Abstract
In this work, electrodialysis (ED) and electro-electrodialysis (EED) were investigated as technologies for the recovery of Sb from wastes and effluents generated during the pyrometallurgical processing of copper sulfide minerals and the hydrometallurgical treatment of low-copper-content mixed minerals. This work addresses the challenge of applying electrochemical methods for recovering these valuable materials and recycling highly concentrated acid solutions used in the latter separation stages of the electrorefining process. The electrochemical characterization of the solutions was conducted, and the electrodeposition of Sb and Bi was performed in electrochemical cells. Also, the implementation of membrane processes in the recovery of such materials was investigated.
1. Introduction
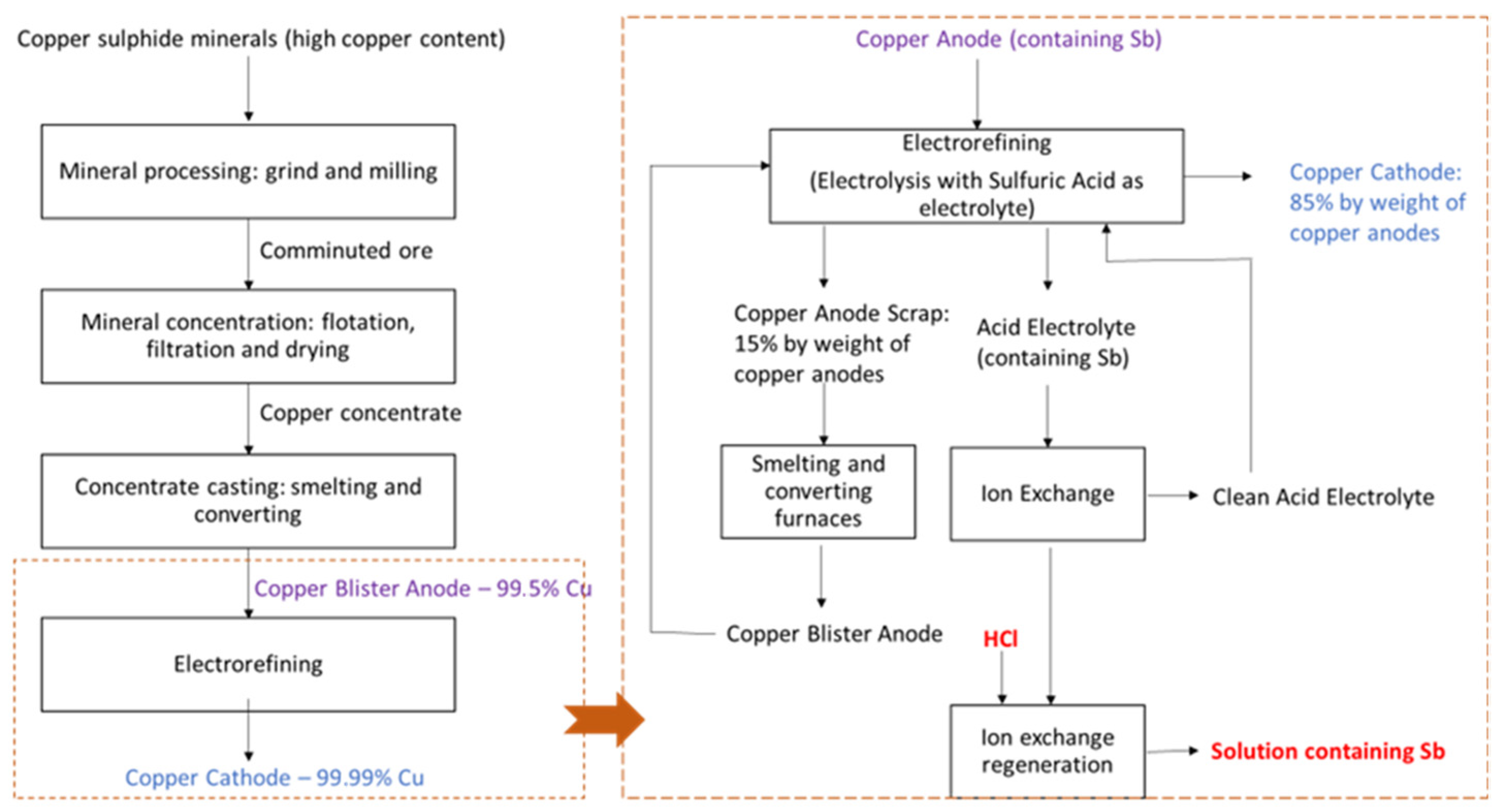
The copper production industry generates several waste effluents that contain valuable elements, among which, antimony, bismuth, iron, lead and arsenic. Impurities, such as antimony and bismuth, decrease the purity of the obtained copper cathodes. Figure 1 shows a general scheme of the copper production process, where at the right side, the steps involved after the electrorefining step are indicated. Copper electrorefining industries have incorporated separation processes that make possible the selective separation of mainly antimony, but also bismuth, from an acidic electrolyte, by, for example, the use of ion-exchange resins (IX). These resins are selective for antimony and bismuth, thus allowing the reuse of a clean acidic electrolyte rich in copper for the production of copper cathodes [1,2].
