Abstract
This paper investigated the potential of thiosulfate as an alternative to cyanide for gold recovery from pressure oxidation residues. Thiosulfate leaching experiments were conducted under controlled conditions, varying parameters such as initial thiosulfate concentration, initial pH, temperature and solid–liquid mixing ratio (S/L). The results indicate that thiosulfate demonstrates promising gold recovery rates, with optimization potential through parameter adjustments. This study aimed to provide valuable insights into the feasibility of adopting thiosulfate as a gold recovery agent, promoting environmentally responsible practices in the metallurgical industry while addressing the challenges associated with pressure oxidation residues.
1. Introduction
The driving force behind the research into the leaching and recovery of gold using lixiviant alternatives to cyanide arises from the environmental hazards potentially caused by cyanide’s toxicity and the ban on its use imposed in various countries in the world. Many alternative lixiviants to cyanide for gold leaching have been investigated. In total, 27 possible gold solvents as alternatives to cyanide have been researched for gold leaching [1]. The rate of gold dissolution generally follows the order of cyanide > thiosulfate > thiourea [2,3,4]. Faster kinetics have been also reported for the thiosulfate–ammonia [3], thiourea–ammonia and thiocyanate/iodine systems [2]. An important concern in the case of sulfur-containing ligands (thiosulfate, thiourea) is the fact that these compounds are susceptible to degradation in an oxidizing environment with the formation of products that may affect gold extraction [3]. It is therefore important to properly select the leaching conditions so that gold is oxidized and lixiviant losses are minimal.
In recent years, thiosulfate has been considered an attractive alternative to cyanide for gold leaching. A process using thiosulfate was developed and commercialized for Barrick Gold’s Goldstrike operation in Eureka County, NV, USA, in 2015. The primary attraction of thiosulfate is its low toxicity and its potential use on ores that cannot be readily treated by conventional cyanidation. The chemistry of the thiosulfate system is relatively complicated compared with cyanide. However, by maintaining suitable Eh and pH conditions and by controlling the concentrations of thiosulfate, oxidant and oxygen in the leaching solution, high gold extractions can be achieved with low reagent consumption for some ores, namely copper sulfide and iron sulfide ores [5]. Several different oxidants have been proposed for the thiosulfate system, including oxygen, Cu(II) ammine complexes, Co(III) ammine complexes and various Fe(III) complexes.
Thiosulfates were selected for evaluation in the framework of the present work, as promising non-cyanide reagents for the recovery of gold from the pressure oxidation (POX) residue of gold-bearing sulfide concentrates. The investigated parameters included the solid–liquid mixing ratio (S/L), temperature (T), initial pH and initial thiosulfate concentration.
2. Materials and Methods
2.1. Materials
A pressure oxidation (POX) residue from Olympias mixed concentrate was produced by Metso-Outotec under the following conditions: S/L = 14%. The liquid was deionized water, T = 200 °C, pO2 = 400 kPa, t = 3 h, and this residue was used as feed material. The following chemicals were used during the tests: calcium thiosulfate, pure 30–50% solution in water (Thermoscientific, Belgium), copper sulfate pentahydrate (ChemLab, Belgium), calcium hydroxide, >95% (ChemLab, Belgium).
2.2. Experimental Conditions
The central operating conditions used during the experimental work were as follows: solid–liquid ratio, S/L = 20% w/v, pH = 7.0, initial concentration of calcium thiosulfate, CaTS = 0.1 M, Cu(II) concentration 0.8 mM, temperature T = 40 °C. The investigated parameters included the following:
- The initial concentration of CaTS, 0.05, 0.10 and 0.15 M;
- The initial pH, 7, 8.5 and 10;
- The solid to liquid ratio, 10, 20 and 30% w/v;
- The operating temperature, 30, 40 and 50 °C.
