Application of Portable Spectroscopic Tools in the Exploration of Manganese Oxide Minerals: Preliminary Results from the Case Study of Drama Mn-Oxide Deposits, Northern Greece †
Abstract
:1. Introduction
2. Materials and Methods
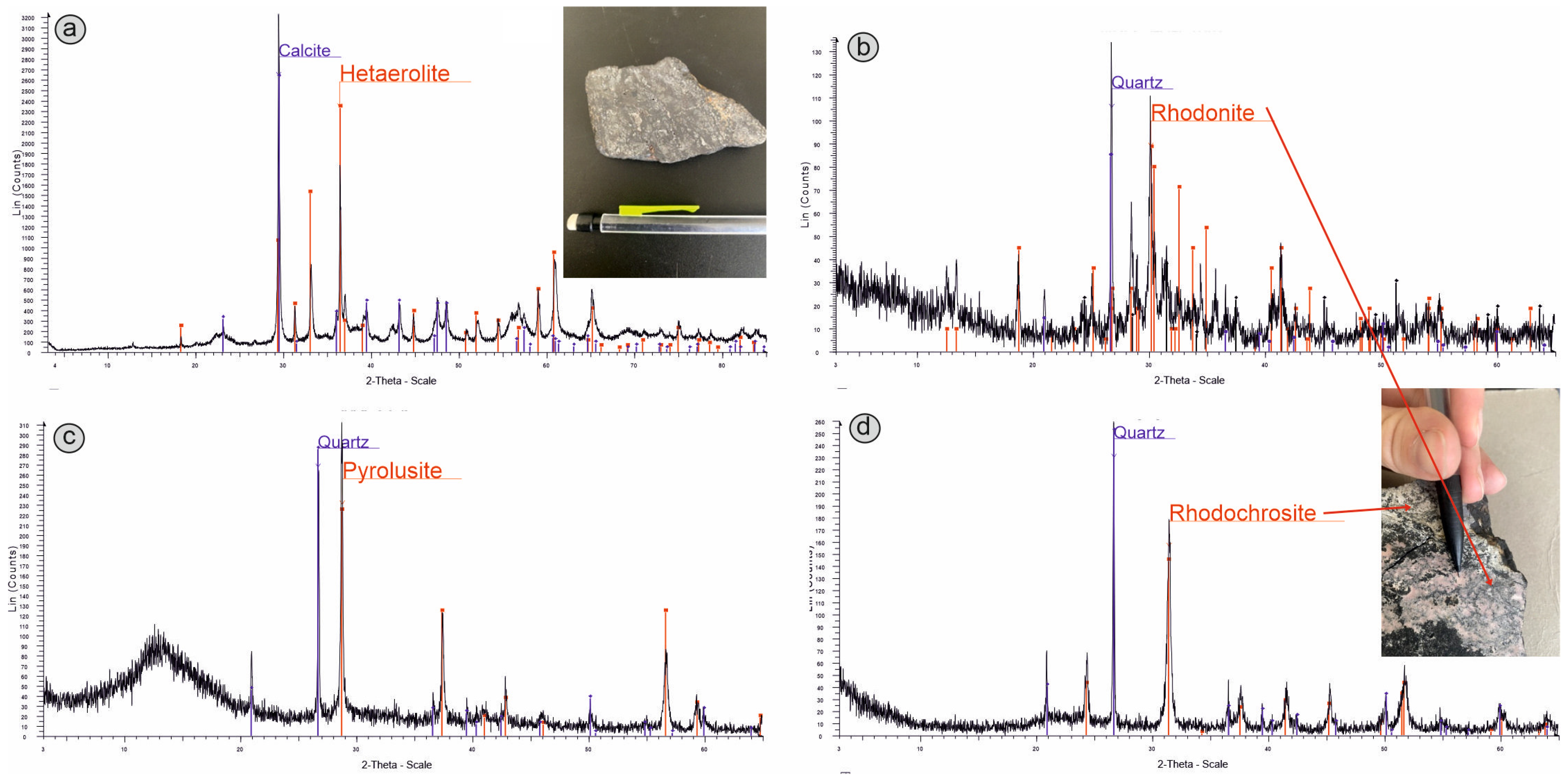
3. Results and Discussion
3.1. Portable X-ray Fluorescence
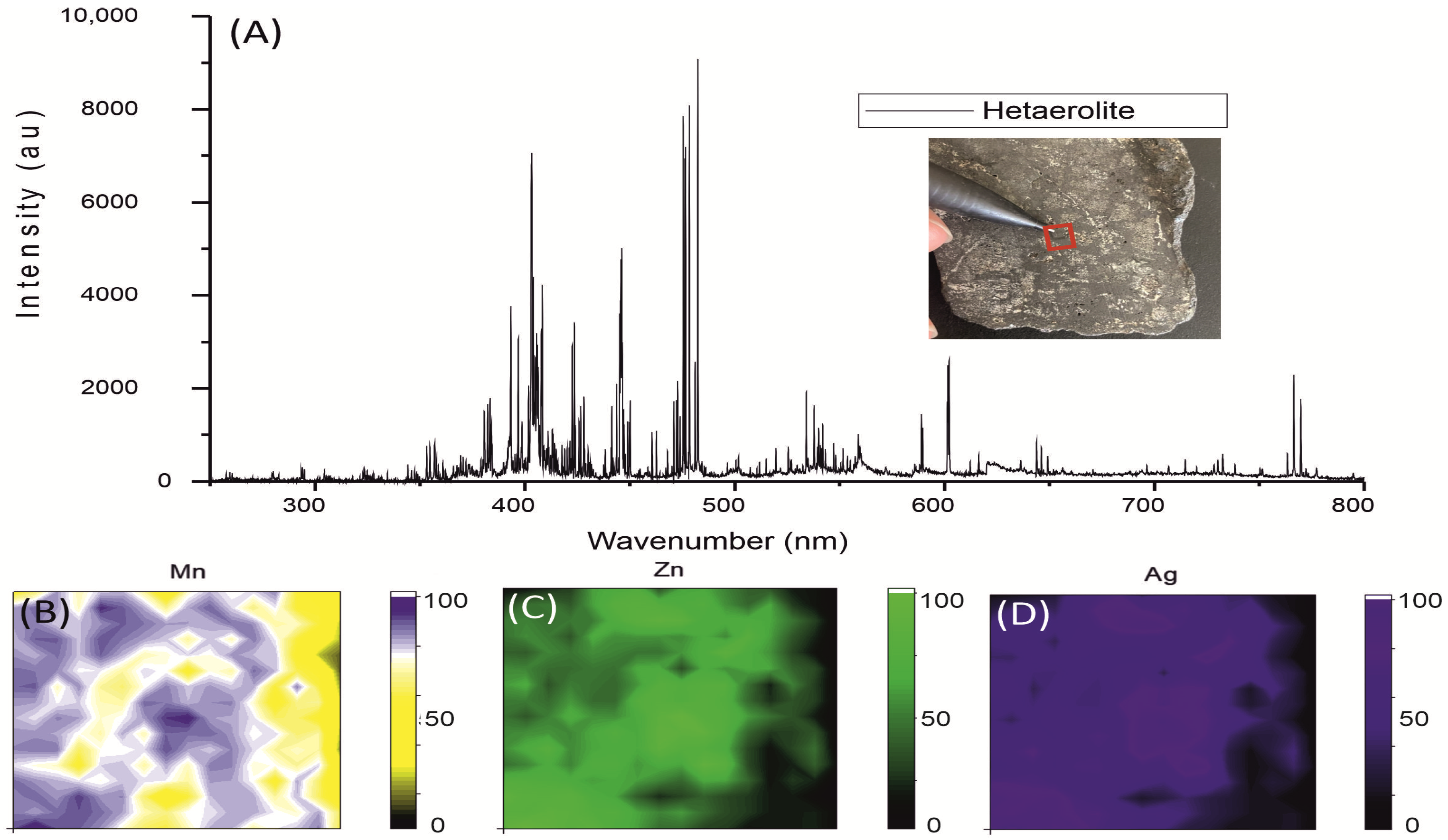
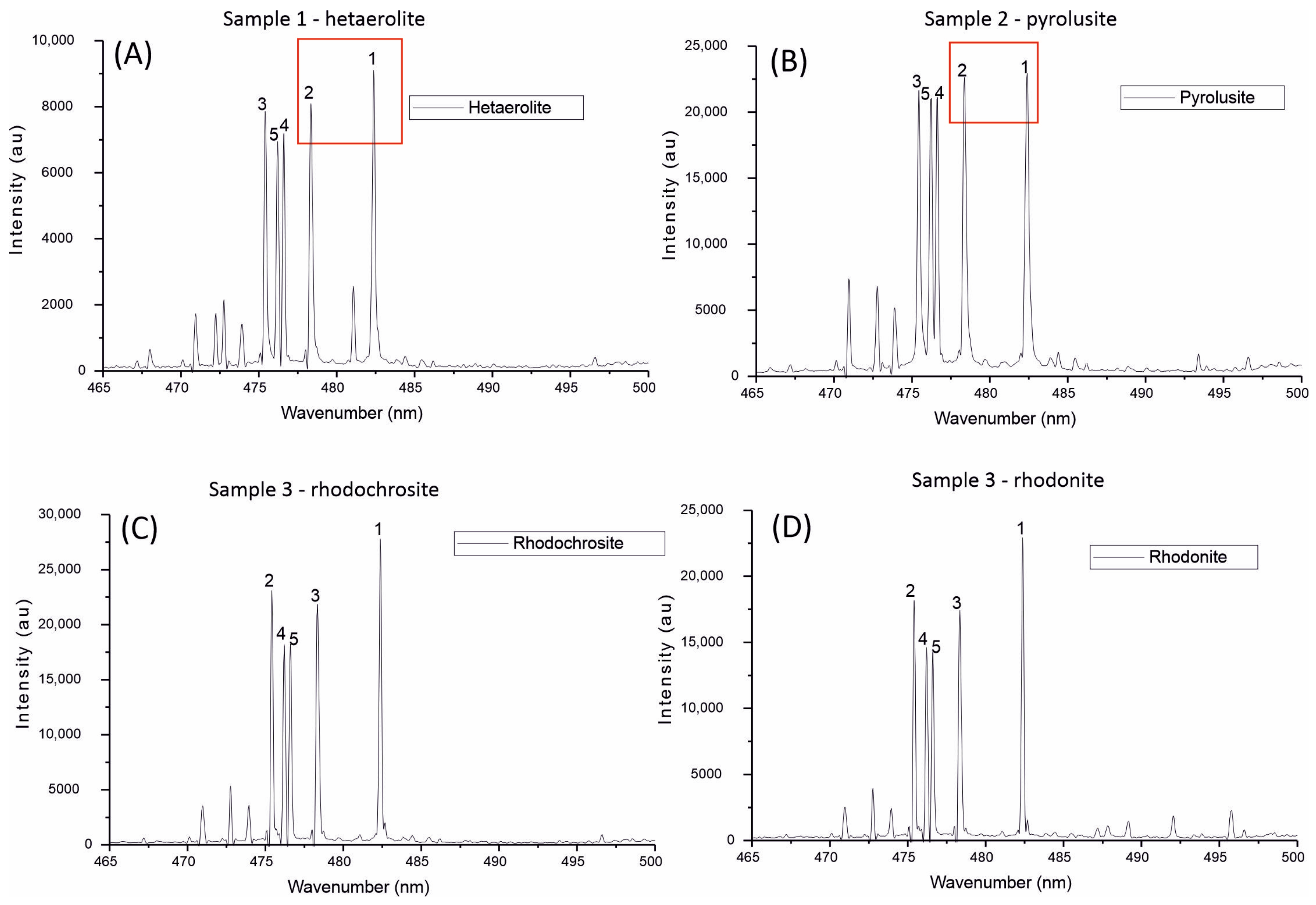
