Abstract
Manganese oxide minerals have a remarkable range of applications. This investigation delves into their potential utility in energy storage, particularly as supercapacitors. The study centers on natural manganese oxides sourced from the Drama region (Greece), evaluating their electrochemical promise and devising strategies for addressing the remediation of mining waste sites. Samples were collected from abandoned mining sites at Kato Nevrokopi area, Drama region. Techniques such as X-ray diffraction (XRD) were employed to probe the structural characteristics of the minerals. Electrochemical studies involved the preparation of electrodes using natural and heat-treated nsutite (hausmannite). Then, the designed electrodes were subjected to cyclic voltammetry tests and charge-discharge measurements. Results showed superior electrochemical performance for the hausmannite-based electrode due to its greater structural homogeneity.
1. Introduction
Manganese oxides are well known for a wide range of applications, such as wastewater treatment [1], metal adsorption ([2,3]), energy storage applications, and rechargeable lithium batteries [4]. However, the mineral identification of manganese oxide minerals remains challenging due to their nano-crystallinity and their structural and oxidation-state diversity from Mn2+ to Mn7+ [5].
Current research endeavors seek to explore the possible applications of various manganese oxides in different battery cell setups. This paper focuses on assessing the electrochemical capabilities of naturally occurring manganese oxides sourced from the manganese ore deposit located in the Drama region of Greece.
There are several modern uses proposed for the manganese mining wastes, mainly as fillers in cement [6], as pyrolusite mine waste for the chemical preparation of manganese oxides [7] and recently, the use of final-stage waste from Mn mining for the synthesis of lithium layered manganese oxide (o-LiMnO2) was proposed [8].
This study examines the potential for the valorization of pyrolusite mine waste from the waste piles of the Drama mines in Nevrokopi. Utilization of this mining waste for energy storage applications adds value to low-grade ores by recovery of manganese and helps the environment by offering a destination to mining waste that is abundant around the world.
2. Materials and Methods
Samples of manganese oxide ore were collected from abandoned mining sites at Mavro Xylo and 25th km deposits, in the Drama region, northern Greece. Mineralogical analysis was conducted by X-ray diffraction (Bruker D8 ADVANCE, Bruker Corporation, Billerica, MA, USA) at Cu Κα radiation of 40 kV, 25 mA and λ = 1.5406 Å. Mineral phases were identified using the Joint Committee for Power Diffraction Standards (JCPDS) file and the software of DIFFRAC.EVA (V6.1, Bruker Corporation, Billerica, MA, USA) provided by Bruker. Sample powders of natural nsutite and hausmannite, which was prepared by thermal treatment of natural nsutite, were used separately for electrode preparations. These electrodes were prepared according to the literature [9]. A VersaSTAT 3 Potentiostat Galvanostat (Houten, The Netherlands) was used for cyclic voltammetry (CV) measurements. The measurements were extracted using a three-electrode single-compartment cell including an Ag/AgCl electrode (reference electrode), a Pt wire (counter-electrode), and the prepared material of hausmannite and nsutite (working electrodes), at room temperature, under Argon conditions. The electrochemical properties of these working electrodes were examined in a 0.1 M potassium hydroxide, KOH, solution (potassium hydroxide pellets, Merck, Saint Louis, MO, USA). All the potentials reported are expressed vs. Ag/AgCl.