All experiments were carried out using a constant concentration of Cu(II) = 0.8 mM (50 mg/L). The effect of treatment duration was also investigated under all the examined conditions by conducting tests with a total duration of 2, 6 and 24 h. The experimental conditions of the leaching tests are presented in Table 1.

Table 1.
Experimental conditions of leaching tests (Cu(II) = 0.8 mM, t = 2, 6 and 24 h).
2.3. Experimental Procedure
For the preparation of the experimental slurries, 20 g of POX residue was mixed with 50 mL of deionized water (DW) in conical flasks. The pH of the slurry was raised to the target pH value, 7, 8.5 or 10, by adding a Ca(OH)2 solution (the supernatant of 1% w/v lime suspension) dropwise. After that step, 10 mL of concentrated CaTS (0.5, 1 or 1.5 M) and 1 mL of 80 mM CuSO4 were added in the slurry. Deionized water (DW) was added until the final volume of the leaching solution was equal to 100 mL. The conical flasks were placed into an incubator, maintaining constant temperature conditions and applying agitation of 250 rpm. After the end of the predetermined leaching duration (2 h, 6 h and 24 h), the shaking flasks were removed from the incubator and the solids were separated from the leachate by vacuum filtration.
2.4. Sampling and Analyses
The leachate solution was analyzed for pH, oxidation reduction potential (ORP) and dissolved oxygen (DO). The pH of the leachate solution was measured using a pH meter (Metrohm 827 Ph Lab, Herisau Switcherland), the dissolved oxygen was analyzed by a Microprocessor oximeter (OXI 196, WTW, Weilheim Germany) and the ORP was determined using a Hach multipolymeter (HQ40d, Loveland, CO, USA). The calcium thiosulfate concentration in the leachate solution was analyzed by iodometric titration. Dried solid residues were analyzed for their Au content by the wet acid digestion method with aqua regia combined with solvent extraction using MIBK.
3. Results and Discussion
3.1. Effect of Initial pH on Gold Leaching
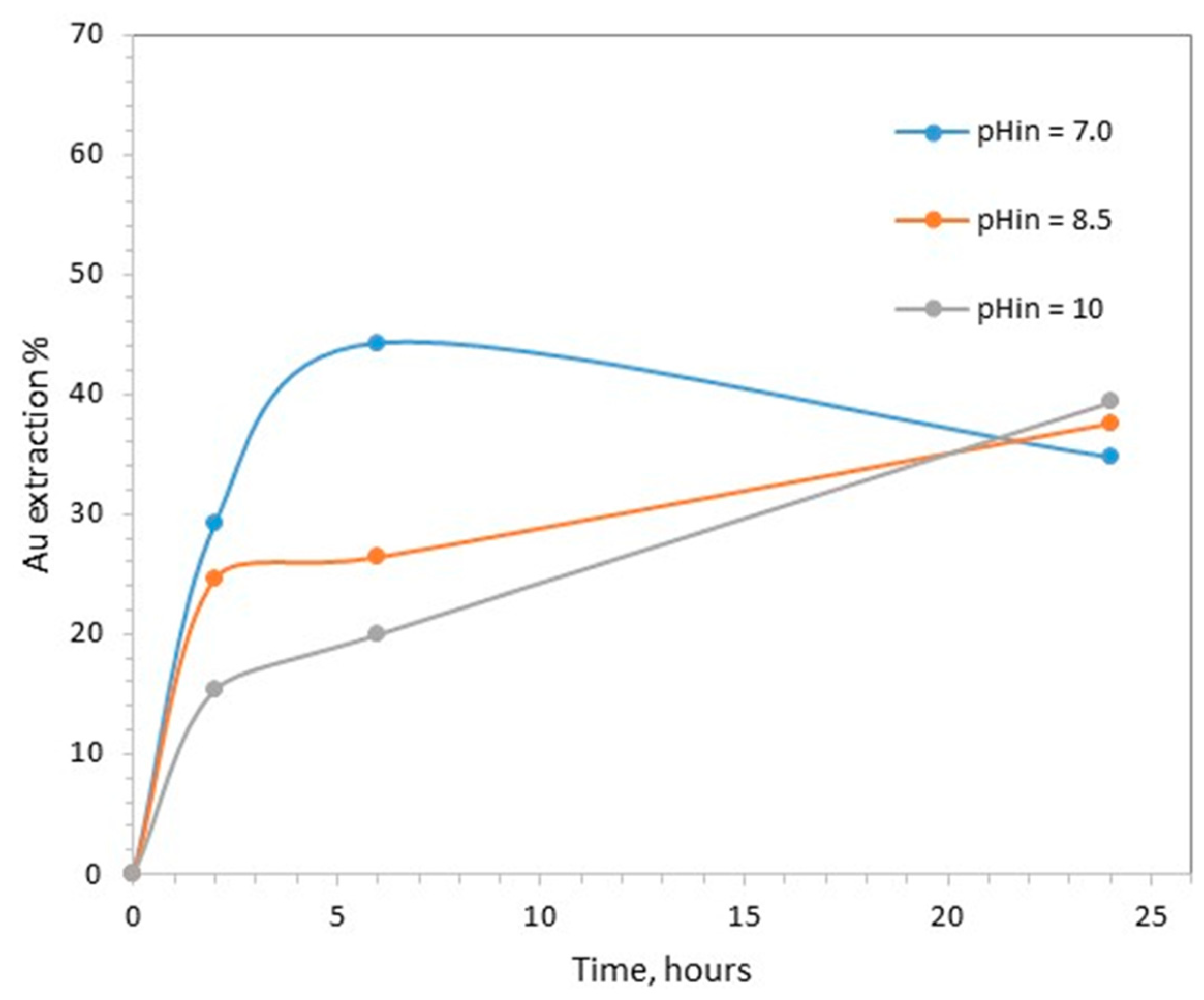
The extraction of gold as a function of time during the tests carried out with different initial pHs is shown in Figure 1. The highest extraction achieved was equal to 40.6% and was observed in the tests with the initial pH of 7.0 at 6 h.
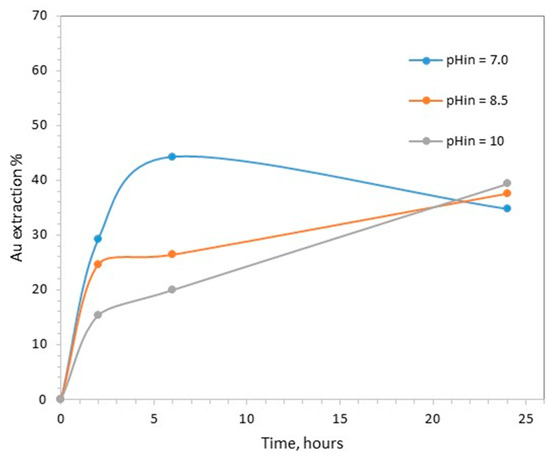
Figure 1.
Effect of initial pH on gold leaching (0.1 M CaTS, 40 °C, S/L = 20%).
When the duration of treatment extended from 6 to 24 h, gold leaching decreased from 40.5% to 34.8%. When the initial pH was adjusted to higher values, i.e., 8.5 and 10, the kinetics of gold extraction were clearly slower.