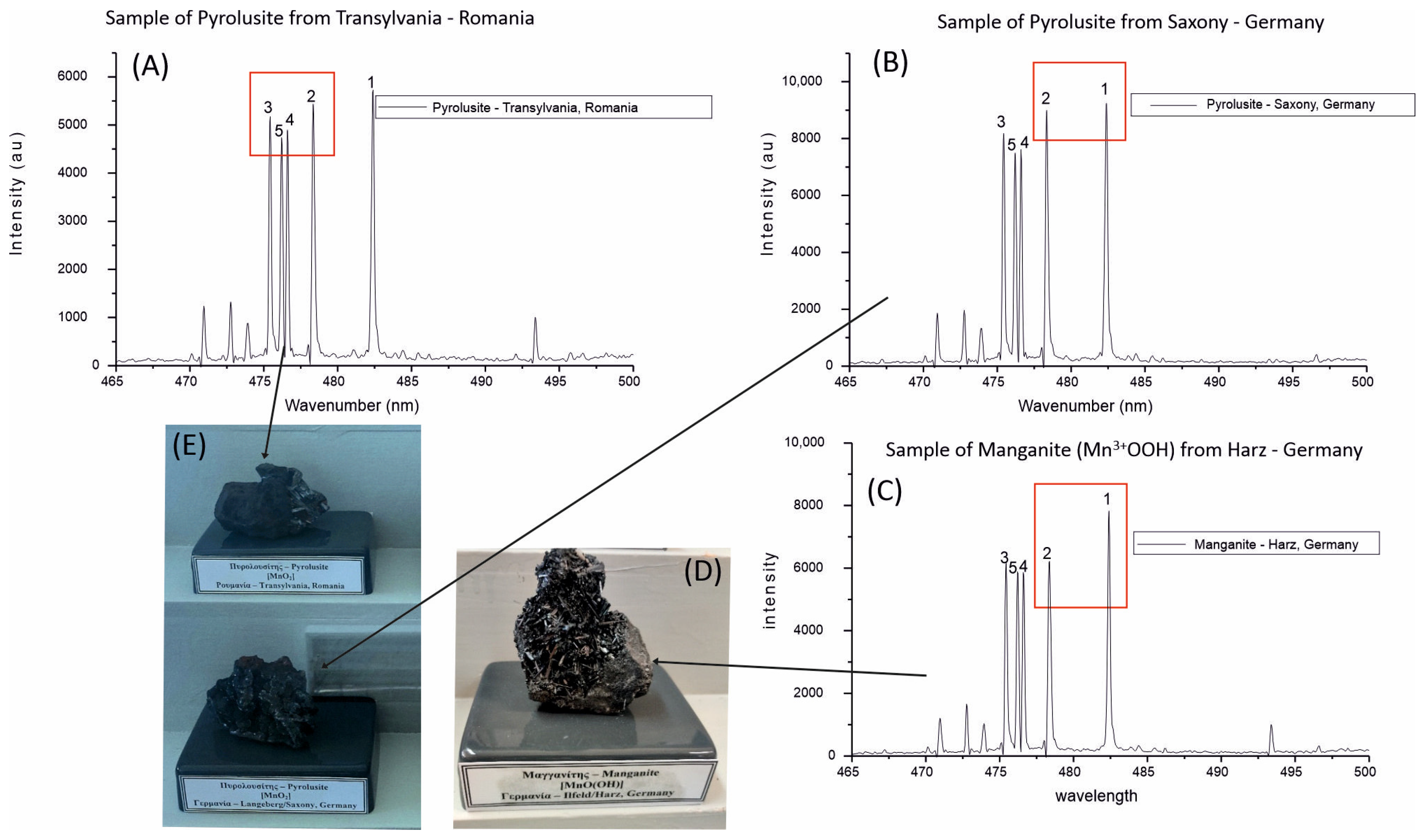
3.2. Portable Laser-Induced Breakdown Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. Regulation of the European Parliament and of the Council Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) 168/2013, (EU) 2018/858, 2018/1724 and (EU) 2019/1020 (COM/2023/160). EU Law—EUR-Lex. Available online: europa.eu (accessed on 10 August 2023).