3. Results
3.1. Mineralogical and Structural Characterization
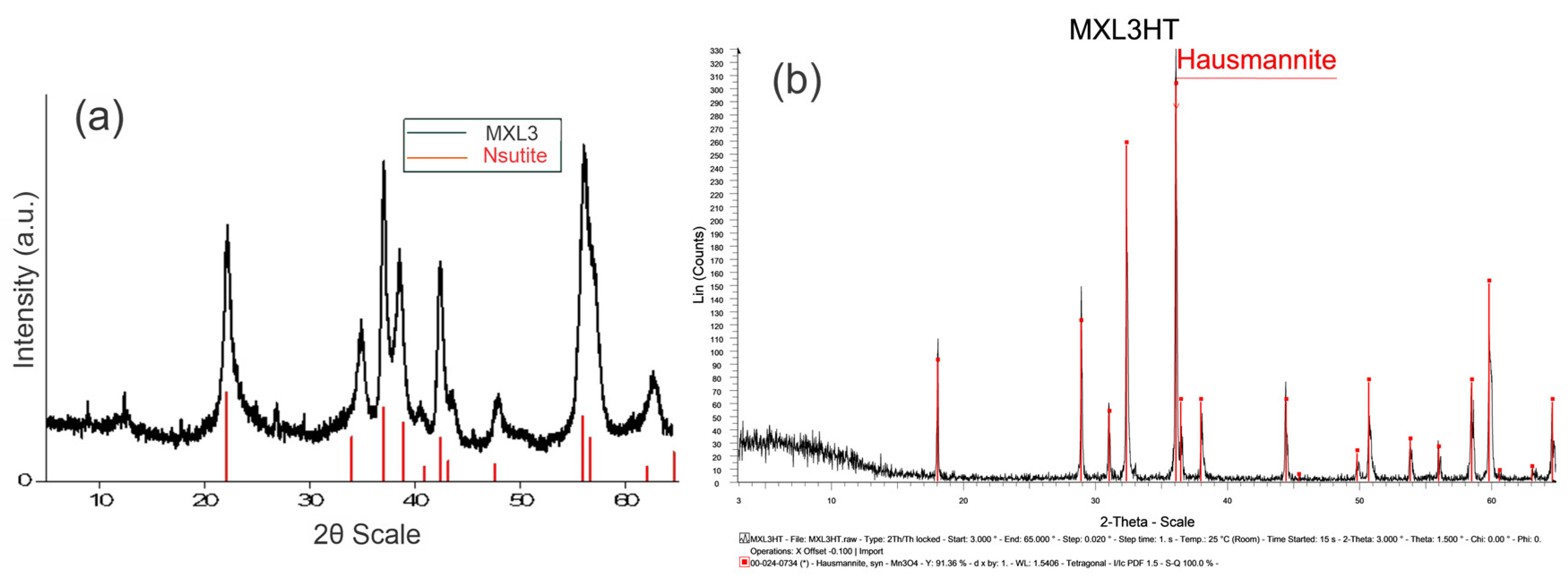
Analysis of the XRD patterns of natural manganese oxide shows that the 2θ values of 22.4°, 23.7°, 34.45°, 36.95°, 38.61°, 40.7°, 42.36°, 43.68°, 55.95°, 57.5°, and 61.81° were attributed to the mineral phase of nsutite (Figure 1a) or also known as γ-MnO2, which consists of an intergrowth structure of β-MnO2 type tunnels [1 × 1] as well as ramsdellite type tunnels [2 × 1] ([5,10]). X-ray diffraction analysis of the heat-treated sample clearly indicates that the primary nsutite successfully transformed into a well-crystallized hausmannite (Figure 1b).
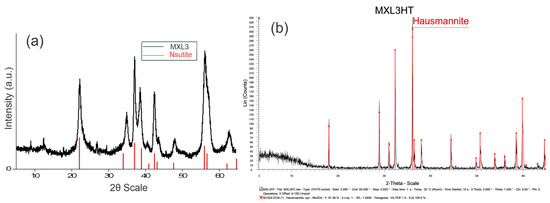
Figure 1.
X-ray Diffraction Patterns of (a) the natural manganese oxide and (b) the heat-treaded nsutite.