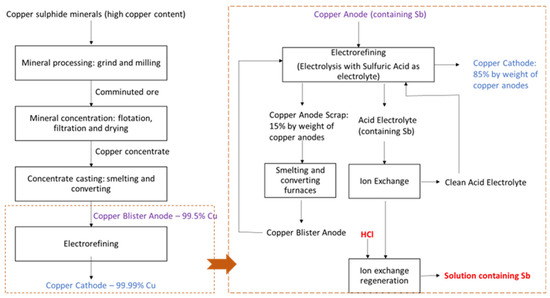
Figure 1.
Copper production by the pyrometallurgical process including the electrorefining process and the use of ion-exchange processes for the regeneration of the baths. The scheme also includes the regeneration of the resins, which is the process by which the effluent considered in the present work is produced.
However, the IX resins need to be regenerated after some time of usage, as their capacity to retain antimony and bismuth reaches a saturation limit. As indicated in Figure 1, highly concentrated HCl solutions are used to regenerate the resins, so that they can be used again in the purification of acidic electrolytes from copper-electrorefining. The result of the cleaning of the ion-exchange beads is an effluent with a high concentrated of HCl, containing mainly Sb and also Bi. One of the main parts of the electro-electrodialysis process is the electrochemical reaction taking place at the cathode. In this work, the recovery of antimony from highly concentrated solutions was investigated. In a lab-scale experiment, the separation of antimony from bismuth (the second most concentrated metal in the solutions) was studied by cyclic voltammetry and electrodeposition experiments.
2. Materials and Methods
Cyclic voltammograms were registered using a 3-electrode configuration in a stirred cell of 50 mL, with a Pt disc as a working electrode, a Pt foil as a counter electrode and a Ag/AgCl reference electrode. The solutions investigated were composed of 6 M HCl, 10 mM Sb(III) and 2.5 mM Bi(III), prepared from Sb2O2 (99%, SigmaAldrich (Saint Louis, MO, USA)) and Bi2O3 (99.9%, SigmaAldrich). These concentrations were established according to typical values measured in the real effluent [2]. The cyclic voltammograms were registered using an Autolab PGSTAT30 galvanostat/potentiostat (Metrohm (Herisau, Switzerland)). Subsequently, the electrodeposition of antimony and bismuth was tested with a divided stirred cell of 250 mL at a stirring rate of 500 rpm. The anode and cathode compartments were separated by a Nafion ion-exchange membrane. The experiments were conducted for 3 h. The samples were extracted from the reactor and measured by atomic absorption spectroscopy. The voltage cell was measured and used to calculate the specific energy consumption for the recovery of both metals.