- Balaram, V.; Sawant, S. Indicator Minerals, Pathfinder Elements, and Portable Analytical Instruments in Mineral Exploration Studies. Minerals 2022, 12, 394. [Google Scholar] [CrossRef]
- Kim, Y. Near Real-Time Reconciliation of Geochemical Data Acquired with Handheld Spectroscopic Devices: Application to Volcanogenic Massive Sulfide (VMS) Deposit from the Iberian Pyrite Belt. Ph.D. Thesis, Université de Lorraine, Lorraine, France, 2022. [Google Scholar]
- Lacroix, E.; Cauzid, J.; Teitler, Y.; Cathelineau, M. Near real-time management of spectral interferences with portable X-ray fluorescence spectrometers: Application to Sc quantification in nickeliferous laterite ores. Geochem. Explor. Environ. Anal. 2021, 21, 1–13. [Google Scholar] [CrossRef]
- Harmon, R.; Senesi, G. Laser-Induced Breakdown Spectroscopy—A geochemical tool for the 21st century. Appl. Geochem. 2021, 128, 104929. [Google Scholar] [CrossRef]
- Fabre, C.; Ourti, N.E.; Mercadier, J.; Cardoso-Fernandes, J.; Dias, F.; Perrotta, M.; Koerting, F.; Lima, A.; Kaestner, F.; Koellner, N.; et al. Analyses of Li-Rich Minerals Using Handheld LIBS Tool. Data 2021, 6, 68. [Google Scholar] [CrossRef]
- Fabre, C. Advances in Laser-Induced Breakdown Spectroscopy analysis for geology: A critical review. Spectrochem. Acta Part B At. Spectrosc. 2020, 166, 105799. [Google Scholar] [CrossRef]
- Bal, B.; Ghosh, S.; Das, A.P. Microbial recovery and recycling of manganese waste and their future application: A review. Geomicrobiol. J. 2018, 36, 85–96. [Google Scholar] [CrossRef]
- Guo, X.; Yang, S.; Wang, D.; Chen, A.; Wang, Y.; Li, P.; Liang, G.; Zhi, C. The energy storage mechanisms of MnO2 in batteries. Curr. Opin. Electrochem. 2021, 30, 100769. [Google Scholar] [CrossRef]
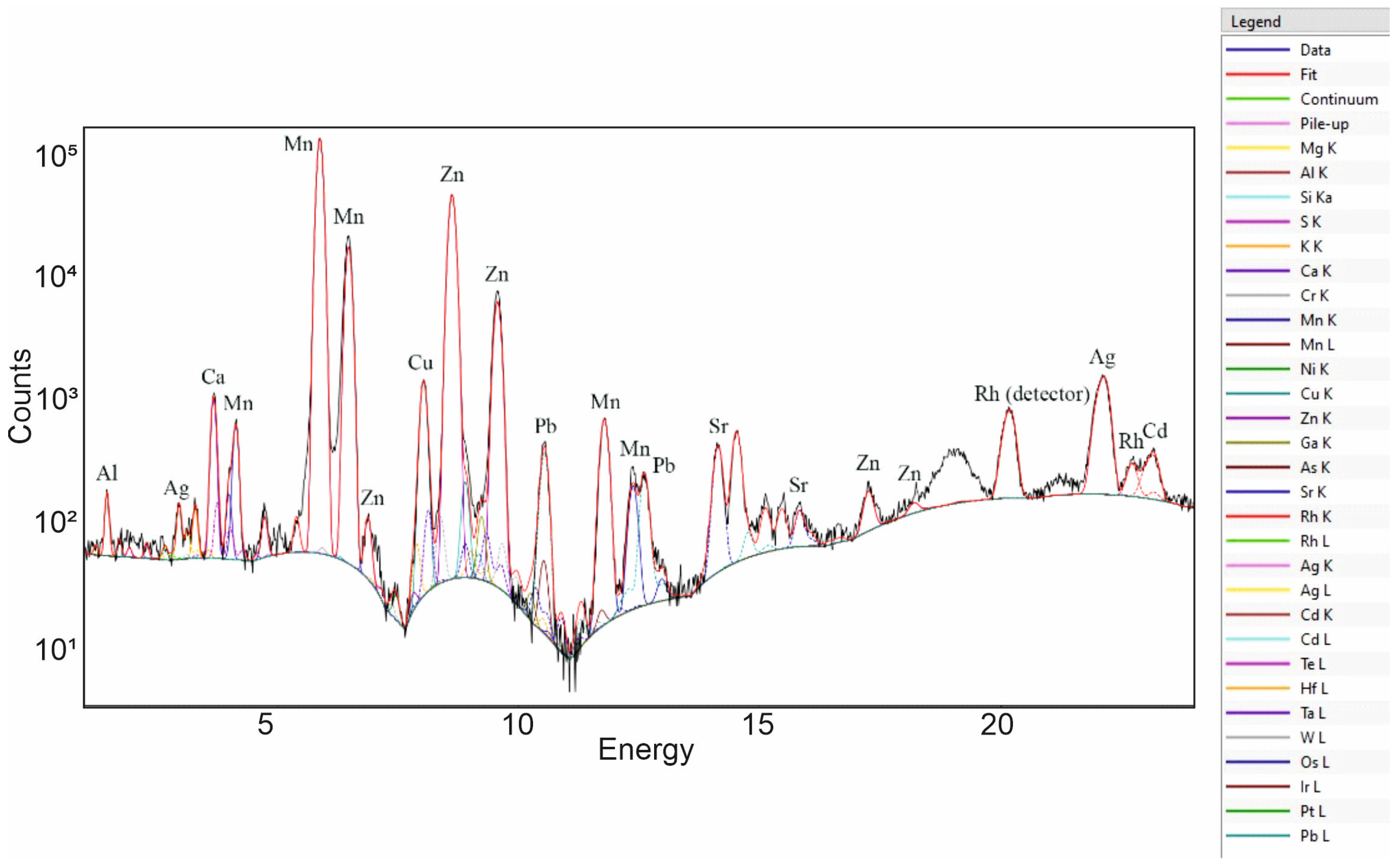

| Spot Analysis | Mn (wt%) | Zn (wt%) | Ca (wt%) | Ag (wt%) | Cu (wt%) | Pb (wt%) | Cd (wt%) |
|---|---|---|---|---|---|---|---|
| 1 | 54.57 | 14.07 | 18.55 | 0.22 | 0.41 | 0.07 | 0.08 |
| 2 | 56.11 | 13.37 | 11.38 | 0.25 | 0.45 | 0.08 | 0.07 |