3.2. Effect of CaTS Concentration
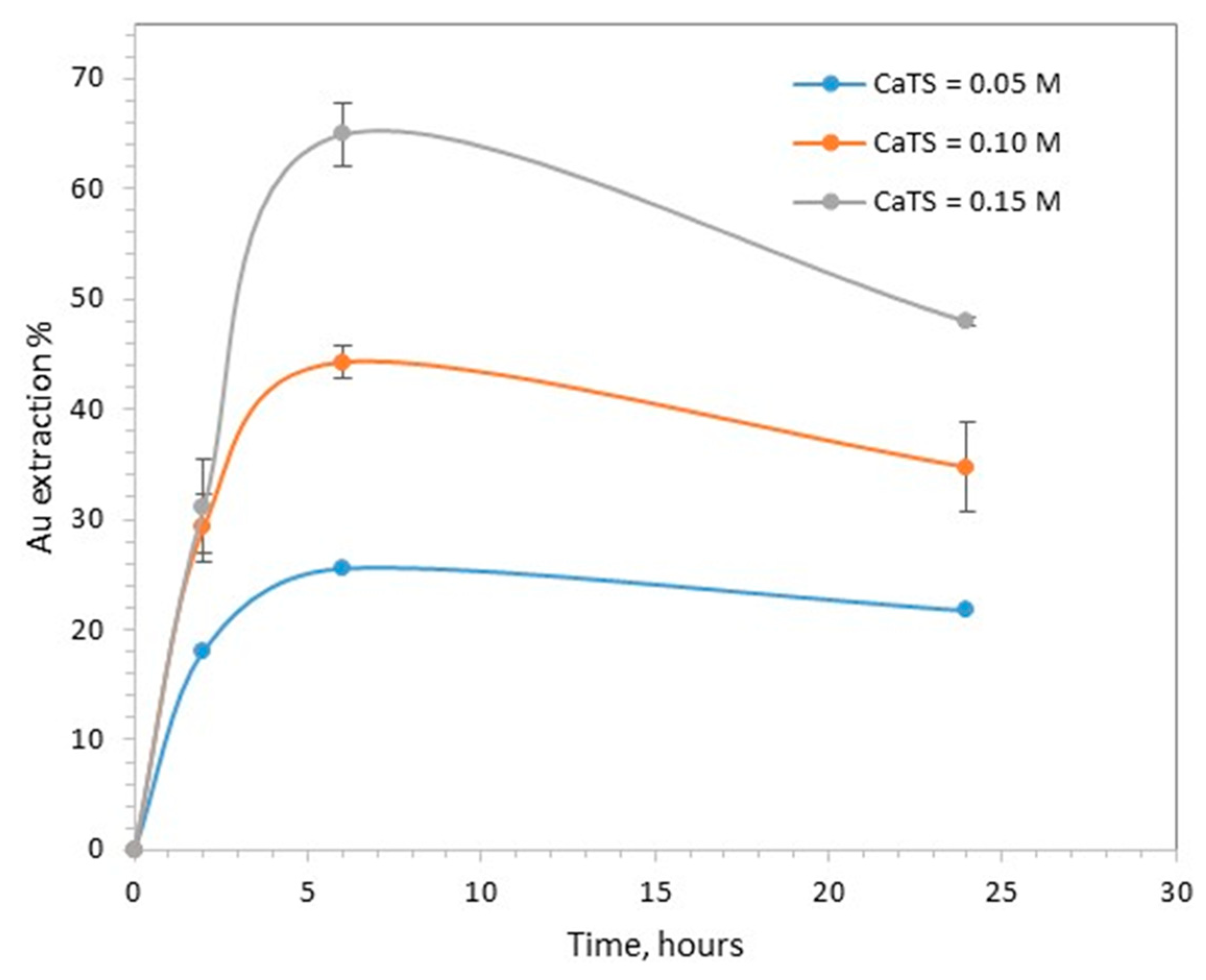
The effect of CaTS concentration on gold leaching is presented in Figure 2. The maximum gold extraction, 65%, was obtained at 6 h, using 0.15 M CaTS. At the same time interval, gold extraction was 25.5% with 0.05 M CaTS and 44.3% with 0.1 M CaTS.
