Joint Forces of HR-Spicp-MS and EAF4-MALS for Characterization of Gold Nanorods Conjugated with Synthetic Glycopolymers †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Glycopolymer Conjugated Gold Nanorods
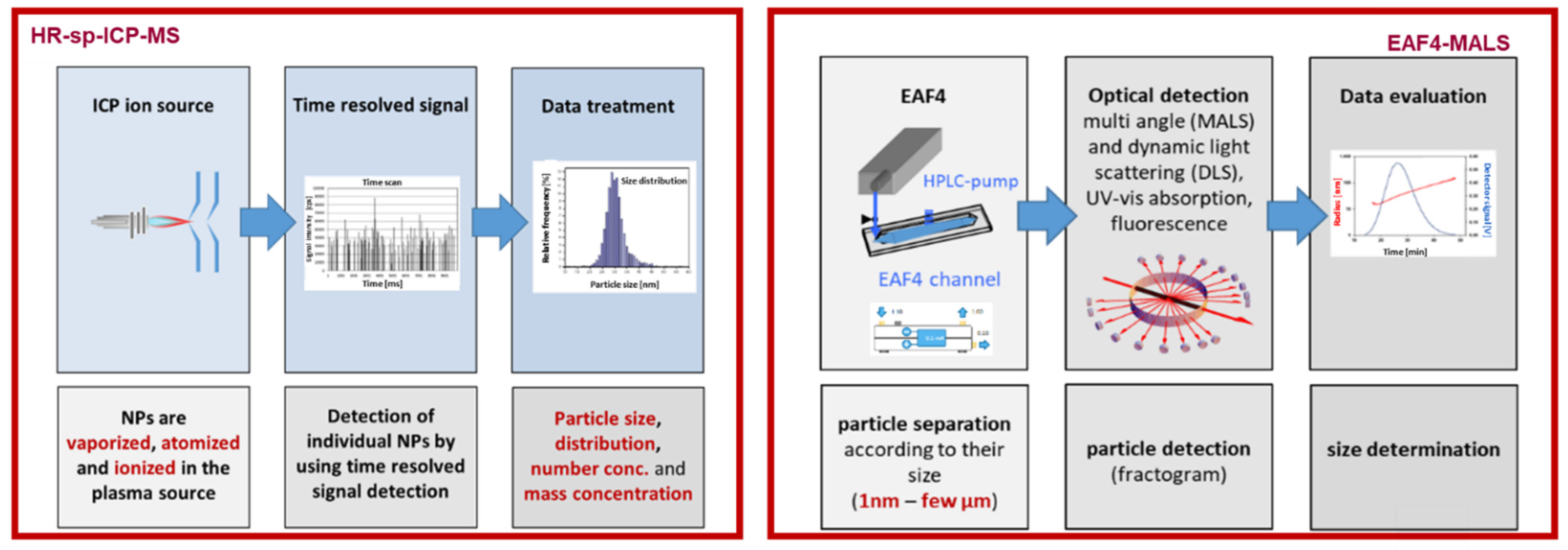
2.2. HR-Spicp-MS
2.3. EAF4-MALS
3. Results
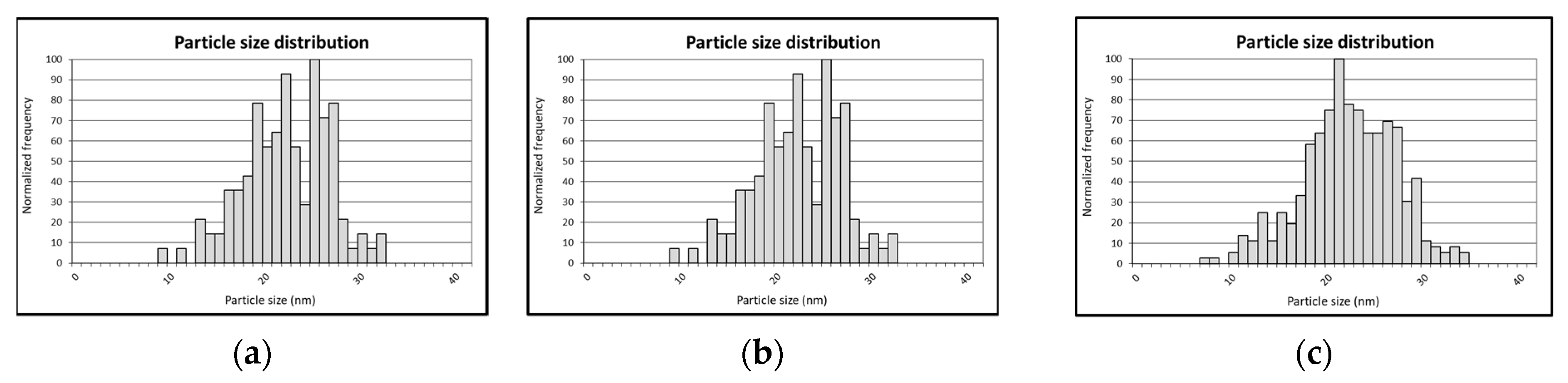
3.1. HR-spICP-MS for Glycoconjugated GNRs Characterization
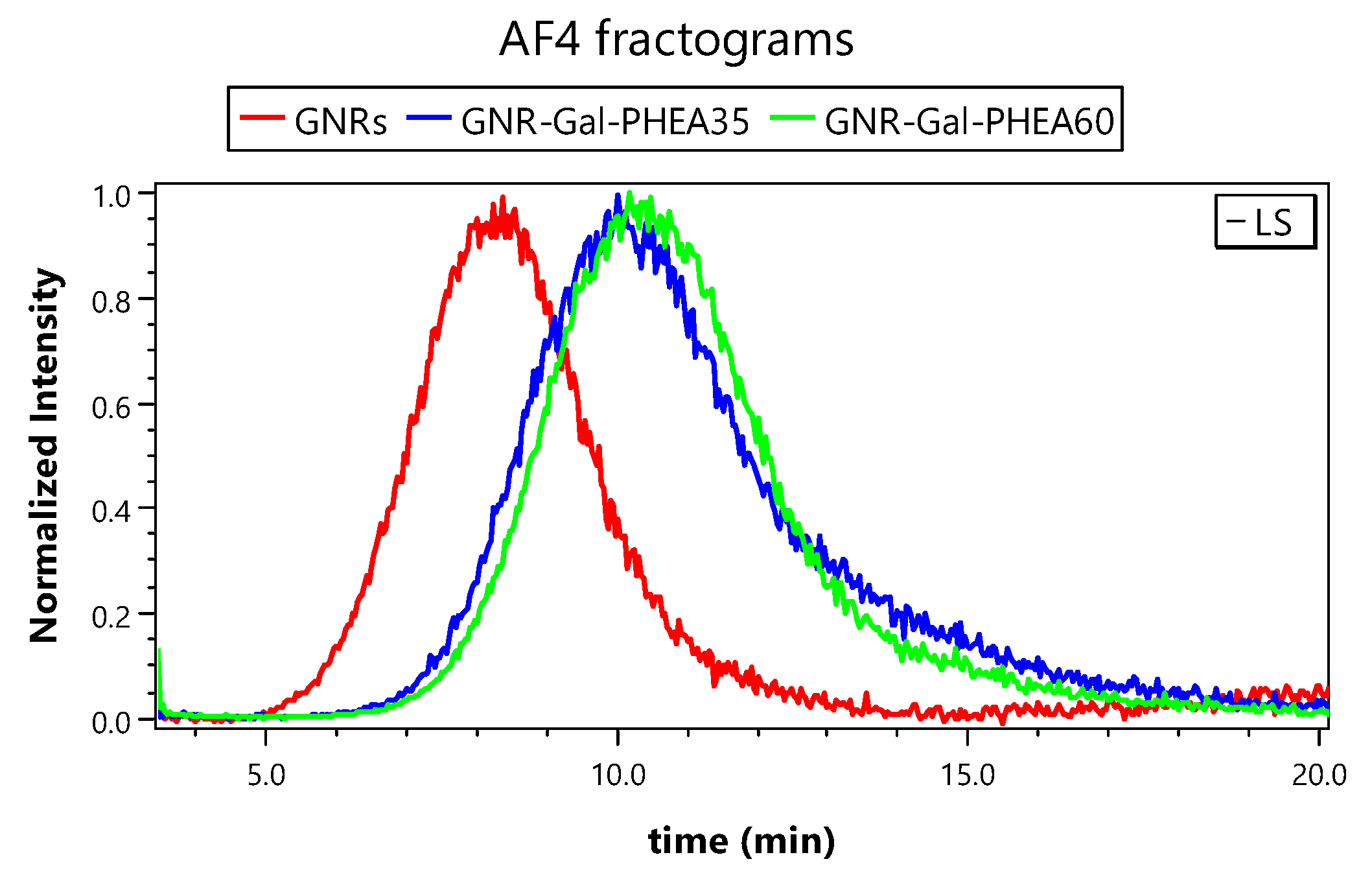
3.2. EAF4-MALS for Glycoconjugated GNRs Characterization
4. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
References
- Curtis, A.S.G.; Wilkinson, C. Nantotechniques and approaches in biotechnology. Trends Biotech. 2001, 19, 97. [Google Scholar] [CrossRef]
- Mülhopt, S.; Diabaté, S.; Dilger, M.; Adelhelm, C.; Anderlohr, C.; Bergfeldt, T.; de la Torre, J.G.; Jiang, Y.; Valsami-Jones, E.; Langevin, D.; et al. Characterization of Nanoparticle Batch-To-Batch Variability. Nanomaterials 2013, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Mancini, M.C.; Nie, S.M. Bioimaging second window for in vivo imaging. Nat. Nanotechnol. 2009, 4, 710. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Yong, T.; Roy, I.; Hu, R.; Ding, H.; Zhao, L.L.; Swihart, M.T.; He, G.S.; Cui, Y.P.; Prasad, P.N. Additive controlled synthesis of gold nanorods (GNRs) for two-photon luminescence imaging of cancer cells. Nanotechnology 2010, 21, 285106. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Gu, M. Surface plasmonic gold nanorods for enhanced two-photon microscopic imaging and apoptosis induction of cancer cells. Biomaterials 2010, 31, 9492. