Possibilities and Limitations of the Use of Hydrogen in Different Metallurgical Sectors †
Abstract
:1. Introduction
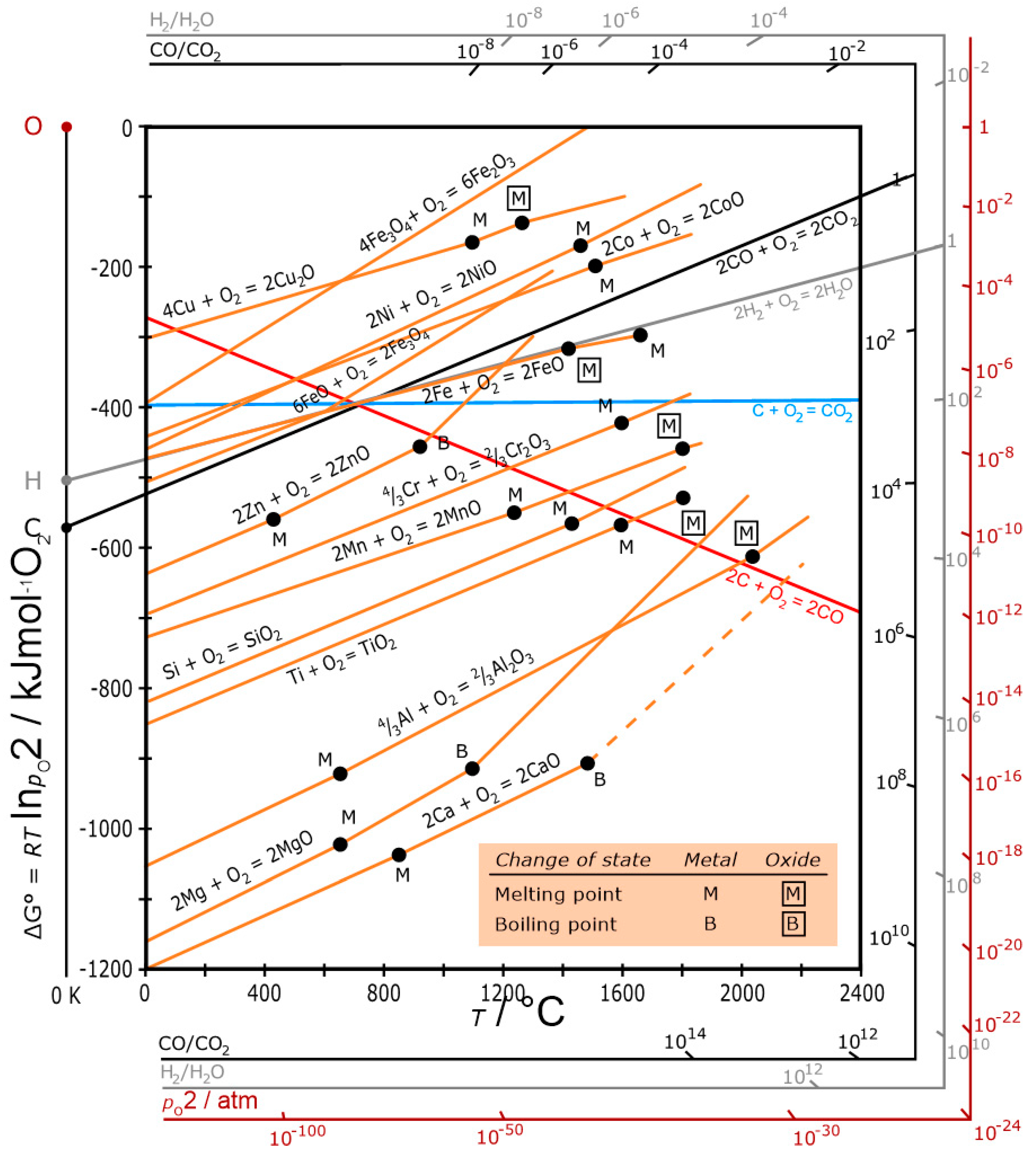
2. Thermodynamics
3. Hydrogen Reduction Treatment of Copper-Containing Slags
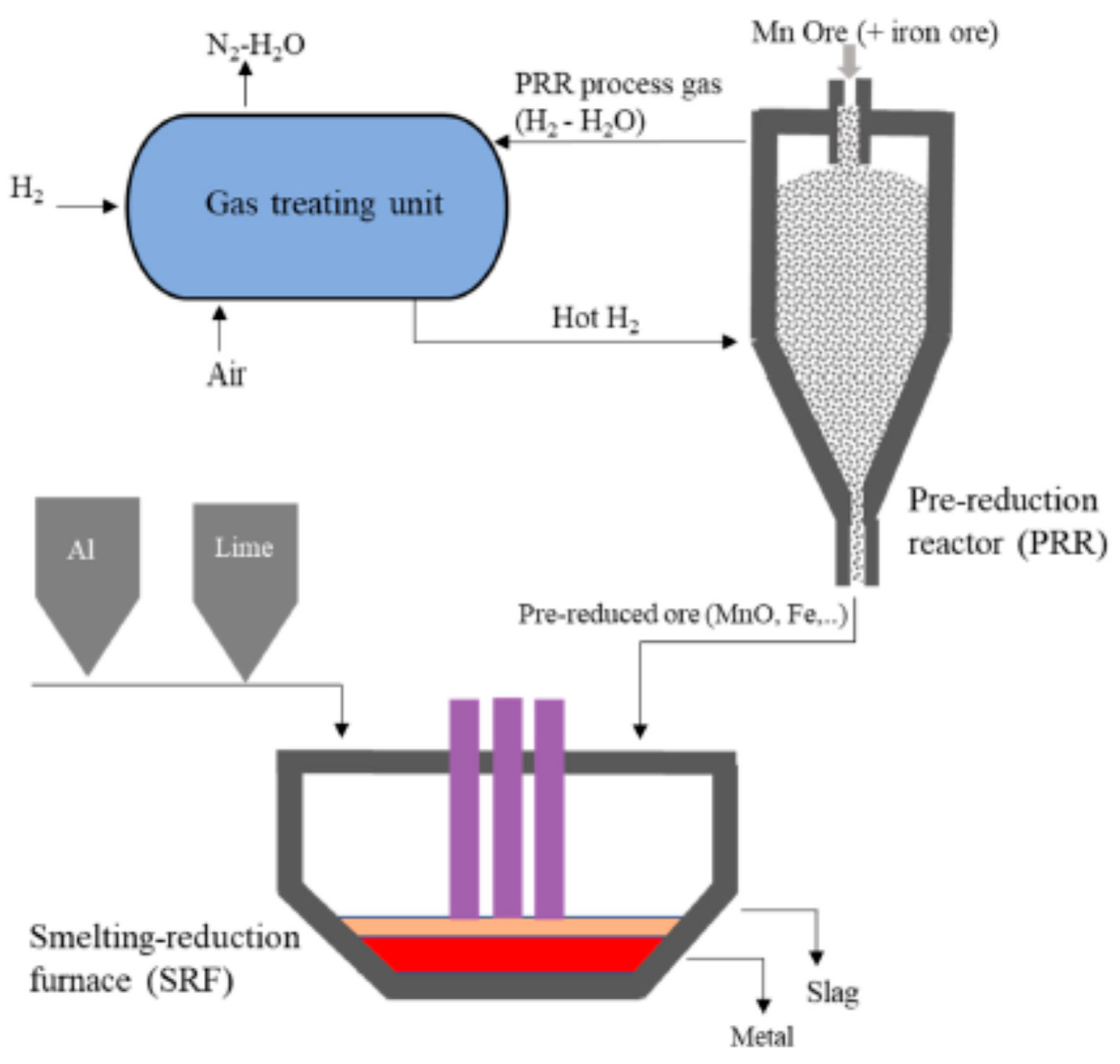
4. Prereduction of Mn Ore with Hydrogen and Aluminothermic Reduction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skibelid, O.B.; Velle, S.O.; Vollan, F.; Van der Eijk, C.; Hoseinpur-Kermani, A.; Safarian, J. Isothermal hydrogen reduction of a lime-added bauxite residue agglomerate at elevated temperatures for iron and alumina recovery. Materials 2022, 15, 6012. [Google Scholar] [CrossRef] [PubMed]
- Ellingham, H.J.T. Reducibility of oxides and sulphides in metallurgical processes. J. Soc. Chem. Ind. 1944, 63, 125–133. [Google Scholar]
- Wikipedia: Creative Commons Licence. Available online: https://en.wikipedia.org/wiki/Ellingham_diagram (accessed on 30 June 2023).
- Van der Eijk, C.; Dalaker, D. Recovery of Copper, Iron, and Alumina from Metallurgical Waste by Use of Hydrogen. In REWAS 2022: Developing Tomorrow’s Technical Cycles; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; Volume I, pp. 729–737. [Google Scholar]
- HARARE Website. Available online: https://h2020harare.eu/ (accessed on 30 June 2023).
- Hovestadt, G.; Coldewe, M.; Friedrich, B. Determining critical parameters of hydrogen reduction treatment of low copper-containing primary slags. In Proceedings of the European Metallurgical Conference 2023, Düsseldorf, Germany, 11–14 June 2023. [Google Scholar]
