samos tuffs containing mostly clinoptilolite and mordenite, respectively, and a synthetic one (Na-P1) prepared from Meliti fly ash were selected as low-cost amendments to restore the in situ negative impacts of Lavrion mine soil.
Soil amelioration was observed via pH, and CEC increase was achieved by all three different zeolitic materials. Regarding heavy metal leaching restoration, the synthetic zeolite proved more efficient, decreasing the metal concentrations in the leachates by 38%, 72%, 61%, 67%, 77% and 33% for Pb, Cd, Zn, Cu, Mn and Fe, respectively, followed by Samos mordenite.
Thus, natural and synthetic zeolitic materials as soil amendments have proved to be promising, low-cost, eco-friendly materials for an in situ treatment of contaminated soil in abandoned mine areas, reducing the adverse effects of pH decline and reversing the picture of desertification and aridity that exists in abandoned mines. The quantity of toxic metals retained is analogous to the adsorbent mass; the scaling up of this process includes calculation of the appropriate zeolite quantities. Further research on additives that could facilitate the process could be of significant importance.
Author Contributions
Conceptualization, C.V. and M.R.; methodology, C.V. and M.R.; formal analysis, C.V. and M.R.; investigation, C.V. and M.R.; resources, C.V. and M.R.; data curation, C.V. and M.R.; validation, C.V. and M.R.; writing—original draft preparation, M.R.; writing—review and editing, C.V. and M.R.; visualization, C.V. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not relevant.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting reported results have been included in the manuscript.
Acknowledgments
National and Kapodistrian University of Athens (Special Account for Research Grands) is kindly thanked for funding the presentation of this work. Vasilis Skounakis, scanning electron microscopy technician, is kindly thanked for his assistance with the SEM-EDS analyses and SEM micrographs.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vasilatos, C.; Koukouzas, N.; Alexopoulos, D. Geochemical Control of Acid Mine Drainage in Abandoned Mines: The Case of Ermioni Mine, Greece. Procedia Earth Planet. Sci. 2015, 15, 945–950. [Google Scholar] [CrossRef]
- Zang, K.; Yang, K.; Wu, X.; Bai, L.; Zhao, J.; Zheng, X. Effects of Underground Coal Mining on Soil Spatial Water Content Distribution and Plant Growth Type in Northwest China. ACS Omega 2022, 7, 18688–18698. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhang, H.; Xiao, L. Formation mechanism of carbide slag composite sustained-alkalinity-release particles for the source control of acid mine drainage. Sci. Rep. 2021, 11, 23793. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumar, D. Water in mining and processing. In Phosphate Rock An Industry in Transition; Elsevier Inc.: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Aguilar-Garrido, A.; Paniagua-López, M.; Sierra-Aragón, M.; Martínez Garzón, F.J.; Martín-Peinado, F.J. Remediation potential of mining, agro-industrial, and urban wastes against acid mine drainage. Sci. Rep. 2023, 13, 12120. [Google Scholar] [CrossRef] [PubMed]
- Favas, P.J.C.; Martino, L.E.; Prasad, M.N.V. Abandoned mine land reclamation—Challenges and opportunities (holistic approach). In Bio-Geotechnologies for Mine Site Rehabilitation; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Roulia, M.; Koukouza, K.; Stamatakis, M.; Vasilatos, C. Fly-ash derived Na-P1, natural zeolite tuffs and diatomite in motor oil retention. Clean. Mater. 2022, 4, 100063. [Google Scholar] [CrossRef]
- Roulia, M.; Alexopoulos, D.; Itskos, G.; Vasilatos, C. Lignite fly ash utilization for acid mine drainage neutralization and clean-up. Clean. Mater. 2022, 6, 100142. [Google Scholar] [CrossRef]
- Giannatou, S.; Vasilatos, C.; Mitsis, I.; Koukouzas, N.; Itskos, G.; Stamatakis, G. Reduction of Toxic Element Mobility in Mining Soil by Zeolitic Amendments. Bull. Geol. Soc. Greece 2016, 50, 2127–2136. [Google Scholar] [CrossRef]
- Giannatou, S.; Vasilatos, C.; Mitsis, I.; Koukouzas, N. Utilization of natural and synthetic zeolitic materials as soil amendments in abandoned mine sites. Bull. Geol. Soc. Greece 2018, 53, 78–98. [Google Scholar] [CrossRef]