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, E.B.; Dreaden, E.C.; Huang, X.H.; El-Sayed, I.H.; Chu, H.H.; Pushpanketh, S.; McDonald, J.F.; El-Sayed, M.A. Gold nanorod assisted near-infrared plasmonic photothermal therapy (PPTT) of squamous cell carcinoma in mice. Cancer Lett. 2008, 269, 57. [Google Scholar] [CrossRef] [PubMed]
- XHuang, H.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 2006, 128, 2115. [Google Scholar]
- Okuno, T.; Kato, S.; Hatakeyama, Y.; Okajima, J.; Maruyama, S.; Sakamoto, M.; Mori, S.; Kodama, T. Photothermal therapy of tumors in lymph nodes using gold nanorods and near-infrared laser light. J. Control. Release 2013, 172, 879. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.S.; Chang, C.N.; Chang, Y.T.; Yang, M.H.; Chien, Y.H.; Chen, S.J.; Yeh, C.S. Gold nanorods in photodynamic therapy, as hyperthermia agents, and in near-infrared optical imaging. Angew. Chem. Int. Ed. 2010, 49, 2711. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, J.Y.; Wu, X.; Wang, P.N.; Peng, Q.A. Plasmonic gold nanorods can carry sulfonated aluminum phthalocyanine to improve photodynamic detection and therapy of cancers. J. Phys. Chem. B 2010, 114, 17194. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Lin, Y.P.; Wang, C.W.; Tzeng, H.C.; Wu, C.H.; Chen, Y.C.; Chen, C.P.; Chen, L.C.; Wu, Y.C. DNA-gold nanorod conjugates for remote control of localized gene expression by near infrared irradiation. J. Am. Chem. Soc. 2006, 128, 3709. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Barua, S.; Kay, D.B.; Rege, K. Simultaneous enhancement of photothermal stability and gene delivery efficacy of gold nanorods using polyelectrolytes. ACS Nano 2009, 3, 2941. [Google Scholar] [CrossRef] [PubMed]