- Hovestadt, G.; Friedrich, B. Fuming process of zinc-rich slags by hydrogen injection. In Proceedings of the 8th Slag Valorizazion Symposium, Mechelen, Belgium, 18–20 April 2023. [Google Scholar]
- Olsen, S.E.; Tangstad, M.; Lindstad, T. Production of Manganese Ferroalloys; Tapir Academic Press: Trondheim, Norway, 2007. [Google Scholar]
- Safarian, J. A Sustainable Process to Produce Manganese and Its Alloys through Hydrogen and Aluminothermic Reduction. Processes 2022, 10, 27. [Google Scholar] [CrossRef]
- Halman Website. Available online: https://halman-project.eu/ (accessed on 30 June 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eijk, C.v.d.; Dalaker, H.; Safarian, J. Possibilities and Limitations of the Use of Hydrogen in Different Metallurgical Sectors. Mater. Proc. 2023, 15, 63. https://doi.org/10.3390/materproc2023015063
Eijk Cvd, Dalaker H, Safarian J. Possibilities and Limitations of the Use of Hydrogen in Different Metallurgical Sectors. Materials Proceedings. 2023; 15(1):63. https://doi.org/10.3390/materproc2023015063
Chicago/Turabian StyleEijk, Casper van der, Halvor Dalaker, and Jafar Safarian. 2023. "Possibilities and Limitations of the Use of Hydrogen in Different Metallurgical Sectors" Materials Proceedings 15, no. 1: 63. https://doi.org/10.3390/materproc2023015063
APA StyleEijk, C. v. d., Dalaker, H., & Safarian, J. (2023). Possibilities and Limitations of the Use of Hydrogen in Different Metallurgical Sectors. Materials Proceedings, 15(1), 63. https://doi.org/10.3390/materproc2023015063