- Chandey, V.P. Fly ash application in reclamation of degraded land: Opportunities and challenges. In Phytomanagement of Fly Ash; Elsevier Inc.: Amsterdam, The Netherlands, 2020; Chapter 6. [Google Scholar]
- Kar, K.K. Handbook of Fly Ash; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Mansouri, N.; Rikhtegar, N.; Panahi, H.A.; Atabi, F.; Shahraki, K.B. Porosity, characterization and structural properties of natural zeolite-clinoptilolite-as a sorbent. Environ. Prot. Eng. 2013, 39, 139–152. [Google Scholar]
- Cundy, C.S.; Cox, P.A. The Hydrothermal Synthesis of Zeolites: History and Development from the Earliest Days to the Present Time. Chem. Rev. 2003, 103, 663–702. [Google Scholar] [CrossRef] [PubMed]
- Dyer, A. Encyclopedia of Materials: Science and Technology; Jürgen Buschow, K.H., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Stamatakis, M.G.; Stamataki, I.S.; Giannatou, S.; Vasilatos, C.; Drakou, F.; Mitsis, I.; Xinou, K. Characterization and evaluation of chabazite- and mordenite-rich tuffs, and their mixtures as soil amendments and slow release fertilizers. Arch. Agron. Soil Sci. 2017, 63, 735–747. [Google Scholar] [CrossRef]
- Kitsopoulos, K.P.; Dunham, A.C. Compositional variations of mordenite from Polyegos Island, Greece: Na-Ca and K-rich mordenite. Eur. J. Mineral. 1998, 10, 569–577. [Google Scholar] [CrossRef]
- SW-846 Test Method 9045D: Soil and Waste pH. Available online: https://www.epa.gov/sites/default/files/2015-12/documents/9045d.pdf (accessed on 17 August 2023).
- Chapman, H.D. Cation-exchange capacity. In Method of Soil Analysis, Part 2: Chemical and Microbiological Properties; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 891–900. [Google Scholar] [CrossRef]
- Rhoades, J.D. Cation exchange capacity. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties, 2nd ed.; Page, A.L., Ed.; 1982; Volume 9, pp. 149–157. [Google Scholar]
- Argyraki, A.; Kelepertzis, E. Urban soil geochemistry in Athens, Greece: The importance of local geology in controlling the distribution of potentially harmful trace elements. Sci. Total Environ. 2014, 482–483, 366–377. [Google Scholar] [CrossRef] [PubMed]
- EN 12457-2:2002; Characterisation of Waste-Leaching-Compliance Test for Leaching of Granular Waste Materials and Sludges-Part 2: One Stage Batch Test at a Liquid to Solid Ratio of 10 L/kg for Materials with Particle Size below 4 mm (without or with Size Reduction). CEN: Brussels, Belgium, 2023.
- Howe, J.A.; Peyton Smith, A. Principles and Applications of Soil Microbiology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Mobilian, C.; Craft, C.B. Encyclopedia of Inland Waters, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Zhang, S.; Zhu, Q.; de Vries, W.; Ros, G.H.; Chen, X.; Muneer, M.A.; Zhang, F.; Wu, L.J. Effects of soil amendments on soil acidity and crop yields in acidic soils: A world-wide meta-analysis. J. Environ. Manag. 2023, 345, 118531. [Google Scholar]
- Vasilatos, C.; Kypritidou, Z.; Anastasatou, M.; Aspiotis, K. Sustainable Restoration of Depleted Quarries by the Utilization of Biomass Energy By-Products: The Case of Olive Kernel Residuals. Sustainability 2023, 15, 1642. [Google Scholar] [CrossRef]
- Jeon, I.; Chung, H.; Kim, S.H.; Nam, K. Use of clay and organic matter contents to predict soil pH vulnerability in response to acid or alkali spills. Helyion 2023, 9, 17044. [Google Scholar] [CrossRef] [PubMed]
- Durães, N.; Novo, L.A.B.; Candeias, C.; Silva, E.F. Distribution, Transport and Fate of Pollutants. In Soil Pollution: From Monitoring to Remediation; Duarte, A.C., Cachada, A., Rocha-Santos, T., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Pan, Z.; Xie, R.; Chen, Z. One-step simultaneous biomass synthesis of iron nanoparticles using tea extracts for the removal of metal(loid)s in acid mine drainage. Chemosphere 2023, 337, 139366. [Google Scholar] [CrossRef] [PubMed]
- Hajihashemi, S.; Rajabpoor, S.; Schat, H. Acid mine drainage (AMD) endangers pomegranate trees nearby a copper mine. Sci. Total Environ. 2023, 889, 164269. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).