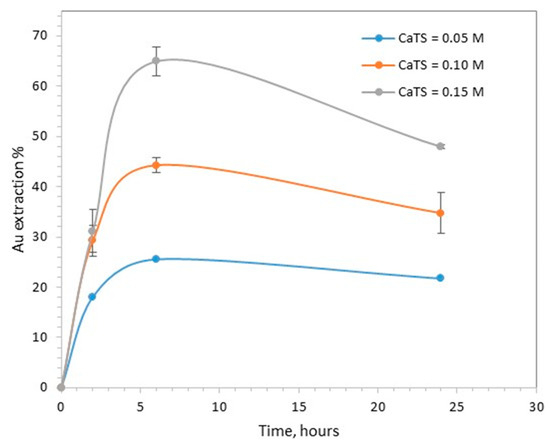
Figure 2.
The effect of CaTS concentration on gold extraction (pH 7, 40 °C, S/L 20%).
The dissolution of gold was lower when the duration of treatment increased from 6 to 24 h. This can be attributed to the reprecipitation or adsorption of dissolved gold, a phenomenon which has often been reported in the literature [6,7,8]. According to Daenzer et al., the stability of dissolved gold in CaTS solutions is negatively affected by the presence of pyrite, activated carbon or gypsum [8]. The pressure-oxidized residue used as feed material in this work did not contain any pyrite or carbonaceous