- Nusz, G.J.; Curry, A.C.; Marinakos, S.M.; Wax, A.; Chilkoti, A. Rational selection of gold nanorod geometry for label-free plasmonic biosensors. ACS Nano. 2009, 3, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Sun, G.M.; Zhang, L.; Tang, Y.J.; Luo, J.J.; Yang, P.H. Multifunctional optical probe based on gold nanorods for detection and identification of cancer cells. Sens. Actuator B Chem. 2014, 191, 741. [Google Scholar] [CrossRef]
- Truong, P.L.; Cao, C.; Park, S.; Kim, M.; Sim, S.J. A new method for non-labeling attomolar detection of diseases based on an individual gold nanorod immunosensor. Lab Chip. 2011, 11, 2591. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, P.G.; Baker, A.N.; Richards, S.J.; Laezza, A.; Walker, M.; Gibson, M. Tuning aggregative versus non-Aggregative lectin binding with glycosylated nanoparticles by the nature of the polymer ligand. J. Mater. Chem. B 2019, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Kálomista, I.; Kéri, A.; Ungor, D.; Csapó, E.; Dékány, I.; Prohaska, T.; Galbács, G. Dimensional characterization of gold nanorods by combining millisecond and microsecond temporal resolution single particle ICP-MS measurements. J. Anal. At. Spectrom. 2017, 32, 2455. [Google Scholar] [CrossRef]
- Johann, C. WP2606: Exosome Characterization with FFF-MALS-DLS.; Wyatt: Dernbach, Germany, 2018. [Google Scholar]
| Parameter | |
|---|---|
| RF power | 1300 W |
| Plasma gas flow rate | 13 L min−1 |
| Carrier gas flow rate | 0.93 L min−1 |
| Measurement mode | TRA |
| Nuclide monitored | 197Au |
| Dwell time | 40 µs |
| Acquisition time | 60 s |
| Nebulizer | MicroMist |