| 3 | 55.84 | 13.23 | 24.29 | 0.22 | 0.18 | 0.17 | 0.08 |
| 4 | 32.30 | 3.24 | 37.43 | 0.13 | 0.12 | 0.03 | 0.06 |
| 5 | 47.63 | 3.24 | 24.96 | 0.16 | 0.17 | 0.11 | 0.06 |
| 6 | 66.89 | 10.54 | 16.88 | 0.33 | 0.34 | 0.06 | 0.07 |
| 7 | 66.02 | 18.85 | 2.75 | 0.45 | 0.70 | 0.09 | 0.07 |
| 8 | 44.95 | 8.74 | 33.23 | 0.16 | 0.15 | 0.10 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soulamidis, G.; Jatteau, M.; Stouraiti, C.; Voudouris, P.; Mavrogonatos, C.; Soukis, K.; Fabre, C.; Caumon, M.-C.; Cauzid, J.; Tarantola, A. Application of Portable Spectroscopic Tools in the Exploration of Manganese Oxide Minerals: Preliminary Results from the Case Study of Drama Mn-Oxide Deposits, Northern Greece. Mater. Proc. 2023, 15, 54. https://doi.org/10.3390/materproc2023015054
Soulamidis G, Jatteau M, Stouraiti C, Voudouris P, Mavrogonatos C, Soukis K, Fabre C, Caumon M-C, Cauzid J, Tarantola A. Application of Portable Spectroscopic Tools in the Exploration of Manganese Oxide Minerals: Preliminary Results from the Case Study of Drama Mn-Oxide Deposits, Northern Greece. Materials Proceedings. 2023; 15(1):54. https://doi.org/10.3390/materproc2023015054
Chicago/Turabian StyleSoulamidis, George, Marjolene Jatteau, Christina Stouraiti, Panagiotis Voudouris, Constantinos Mavrogonatos, Konstantinos Soukis, Cécile Fabre, Marie-Camille Caumon, Jean Cauzid, and Alexandre Tarantola. 2023. "Application of Portable Spectroscopic Tools in the Exploration of Manganese Oxide Minerals: Preliminary Results from the Case Study of Drama Mn-Oxide Deposits, Northern Greece" Materials Proceedings 15, no. 1: 54. https://doi.org/10.3390/materproc2023015054
APA StyleSoulamidis, G., Jatteau, M., Stouraiti, C., Voudouris, P., Mavrogonatos, C., Soukis, K., Fabre, C., Caumon, M.-C., Cauzid, J., & Tarantola, A. (2023). Application of Portable Spectroscopic Tools in the Exploration of Manganese Oxide Minerals: Preliminary Results from the Case Study of Drama Mn-Oxide Deposits, Northern Greece. Materials Proceedings, 15(1), 54. https://doi.org/10.3390/materproc2023015054










