Abstract
The presence of chemicals, heavy metals, and colorants from textile effluents in water represent a significant health risk. To address this, a range of treatment methods, including ion exchange, membrane processes, physicochemical approaches, and biological techniques, are employed to eliminate these contaminants. Activated carbon, distinguished by its porous structure and effective pollutant, is a notable solution. An environmentally sustainable method involves producing activated carbon from agricultural and industrial waste to combat water pollution. For instance, the recycling of industrial waste, like glass fiber-reinforced plastic, can be used for water treatment via pyrolysis with a ZnCl2 activation process. The quality of the activated carbon is confirmed through characterization methods such as XRD, FTIR, SEM, and Raman spectroscopy, suggesting potential applications extending beyond water filtration, encompassing supercapacitors, fuel storage, and CO2 absorption. This study underscores the environmentally friendly potential of repurposing industrial waste for efficient and eco-conscious water purification.
1. Introduction
Various textile industry chemicals, including dyes, compounds, and heavy metals, can harm human health, causing skin irritation, allergies, and potential carcinogenic effects []. To treat textile effluents and ensure safe discharge or reuse, methods like ion exchange, membrane processes, and biological treatments are employed [,,]. Activated carbon, clay minerals, and nanomaterials effectively purify heavily contaminated wastewater by adsorption [,]. Activated carbon, widely used for its exceptional porosity and surface area, plays a crucial role in various applications [,]. GFRP used widely in transportation, wind energy, and aerospace, is projected to grow to $14.3 billion by 2025 from $11.5 billion in 2020. Rapid production will soon result in sizable global waste, posing risks to the environment and human health. For sustainable development, we have sizeable waste to synthesize activated carbon for methylene blue adsorption [].
2. Material and Methodology
Waste GFRP material was obtained and cut into 15 mm × 15 mm samples. Pyrolysis was conducted in a controlled argon environment at three temperatures (700 °C, 600 °C, and 500 °C) for 20 min with no pre-treatment applied. Another sample set was chemically treated with a 20% ZnCl2 solution for 24 h, re-weighed, and dried at 80 °C. Subsequent pyrolysis was conducted under the same conditions. Etching with a 10% HCl solution followed, and samples were dried for 24 h. Comprehensive characterization included morphology, composition, and structure analysis, providing insights into material behavior []. A total of 50 mg of each sample was incubated in a methylene blue solution for up to 2 h, followed by UV-Vis spectroscopy analysis at 665 nm for concentration determination.
3. Result and Discussion
3.1. Fourier Transform Infrared Spectroscopy
FTIR spectra in Figure 1a display structural changes from untreated to treated samples as the pyrolysis temperature increases. In the GFRP spectra, peaks observed at 1029 cm−1, 1270 cm−1, and 1731 cm−1 are attributed to ester groups, which diminish after pyrolysis. The emergence of peaks at 747 cm−1 and 866 cm−1, indicative of -CH bending, along with the appearance of the 1608 cm−1 peak associated with aromatic C=O and C=C bond stretching is noteworthy. As the temperature rises, the 1608 cm−1 peak diminishes, and this effect is more pronounced in treated samples. A faint peak at 1370 cm−1 suggests phenolic -OH bending, and an additional peak at 3422 cm−1 signifies -OH group stretching. Notably, the presence of the 1428 cm−1 peak in the treated sample, associated with aliphatic α-CH2 bending, suggests a heightened degree of carbonization on its surface [,,,,].
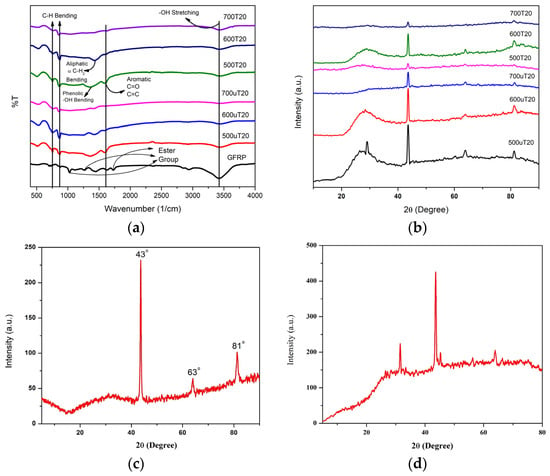
Figure 1.
(a) FTIR spectra of treated and untreated samples. (b) XRD spectra of treated and untreated samples. (c) XRD plot of glass fiber. (d) XRD plot of stub/sample holder.