material, but the gypsum content was high, i.e., close to 15%, and may have explained the observed decrease in gold extraction during longer treatment times.
3.3. The Effect of Solid to Liquid Ratio
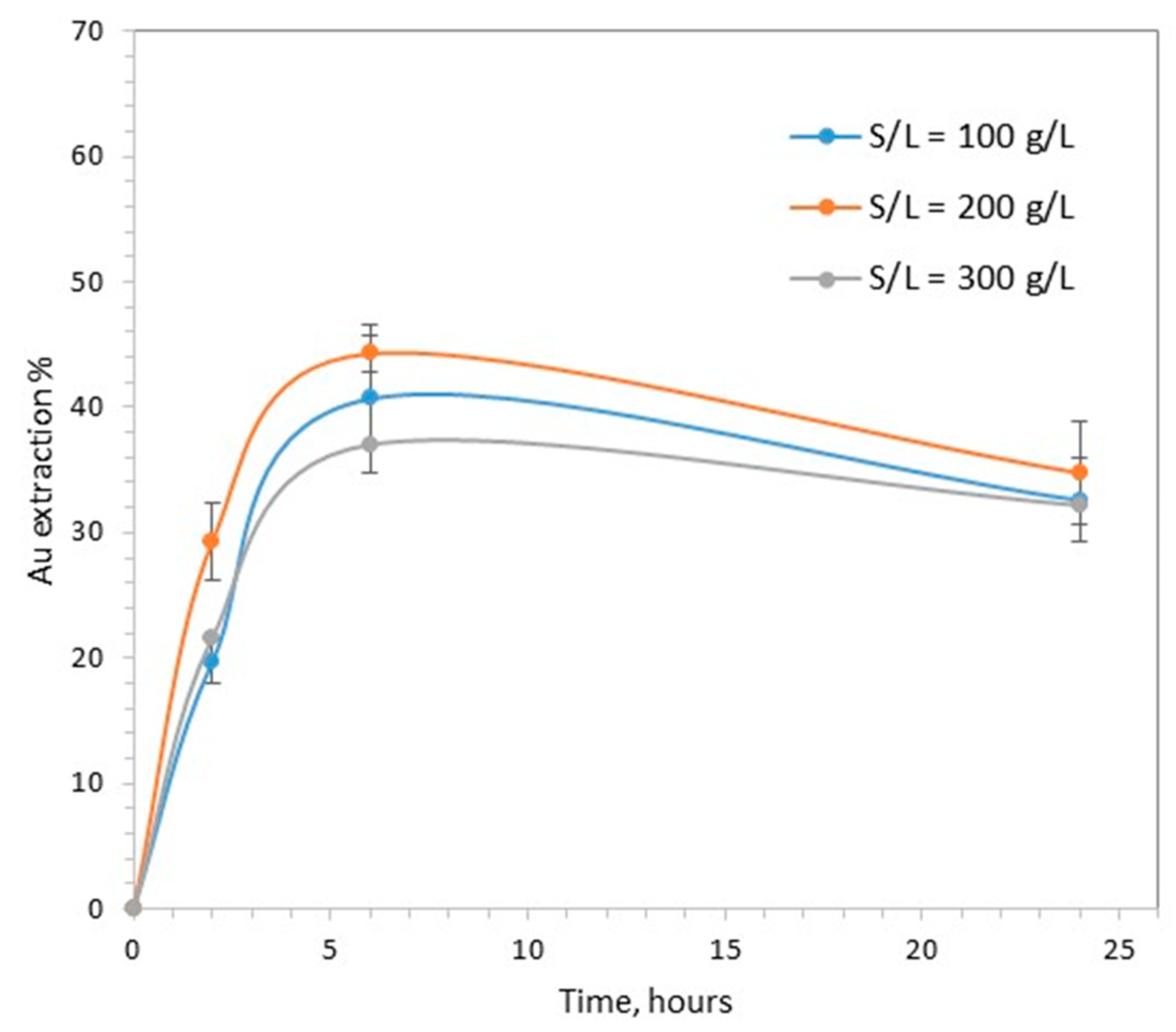
As shown in Figure 3, the increase in the solid to liquid ratio from 100 to 300 g/L had a limited effect on the percentage of gold extraction. The maximum extraction was observed at 6 h, and the observed variation in values, 37–44%, was not very different from the variation between the reproducibility experiments.
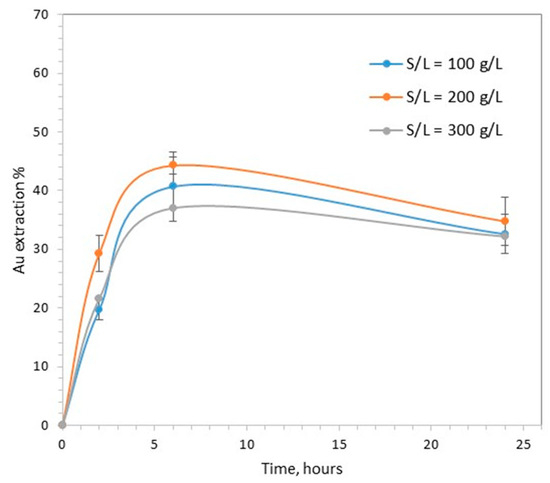
Figure 3.