3. Results and Discussion
3.1. Cyclic Voltammograms of HCl Solutions Containing Antimony and Bismuth
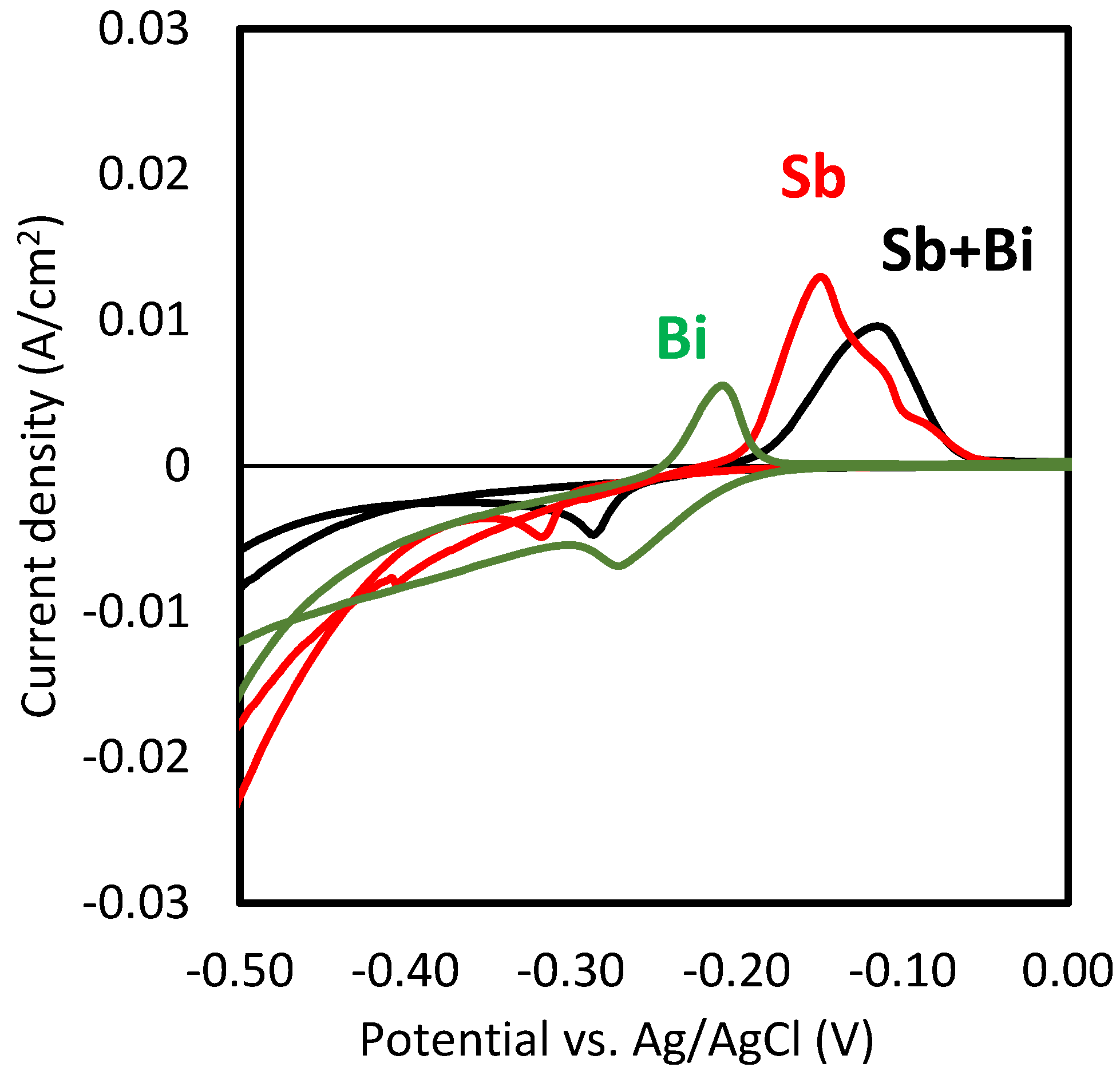
Figure 2 shows the cyclic voltammograms obtained with HCl solutions and each of the metals and their mixtures. The reduction peak for the bismuth electrodeposition was observed at −0.27 V, that of Sb at −0.32 V, and that of the mixture of antimony and bismuth at the intermediate potential value of −0.30 V. Here, it is to note that only one reduction peak was obtained with the mixtures of antimony and bismuth, which may indicate that the separate electrodeposition of both metals was difficult to achieve. However, since the relative concentration of antimony was four times higher than that of bismuth [2], the galvanostatic recovery of both metals by electrodeposition was evaluated. At potentials more negative than −0.4 V, a sharp increase in the cathodic current (negative values) took place, which could be associated with the evolution of hydrogen bubbles at the surface of the cathode [3]. Anodic peaks were observed in all three cases during the anodic scan of the cyclic voltammograms (from −0.5 to 0 V). These peaks revealed that the redissolution of the previously deposited metals took place.