3.2. X-ray Diffraction
X-ray diffraction (XRD) is a valuable technique for studying material composition and crystalline structure. Figure 1b exhibits a pronounced broad hump within the 20° to 30° range, indicative of the disordered atomic arrangement characteristic of amorphous carbon. Figure 1c displays XRD spectra of glass fibers, affirming their amorphous character. X-ray diffraction provides insights into sample composition and structure. The broad peak in Figure signifies the amorphous nature of the synthesized carbon and the peak located at the theta value 43° in Figure 1d was associated with the sample holder.
3.3. Scanning Electron Microscope
The SEM examination results indicate that samples treated with ZnCl2 exhibited significantly increased porosity compared to untreated samples. This enhanced porosity suggests their potential as effective adsorbent materials, particularly for water treatment applications to remove contaminants. Figure 2a–c demonstrates that samples that were not treated with ZnCl2 lack in flaky structure as compared to treated ones.
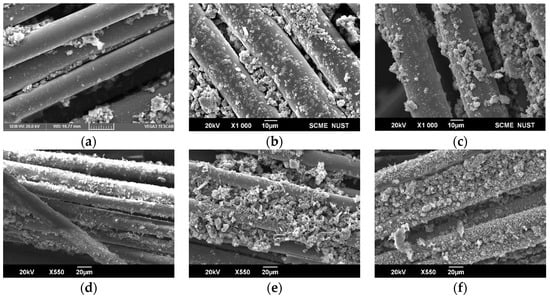
Figure 2.
SEM images: (a) 500 °C untreated; (b) 600 °C untreated; (c) 700 °C untreated; (d) 500 °C treated; (e) 600 °C treated; and (f) 700 °C treated.
Figure 2d–f demonstrates the porous nature of the ZnCl2-treated sample pyrolyzed at 600 °C for 20 min; figures also highlight the trade-off between porosity and carbon content, with ZnCl2-treated samples displaying a flakier structure. While the activation process increased porosity and active adsorption sites, it resulted in a loss of carbon content on the fiber surface.
3.4. Raman Spectroscopy
In Figure 3a, the Raman spectrum reveals two distinct peaks: the D band at 1332 cm−1 and the G band at 1599 cm−1. The D band is associated with disordered or defective carbon structures, indicating the presence of defects, vacancies, or impurities in the material. It is also related to the sp2 carbon network and carbon–carbon double bonds or aromatic carbon types. Analyzing the intensity and ratio (ID/IG) of these two peaks provides insights into the material’s structural characteristics. A higher ID/IG ratio indicates greater disorder, while a lower ratio signifies a more organized and graphitic carbon structure. It is important to note that variations in ID/IG ratios could be influenced by the presence of glass fibers in the samples.
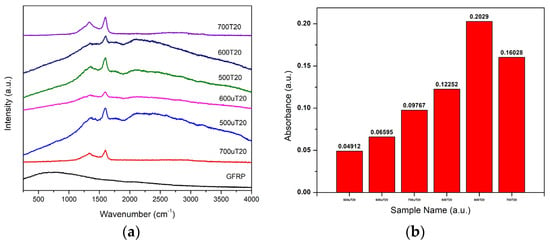
Figure 3.
(a) Raman spectrum of treated and untreated samples. (b) UV-Vis spectroscopy results.
3.5. Adsorption of Methylene Blue
In Figure 3b, various samples underwent different treatments involving ZnCl2 and pyrolysis. Samples treated with ZnCl2 and pyrolyzed for 20 min showed superior adsorption compared to other samples, suggesting an optimal pyrolysis duration for enhanced adsorption. Higher pyrolysis temperature led to more amorphous carbon, enhancing adsorption effectiveness. However, adsorption values remained lower, likely due to reduced activated carbon content on glass fiber surfaces. Higher carbon content in treated samples correlated with better adsorption, as more carbon indicates more active adsorption sites. Despite increased porosity in treated samples, their lower carbon content negated the porosity advantage. Balancing porosity and carbon content is crucial for optimal activated carbon materials in adsorption applications.