3.2. Electrochemical Characterization
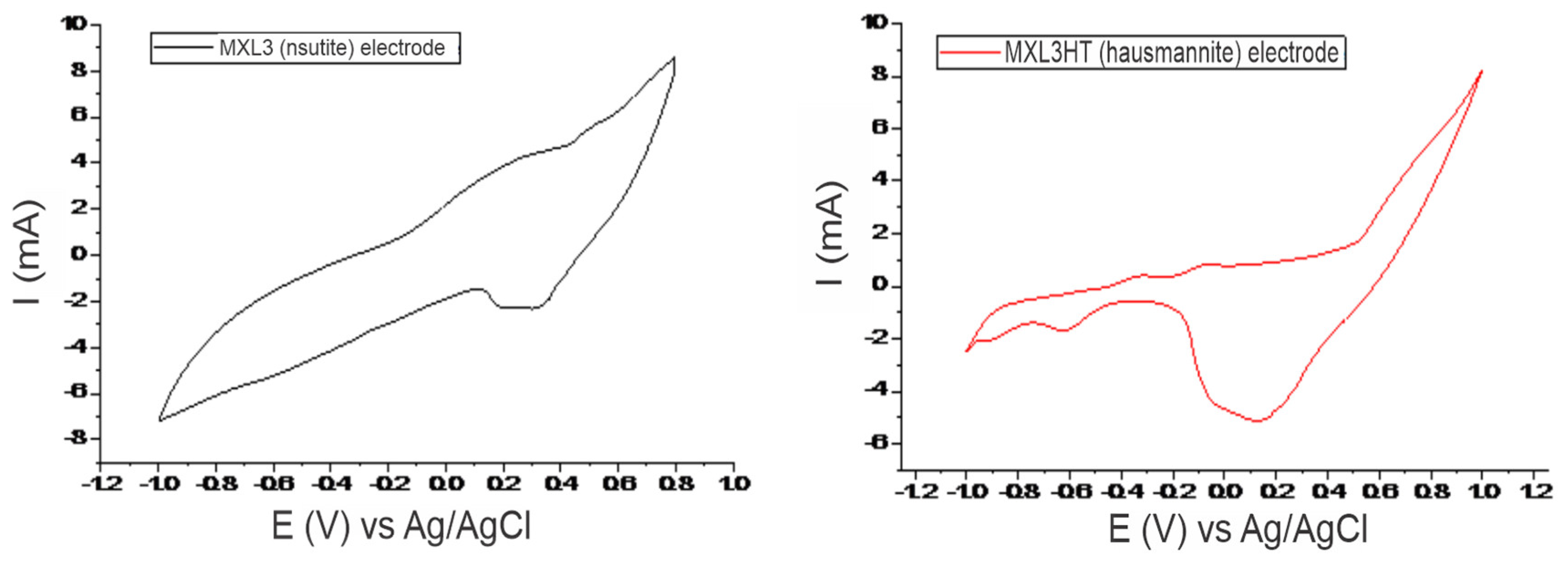
Figure 2 shows the cyclic voltammograms of MXL3 (natural nsutite) and MXL3HT (heat-treated nsutite) electrodes with a sweep rate of 50 mV/s in potential windows of (−1, 0.8) and (−1, 1), respectively. These two electrodes appear to have some noticeable differences in their diagrams due to the different nature of the minerals. In MXL3 and MXL3HT voltammograms, the anodic peaks of +0.53 V (MXL3), +0.7 V (MXL3HT), and the cathodic peaks of +0.31 V (MXL3), +0.13 V (MXL3HT) correspond to the oxygen evolution and oxygen reduction reactions [8]. Both voltammograms’ remaining anodic and cathodic peaks correspond to manganese redox reactions ([11,12]). The electrode of hausmannite, due to its plethora of redox reactions (Figure 2), is more active than the electrode of natural nsutite.
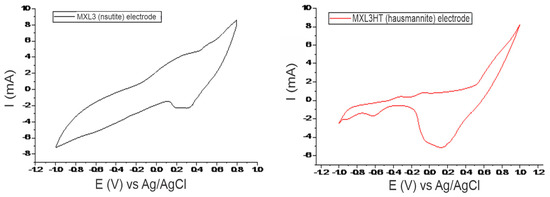
Figure 2.
Cyclic voltammograms of MXL3 (nsutite, left) and MXL3HT (hausmannite, right) in a 0.1 M KOH electrolyte, at a scan rate of 50mV/s and potential window of [−1, 0.8] and [−1, 1], respectively.
4. Conclusions
This study showcased the structural and electrochemical attributes of certain manganese oxides identified in the Mn oxide deposit in Drama, northern Greece. The production of hausmannite was achieved by heat treatment of nsutite. Two distinct electrodes were fabricated, employing nsutite and hausmannite as principal components. The outcome of the electrochemical experiments conducted on these electrodes indicates that further research is needed in investigating the structural aspects of hausmannite. This particular compound holds potential as an electrode, primarily for energy storage applications, while also warranting exploration for other potential applications that could contribute to the shift towards green energy solutions. Despite these materials originating from the waste piles of inactive mines, they have potential for utilization.