| Spray chamber | Cyclonic |
| GNRs | GNR-Gal-PHEA35 | GNR-Gal-PHEA60 | |
|---|---|---|---|
| Spherical equivalent diameter (nm) | 21.0 ± 0.5 | 21.0 ± 0.4 | 22.0 ± 0.0 |
| GNRs | GNR-Gal-PHEA35 | GNR-Gal-PHEA60 | |
|---|---|---|---|
| Dh (nm) | 17.0 | 21.3 | 22.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velimirovic, M.; Pancaro, A.; Mildner, R.; Georgiou, P.G.; Tirez, K.; Nelissen, I.; Johann, C.; Gibson, M.I.; Vanhaecke, F. Joint Forces of HR-Spicp-MS and EAF4-MALS for Characterization of Gold Nanorods Conjugated with Synthetic Glycopolymers. Mater. Proc. 2021, 4, 93. https://doi.org/10.3390/IOCN2020-07923
Velimirovic M, Pancaro A, Mildner R, Georgiou PG, Tirez K, Nelissen I, Johann C, Gibson MI, Vanhaecke F. Joint Forces of HR-Spicp-MS and EAF4-MALS for Characterization of Gold Nanorods Conjugated with Synthetic Glycopolymers. Materials Proceedings. 2021; 4(1):93. https://doi.org/10.3390/IOCN2020-07923
Chicago/Turabian StyleVelimirovic, Milica, Alessia Pancaro, Robert Mildner, Panagiotis G. Georgiou, Kristof Tirez, Inge Nelissen, Christoph Johann, Matthew I. Gibson, and Frank Vanhaecke. 2021. "Joint Forces of HR-Spicp-MS and EAF4-MALS for Characterization of Gold Nanorods Conjugated with Synthetic Glycopolymers" Materials Proceedings 4, no. 1: 93. https://doi.org/10.3390/IOCN2020-07923
APA StyleVelimirovic, M., Pancaro, A., Mildner, R., Georgiou, P. G., Tirez, K., Nelissen, I., Johann, C., Gibson, M. I., & Vanhaecke, F. (2021). Joint Forces of HR-Spicp-MS and EAF4-MALS for Characterization of Gold Nanorods Conjugated with Synthetic Glycopolymers. Materials Proceedings, 4(1), 93. https://doi.org/10.3390/IOCN2020-07923