4. Conclusions
This study investigated the synthesis of activated carbon from glass fiber reinforced polyester (GFRP) waste and its application for methylene blue adsorption in water. Key findings include successful synthesis methods: XRD and FTIR analysis revealing the material’s characteristics, SEM analysis illustrating the impact of pyrolysis and chemical treatment on porous structure, and the discovery that ZnCl2-treated samples demonstrated effective adsorption. However, increasing adsorption temperature reduced adsorption due to decreased carbon content on glass fiber supports. This study highlights the potential of repurposing GFRP waste for environmental cleanup to enhance adsorption performance in treated samples. Further adjustments to the activation process and parameters may be necessary.
Author Contributions
Conceptualization, F.R. and M.I.; methodology, S.A. and Z.A.; validation, S.A. and Z.A.; formal analysis, M.I. and A.U.R.; investigation, F.R., A.R. and B.B.; resources, M.I.; data curation, F.R. and B.B.; writing—original draft preparation, F.R. and M.I.; writing—review and editing, M.S.B. and M.S.; supervision, M.I.; project administration, M.I.; funding acquisition, M.I. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Higher Education Commission (HEC) Government of Pakistan under the NRPU grant (Ref No. 20-15188/NRPU/R&D/HEC/2021 2021). The material was courtesy of Fibre Craft Industries (FCI) Lahore, Pakistan.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data will be available on request.
Acknowledgments
We are thankful to Sofia for facilitating us in UV-spectroscopy.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yadav, A.K.; Jain, C.K.; Malik, D.S. Toxic characterization of textile dyes and effluents in relation to human health hazards. J. Sustain. Environ. Res. 2014, 3, 95–102. [Google Scholar]
- Piaskowski, K.; Świderska-Dąbrowska, R.; Zarzycki, P.K. Dye removal from water and wastewater using various physical, chemical, and biological processes. J. AOAC Int. 2018, 101, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Aqeel, K.; Mubarak, H.A.; Amoako-Attah, J.; Abdul-Rahaim, L.A.; Al Khaddar, R.; Abdellatif, M.; Al-Janabi, A.; Hashim, K.S. Electrochemical removal of brilliant green dye from wastewater. IOP Conf. Ser. Mater. Sci. Eng. 2020, 888, 012036. [Google Scholar] [CrossRef]
- Karimifard, S.; Moghaddam, M.R. Application of response surface methodology in physicochemical removal of dyes from wastewater: A critical review. Sci. Total Environ. 2018, 640, 772–797. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Bulgariu, L.; Escudero, L.B.; Bello, O.S.; Iqbal, M.; Nisar, J.; Adegoke, K.A.; Alakhras, F.; Kornaros, M.; Anastopoulos, I. The utilization of leaf-based adsorbents for dyes removal: A review. J. Mol. Liq. 2018, 276, 728–747. [Google Scholar] [CrossRef]
- Selmani, F.; Cherifi, H.; Hanini, S.; Khalladi, R. Competitive Adsorption of Dyes on Activated Carbon Prepared from Vegetable Fibers. FEB-Fresenius Environ. Bull. 2019, 28, 4598. [Google Scholar]
- Ören, A.H.; Kaya, A. Factors affecting adsorption characteristics of Zn2+ on two natural zeolites. J. Hazard. Mater. 2006, 131, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Ge, F.; Li, C.; Lu, S. Study of the thermal degradation of flame-retardant polyester GFRP using TGA and TG-FTIR-GC/MS. J. Therm. Anal. Calorim. 2022, 147, 5743–5760. [Google Scholar] [CrossRef]
- Yu, X.; Wang, S.; Zhang, J. Preparation of high adsorption performance activated carbon by pyrolysis of waste polyester fabric. J. Mater. Sci. 2018, 53, 5458–5466. [Google Scholar] [CrossRef]
- Gumustas, M.; Sengel-Turk, C.T.; Gumustas, A.; Ozkan, S.A.; Uslu, B. Chapter 5—Effect of Polymer-Based Nanoparticles on the Assay of Antimicrobial Drug Delivery Systems. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 67–108. [Google Scholar]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Hussain, O.A.; Hathout, A.S.; Abdel-Mobdy, Y.E.; Rashed, M.; Rahim, E.A.; Fouzy, A. Preparation and characterization of activated carbon from agricultural wastes and their ability to remove chlorpyrifos from water. Toxicol. Rep. 2023, 10, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Uchimiya, M.; Wartelle, L.H.; Klasson, K.T.; Fortier, C.A.; Lima, I.M. Influence of Pyrolysis Temperature on Biochar Property and Function as a Heavy Metal Sorbent in Soil. J. Agric. Food Chem. 2011, 59, 2501–2510. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).