Studying the Magnetic Properties and Corrosion Resistance of Coated NdFeB Magnets †
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, J.; Jiang, M.; Qiao, L.; Sheng, J.; Li, J.; Jiang, L. Surface modification of spherical NdFeB magnetic powders by a fluid-bed nickel electrodeposition. Mater. Lett. 2008, 62, 4407–4409. [Google Scholar] [CrossRef]
- Bae, K.-H.; Lee, S.-R.; Kim, H.-J.; Lee, M.-W.; Jang, T.-S. Effect of WS2/Al co-doping on microstructural and magnetic properties of Nd-Fe-B sintered magnets. J. Alloys Compd. 2016, 673, 321–326. [Google Scholar] [CrossRef]
- Sueptitz, R.; Uhlemann, M.; Gebert, A.; Schultz, L. Corrosion, passivation and breakdown of passivity of neodymium. Corros. Sci. 2010, 52, 886–891. [Google Scholar] [CrossRef]
- Chitrada, K.; Raja, K.S.; Pesic, B.; Charit, I. Corrosion Behavior of Surface Modified NdFeB Permanent Magnet in Dilute Chloride Environments. Electrochim. Acta 2014, 123, 23–32. [Google Scholar] [CrossRef]
- Zheng, J.; Lin, M.; Xia, Q. A preparation method and effects of Al-Cr coating on NdFeB sintered magnets. J. Magn. Magn. Mat. 2012, 324, 3966–3969. [Google Scholar] [CrossRef]
- Wei, Y.-K.; Li, Y.-J.; Zhang, Y.; Luo, X.-T.; Li, C.-J. Corrosion resistant nickel coating with strong adhesion on AZ31B magnesium alloy prepared by an in-situ shot-peening-assisted cold spray. Corros. Sci. 2018, 138, 105–115. [Google Scholar] [CrossRef]
- Sheng, J.; Jiang, L.; Zheng, J. NdFeB magnetic powders surface modification by a fluid-bed electrodeposition. J. Mater. Sci. 2006, 41, 5735–5738. [Google Scholar] [CrossRef]
- Wang, F.; Chen, X.P.; Qiu, P.; Wang, X.D.; Lu, W. Corrosion Resistance of Electroplating of Cu-Ni/P Coatings on NdFeB Magnet Materials. Int. J. Electrochem. Sci. 2020, 15, 10476–10487. [Google Scholar] [CrossRef]
- Yan, J.; Wu, Q.; Hu, X.; Jia, J.; Zhao, Y.; Zou, M.; Ge, H. The effect of Dy-Cu co-deposition and grain boundary diffusion on the microstructure and magnetic properties of sintered NdFeB magnets. J. Magn. Magn. Mat. 2024, 600, 172129. [Google Scholar] [CrossRef]