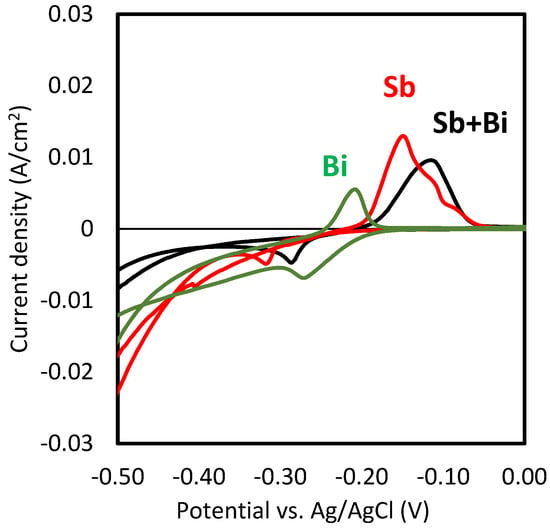
Figure 2.
Cyclic voltammograms obtained with 6 M HCl solutions and different concentrations of Sb and Bi (10 mM Sb, 2.5 mM Bi and mixtures of 10 mM Sb and 2.5 mM Bi).
3.2. Electrorecovery of Antimony and Bismuth
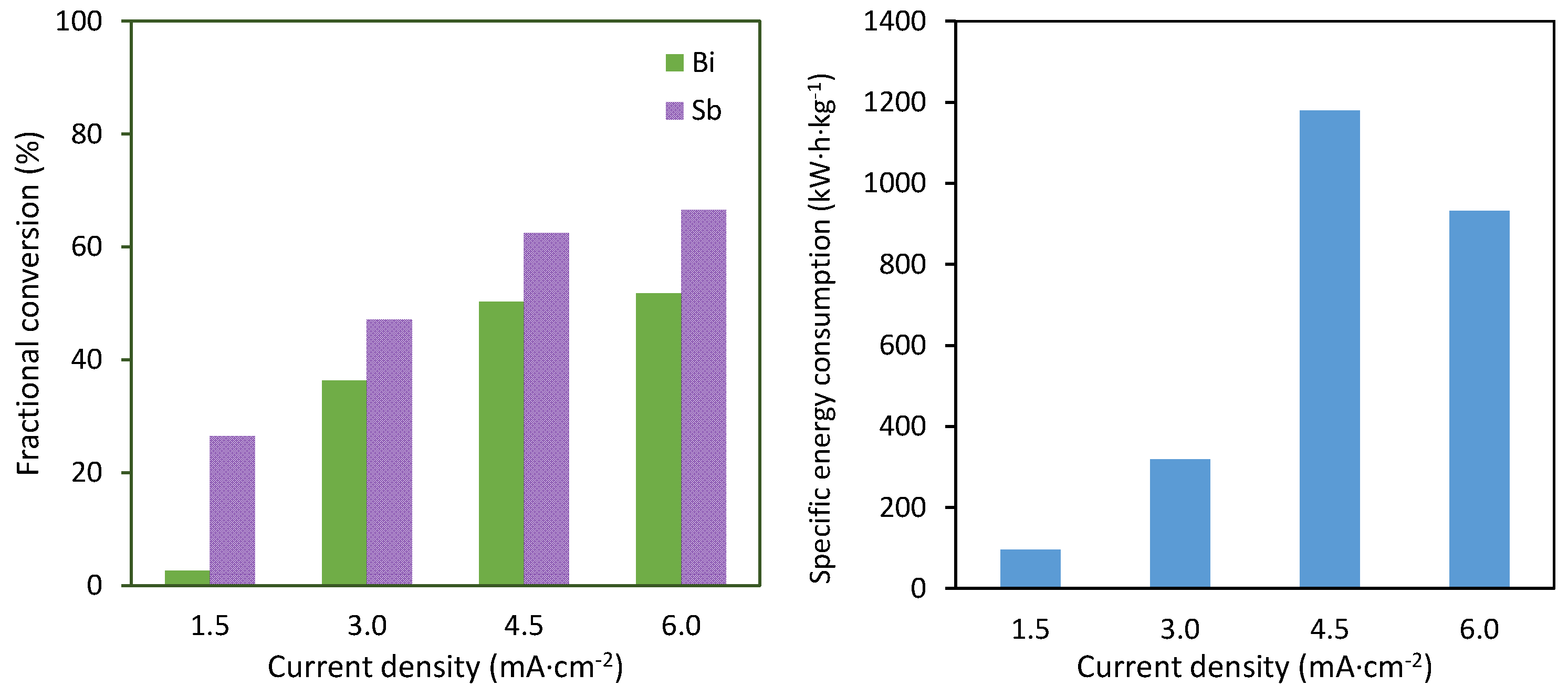
The average results of the electrodeposition of antimony and bismuth are shown in Figure 3. The left panel shows the final values of the fractional conversion obtained for both metals. It can be seen that the deposition of antimony took place preferentially, which can be directly related to the higher proportion of antimony in the mixtures. The fractional conversion of both metals increased with the applied current density, reaching a saturation value at 6 mA·cm−2. The fact that increasing the current density from 4.5 to 6 mA·cm−2 did not imply a proportional increase in the fractional conversion evidenced that the recovery of both metals was limited by the mass transfer from the bulk solution towards the electrode surface. Moreover, these results confirmed that the deposition took place in the form of an alloy. The separation of both metals was only partially possible at the lowest applied current density of 3 mA·cm−2, at which 25% of antimony was deposited, but only 5% of bismuth could be recovered.