The effect of solid to liquid ratio on gold extraction (pH = 7, CaTS = 0.1 M, T = 40 °C).
3.4. The Effect of Temperature
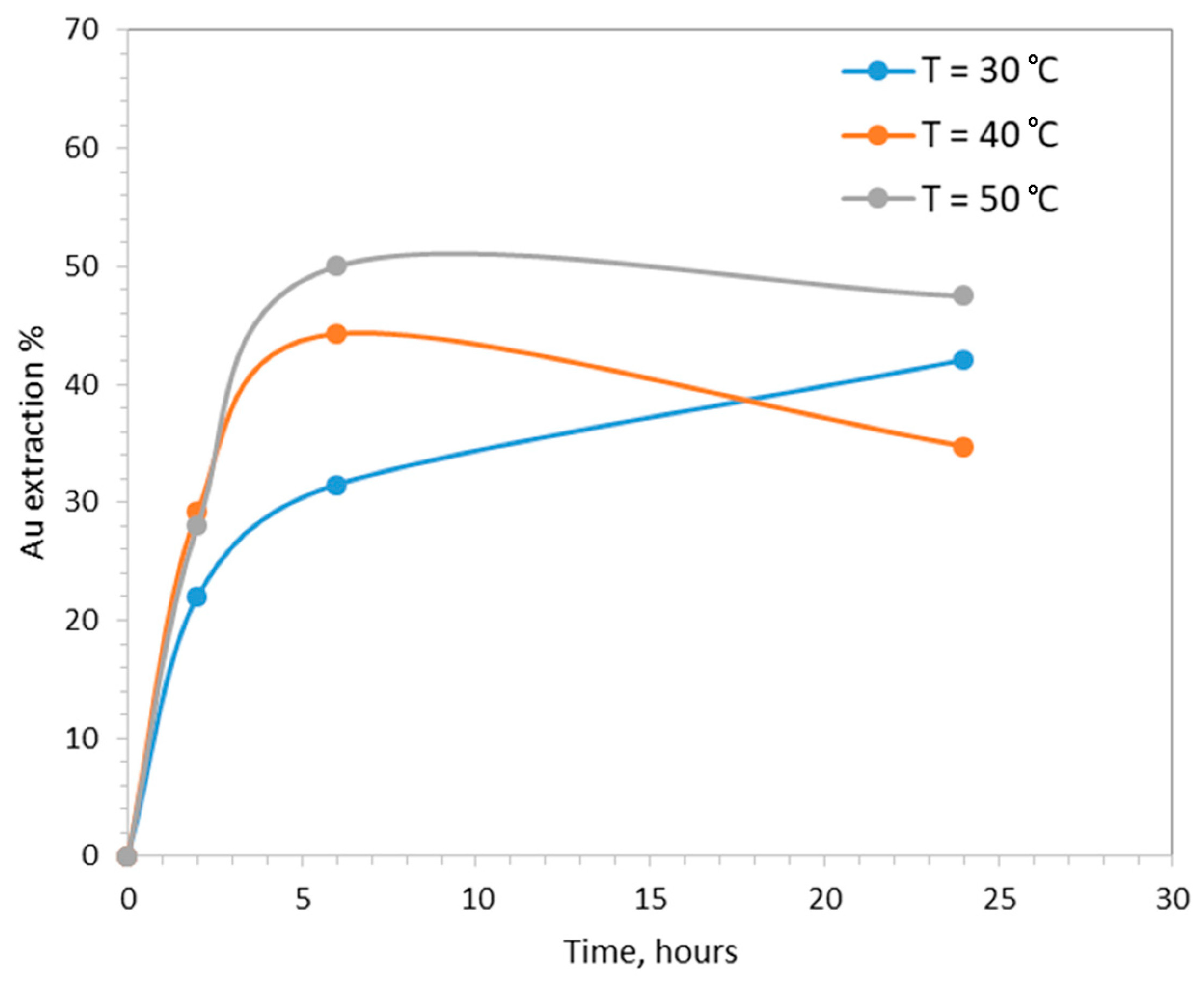
The increase in operating temperature had a positive effect on the kinetics of gold extraction (Figure 4). Increasing the temperature from 30 to 40 and 50 °C, gold extraction after 6 h was equal to 31%, 44% and 50%, respectively. After 24 h, gold extraction was equal to 42% at 30 °C (still at increasing trend), dropped from 44% to 35% at 40 °C and dropped from 50% to 47% at 50 °C.
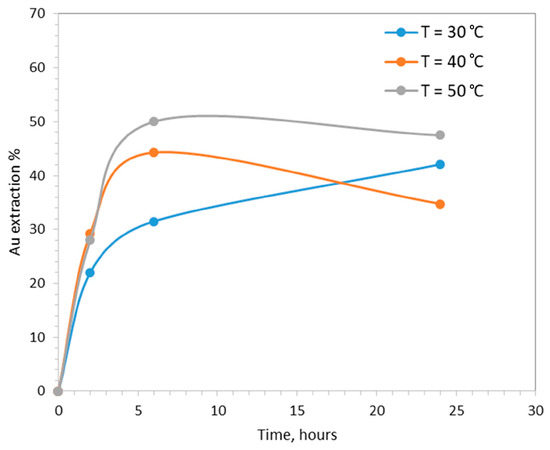
Figure 4.
The effect of temperature on gold extraction (pHin = 7, CaTS = 0.1 M, S/L = 20%.