- Sueptitz, R.; Tschulik, K.; Uhlemann, M.; Katter, M.; Schultz, L.; Gebert, A. Effect of magnetization state on the corrosion behaviour of NdFeB permanent magnets. Corros. Sci. 2011, 53, 2843–2852. [Google Scholar] [CrossRef]
- Cao, R.; Zhu, L.; Liu, H.; Yang, X.; Nan, H.; Li, W. Improvement of corrosion resistance and magnetic properties for sintered NdFeB by alumina sol-containing conversion film. RSC Adv. 2016, 6, 92510–92519. [Google Scholar] [CrossRef]
- Wang, X.; Shen, L.; Qiu, M.; Wang, K.; Tian, Z. Effect of Friction on Preparation of NdFeB Nickel Coating by Jet Electrodeposition. Int. J. Electrochem. Sci. 2018, 13, 7706–7717. [Google Scholar] [CrossRef]
- Périgo, E.A.; de Campos, M.F.; Faria, R.N.; Landgraf, F.J.G. The effects of the pressing step on the microstructure and aging of NdFeB bonded magnets. J. Powder Technol. 2012, 224, 291–296. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.; Guo, D.; Ren, X.; Ma, Y. An Investigation of the Magnetic Properties and Structures of Sr-Ferrite/NdFeB Hybrid Magnets with Cold Pressing and SPS Methods. J. Electron. Mater. 