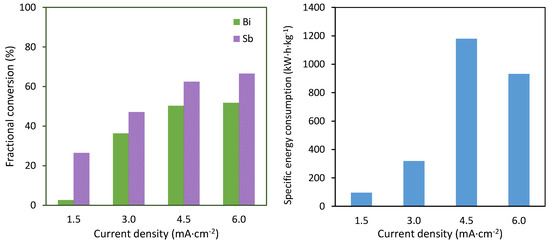
Figure 3.
Average results as a function of the current density. Left: fractional conversion of antimony and bismuth. Right: specific energy consumption per kg of metal recovered.
Regarding the energy needed to recover both metals, the results shown in the right panel of Figure 3 confirmed that current densities higher than 3 mA·cm−2 implied a significant increase in energy consumption. This could be related to the intense generation of hydrogen at very high current densities, as a result of which an important portion of the applied current was not effectively used for the recovery of both metals, but was used for the hydrogen evolution reaction. This phenomenon, together with the increased cell voltages at increasing current densities, led as a result to a notable increase in specific energy consumption at current densities of 4.5 and 6 mA·cm−2.
4. Conclusions
The recovery of antimony and bismuth from highly concentrated HCl solutions by means of electrodeposition is feasible. This process can significantly contribute to the circularity of the copper electrorefining process by enabling the recycling of HCl solutions for the regeneration of ion-exchange resins and also to the recovery of two critical raw materials like antimony and bismuth. The results obtained indicated that current densities of around 1.5 mA·cm−2 will favor the selective deposition of antimony over bismuth and a low specific energy consumption.
Author Contributions
Conceptualization, V.P.-H., M.A.S.R., G.C., G.R. and A.M.B.; methodology, M.C.M.-C.; validation, V.P.-H.; investigation, L.H.-P.; resources, V.P.-H.; data curation, L.H.-P.; writing—original draft preparation, M.C.M.-C., L.H.-P. and A.M.B.; writing—review and editing, V.P.-H.; project administration, A.M.B.; funding acquisition, V.P.-H., M.A.S.R., G.C., G.R. and A.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge the support from the Agencia Estatal de Investigación (AEI/10.13039/501100011033) (Spain) under the project PCI2019-103535 and by FEDER “A way of making Europe”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Acknowledgments
The authors would like to thank the financial support of ERAMIN2 (FINEP-Brazil, ANID-Chile and AEI-Spain) and Cyted (Network 318RT0551).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Arroyo-Torralvo, F.; Rodríguez-Almansa, A.; Ruiz, I.; González, I.; Ríos, G.; Fernández-Pereira, C.; Vilches-Arenas, L.F. Optimizing operating conditions in an ion-exchange column treatment applied to the removal of Sb and Bi impurities from an electrolyte of a copper electro-refining plant. Hydrometallurgy 2017, 171, 285–297. [Google Scholar] [CrossRef]
- Barros, K.S.; Vielmo, V.S.; Moreno, B.G.; Riveros, G.; Cifuentes, G.; Bernardes, A.M. Chemical composition data of the main stages of copper production from sulfide minerals in Chile: A review to assist circular economy studies. Minerals 2022, 12, 250. [Google Scholar] [CrossRef]
- Hernández-Pérez, L.; Carrillo-Abad, J.; Pérez-Herranz, V.; Montañés, M.T.; Martí-Calatayud, M.C. Effluents from the copper electrorefining as a secondary source of antimony: Role of mass transfer on the recovery by electrodeposition. Desalination 2023, 549, 116322. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).