Author Contributions
Methodology, G.S., M.K. and E.C.; validation, G.S., C.S., C.A.M., M.K. and E.C.; resources, G.S., C.S., C.A.M., M.K., E.C., P.T.; data curation, G.S., M.K. and E.C.; writing—original draft preparation, G.S., M.K. and E.C.; writing—review and editing, G.S., C.S., C.A.M., M.K., E.C., P.T.; visualisation, G.S., C.S., M.K. and E.C.; supervision, G.S., C.S., C.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
The Hellenic Foundation for Research and Innovation (HFRI) supported the research under the 4th Call for HFRI PhD Fellowships (Fellowship Number: 11210).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the manuscript.
Acknowledgments
Facility Acknowledgment: All powder diffraction data were collected in the National and Kapodistrian University of Athens X-ray Diffraction Core Facility.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ghosh, S. Diversity in the Family of Manganese Oxides at the Nanoscale: From Fundamentals to Applications. ACS Omega 2020, 5, 25493–25504. [Google Scholar] [CrossRef] [PubMed]
- Rout, K.; Mohapatra, M.; Mohapatra, B.K.; Anand, S. Pb(II), Cd(II) and Zn(II) adsorption on low grade manganese ore. Int. J. Eng. Sci. Technol. 2009, 1, 106–122. [Google Scholar]
- Nguyen, T.Q.; Loganathan, P.; Nguyen, T.V.; Vigneswaran, S. Removing arsenic from water with an original and modified natural manganese oxide ore: Batch kinetic and equilibrium adsorption studies. Environ. Sci. Pollut. Res. 2020, 27, 5490–5502. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, M. Manganese oxides for lithium batteries. Prog. Solid State Chem. 1997, 25, 1–71. [Google Scholar] [CrossRef]
- Post, J. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Pimentel, M.; da Silva, M.R.C.; Viveiros, D.C.S.; Picanço, M.S. Manganese mining waste as a novel supplementary material in Portland cement. Mater. Lett. 2022, 309, 131459. [Google Scholar] [CrossRef]
- Darmane, Y.; Cherkaoui, M.; Kitane, S.; Alaoui, A.; Sebban, A.; Touhami, M.E. Preparation of chemical manganese dioxide from Moroccan pyrolusite mine waste. Hydrometallurgy 2008, 92, 73–78. [Google Scholar] [CrossRef]
- Mendes, K.C.; Figueira, B.A.M.; Lavra, T.C.C.; Fernandez, O.J.C.; Gómez, P.C.; Mercury, J.M.R. Hydrothermal synthesis of o-LiMnO2 employing Mn mining residues from Amazon (Brazil) as starting material. Mater. Lett. X 2019, 2, 100012. [Google Scholar] [CrossRef]
- Stosevski, I.; Bonakdarpour, A.; Fang, B.; Voon, S.; Wilkinson, D. Hausmannite Mn3O4 as a positive active electrode material for rechargeable aqueous Mn-oxide/Zn batteries. Int. J. Energy Res. 2021, 45, 220–230. [Google Scholar] [CrossRef]
- Chabre, Y.; Pannetier, J. Structural and Electrochemical Properties of the proton/γ–MnO2 system. Prog. Solid State Chem. 1995, 23, 1–130. [Google Scholar] [CrossRef]
- Gowda, C.; Mathur, A.; Parui, A.; Kumbhakar, P.; Pandey, P.; Sharma, S.; Chandra, A.; Singh, A.; Halder, A.; Tiwary, C. Understanding the electrocatalysis OER and ORR activity of ultrathin spinel Mn3O4. J. Ind. Eng. Chem. 2022, 113, 153–160. [Google Scholar] [CrossRef]
- Sahoo, R.; Pham, D.; Lee, T.; Luu, T.; Seok, J.; Lee, Y. Redox-Driven Route for Widening Voltage Window in Asymmetric Supercapacitor. ACS Nano 2018, 12, 8494–8505. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).