2024, 53, 1763–1772. [Google Scholar] [CrossRef]
- Lu, Y.; Luo, S.; Ren, Z.; Zou, Y.; Zhong, S.; Wu, Y.; Liu, C.; Yang, M. Corrosion mechanism of Ni deposits on magnets by pulse current electro-deposition. Surf. Coat. Technol. 2021, 409, 126833. [Google Scholar] [CrossRef]
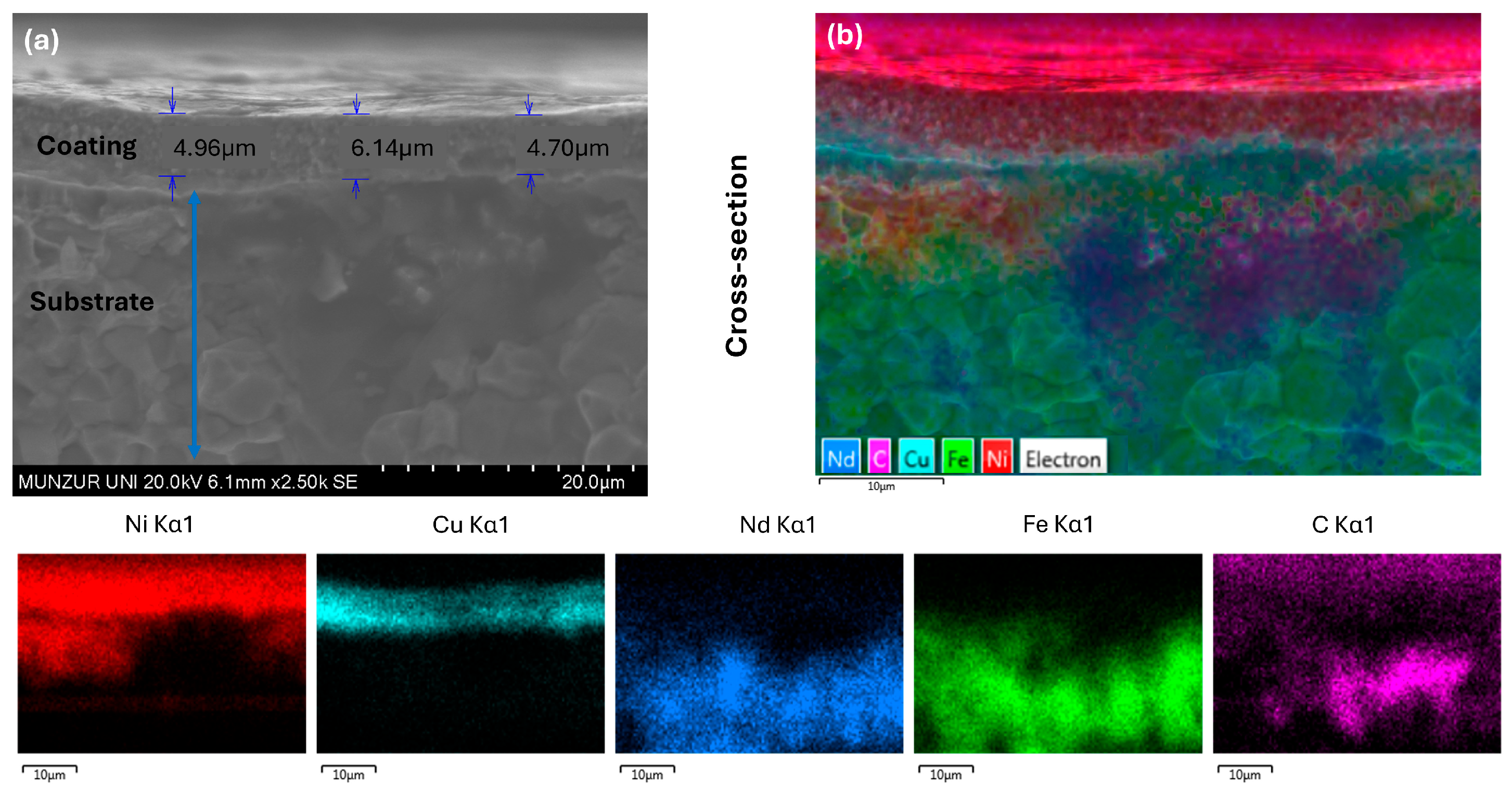
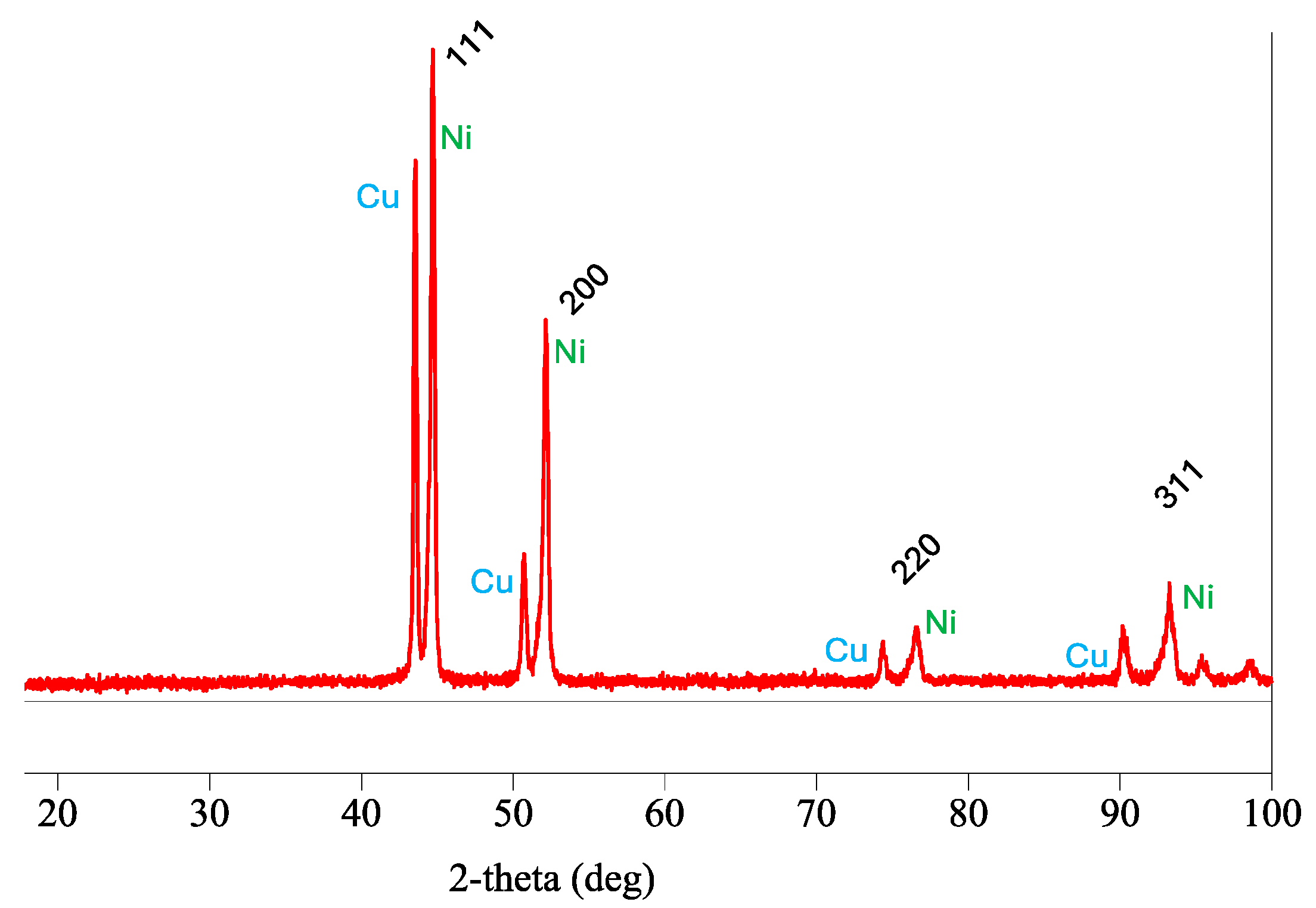
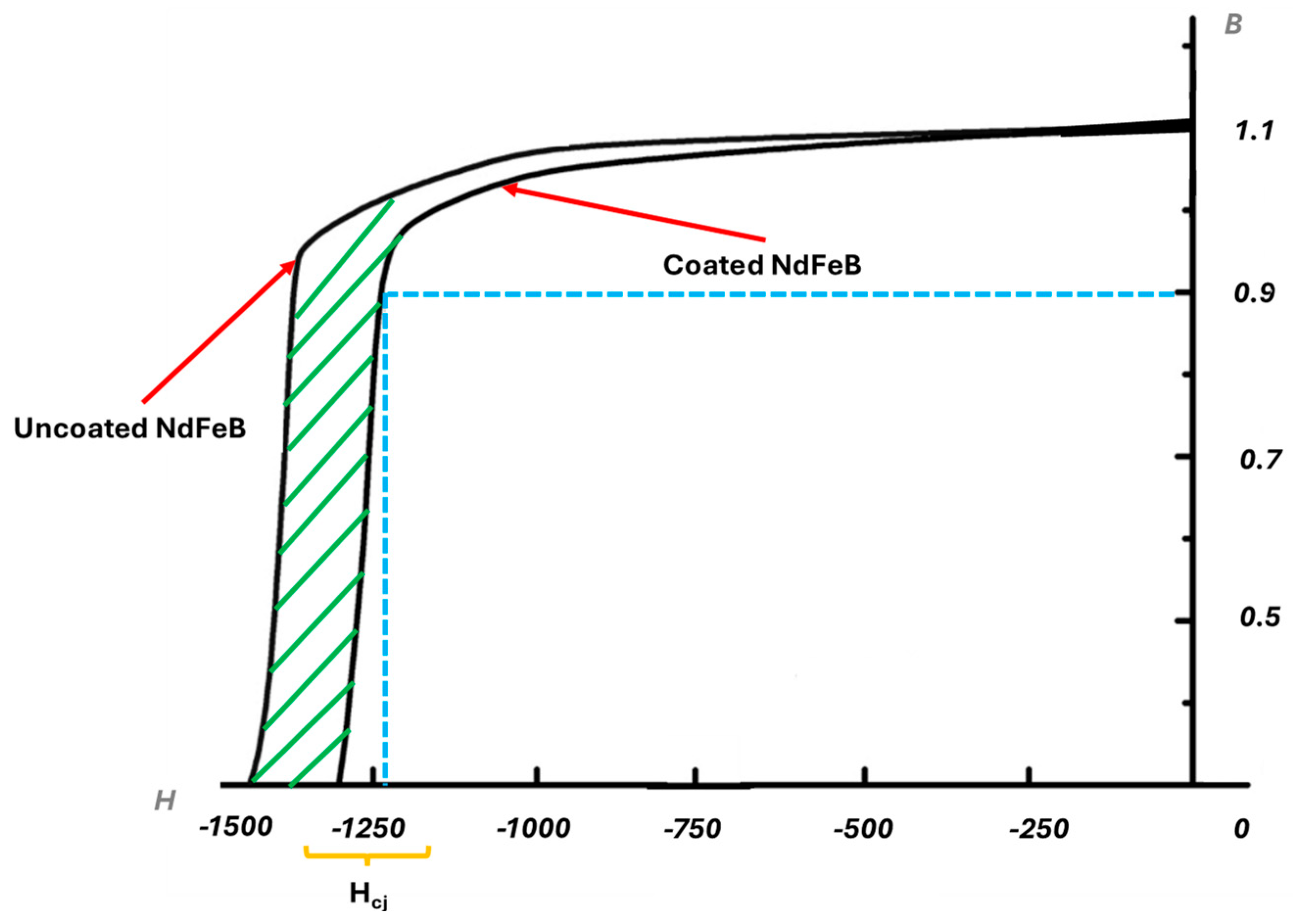
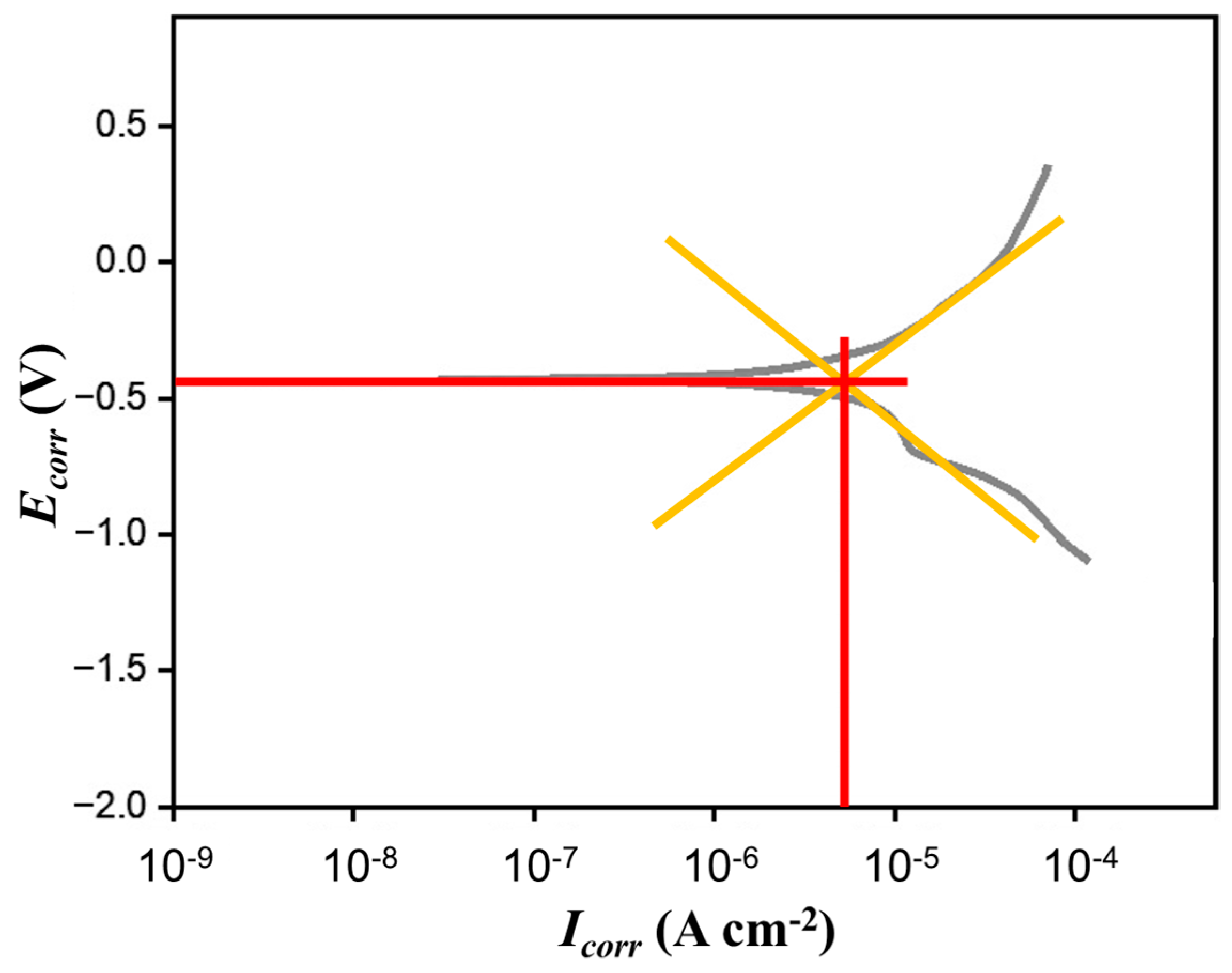
| Composition | Concentration | Operating Parameters | ||
|---|---|---|---|---|
| Ni plating | NiSO4·6H2O | 200 g/L | Temperature | 50 °C |
| NiCl·6H2O | 60 g/L | pH | 4.5 | |
| H3BO3 | 40 g/L | Duty cycle | 50% | |
| C6H5NaO2S | 0.1 g/L | Time | 30 min | |
| C12H25SO4Na | 0.1 g/L | Current density | 1.0 A/dm2 | |
| Cu plating | Cu2P2O7 | 50 g/L | Temperature | 80 °C |
| K4P2O7 | 350 g/L | pH | 10 | |
| NH3·H2O | 0.3 mL/L | Time | 30 min | |