In the present study, the maximum extraction of gold was 65% and was obtained within a relatively short treatment time, i.e., t = 6 h. According to the available published data, where a similar treatment scheme was applied for gold extraction (CaTS = 0.15 M, Cu = 0.8 mM, air, pH = 7–8, T = 40 °C), the extraction obtained at 6 h was lower, ranging between 8.2% and 45%, but the increase in treatment time had, in all cases, a positive effect, with final extraction rates of up to 74% [7,8]. In our study, the extension of treatment duration from 6 to 24 h caused the reprecipitation of dissolved gold. In all experiments, the gold concentration in the leachate was lower at 24 h than at 6 h. The main drawback of the thiosulfate process in our system seems to be the instability of gold–TS complexes, probably related to the high content of gypsum and oxidized Fe(III) phases [9,10,11].
Author Contributions
Conceptualization, N.P.; methodology, N.P.; validation, N.P. and M.T.; formal analysis, C.M.; investigation, C.M. and K.K.; resources, I.P.; data curation, C.M. and N.P.; writing—original draft preparation, C.M.; writing—review and editing, N.P. and K.A.; visualization, C.M.; supervision, N.P.; project administration, I.P.; funding acquisition, M.T. and I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Hellas Gold S.A. (62403200).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aylmore, M.G. Chapter 27—Alternative Lixiviants to Cyanide for Leaching Gold Ores. In Gold Ore Processing, 2nd ed.; Adams, M.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 447–484. [Google Scholar]
- Senanayake, G. Gold leaching in non-cyanide lixiviant systems: Critical issues on fundamentals and applications. Miner. Eng. 2004, 17, 785–801. [Google Scholar] [CrossRef]
- Aylmore, M.G. Alternative lixiviants to cyanide for leaching gold ores. In Developments in Mineral Processing, 1st ed.; Adams, M.D., Wills, B.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 501–539. [Google Scholar]
- Zhang, Y. Current status on leaching precious metals from waste printed circuit boards. Procedia Environ. Sci. 2012, 16, 560–568. [Google Scholar] [CrossRef]
- Wan, R.Y. Importance of solution chemistry for thiosulfate leaching of gold. In Proceedings of the World Gold ’97, Singapore, 1–3 September 1997; The Society for Mining, Metallurgy and Exploration, Inc.: Littleton, CO, USA, 1997; pp. 159–162. [Google Scholar]
- Zhang, H.; Jeffrey, M. A study of pyrite catalysed oxidation of thiosulfate. In Hydrometallurgy Proceedings of the Sixth International Symposium; Society for Mining, Metallurgy, and Exploration (SME): Phoenix, AZ, USA, 2008. [Google Scholar]
- Zhang, H.; Dai, X.; Breuer, P. Factors affecting gold leaching in thiosulfate-O2 solutions. In Proceedings of the ALTA 2013, Perth, Australia, 30–31 May 2013; ALTA Metallurgical Services Publications: Perth, Australia, 2013. [Google Scholar]
- Daenzer, R.; Dreiseinger, D.; Choi, Y. Role of polythionates on the stability gold in the leaching of double refractory ores in the calcium thiosulfate-air leaching system. In ALTA 2016 Gold Pm Proceedings; ALTA Metallurgical Services Publications: Perth, Australia, 2016; pp. 282–298. [Google Scholar]
- Zhu, Y.; Liang, X.; Zhang, M. Thiosulfate leaching of gold: A review. J. Hazard. Mater. 2017, 336, 174–184. [Google Scholar]
- Yildiz, O.; Erdogan, B.M.; Ozkan, G. Thiosulfate leaching of gold from pyrite: The effect of solution pH and redox potential. Miner. Eng. 2018, 124, 145–152. [Google Scholar]
- Heidari, M.; Pourabbas, A. The role of thiosulfate oxidation on gold leaching from a refractory gold ore. Hydrometallurgy 2018, 179, 17–24. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).