| (BH)max/kJm−3 | Br/mT | Hcj/kAm | |
|---|---|---|---|
| Coated | 48.7 | 5.362 | −1312 |
| Uncoated | 46.4 | 5.298 | −1457 |
| Ecorr (Potential vs. SCE V) | Icorr (A cm−2) | βa | βc |
|---|---|---|---|
| −478 | 1.34 × 10−6 | 0.0964 | 0.5173 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doğan, F. Studying the Magnetic Properties and Corrosion Resistance of Coated NdFeB Magnets. Mater. Proc. 2025, 21, 4. https://doi.org/10.3390/materproc2025021004
Doğan F. Studying the Magnetic Properties and Corrosion Resistance of Coated NdFeB Magnets. Materials Proceedings. 2025; 21(1):4. https://doi.org/10.3390/materproc2025021004
Chicago/Turabian StyleDoğan, Fatih. 2025. "Studying the Magnetic Properties and Corrosion Resistance of Coated NdFeB Magnets" Materials Proceedings 21, no. 1: 4. https://doi.org/10.3390/materproc2025021004
APA StyleDoğan, F. (2025). Studying the Magnetic Properties and Corrosion Resistance of Coated NdFeB Magnets. Materials Proceedings, 21(1), 4. https://doi.org/10.3390/materproc2025021004





