Towards Sustainable Inorganic Polymers: Production and Use of Alternative Activator †
Abstract
:1. Introduction
2. Materials and Methods
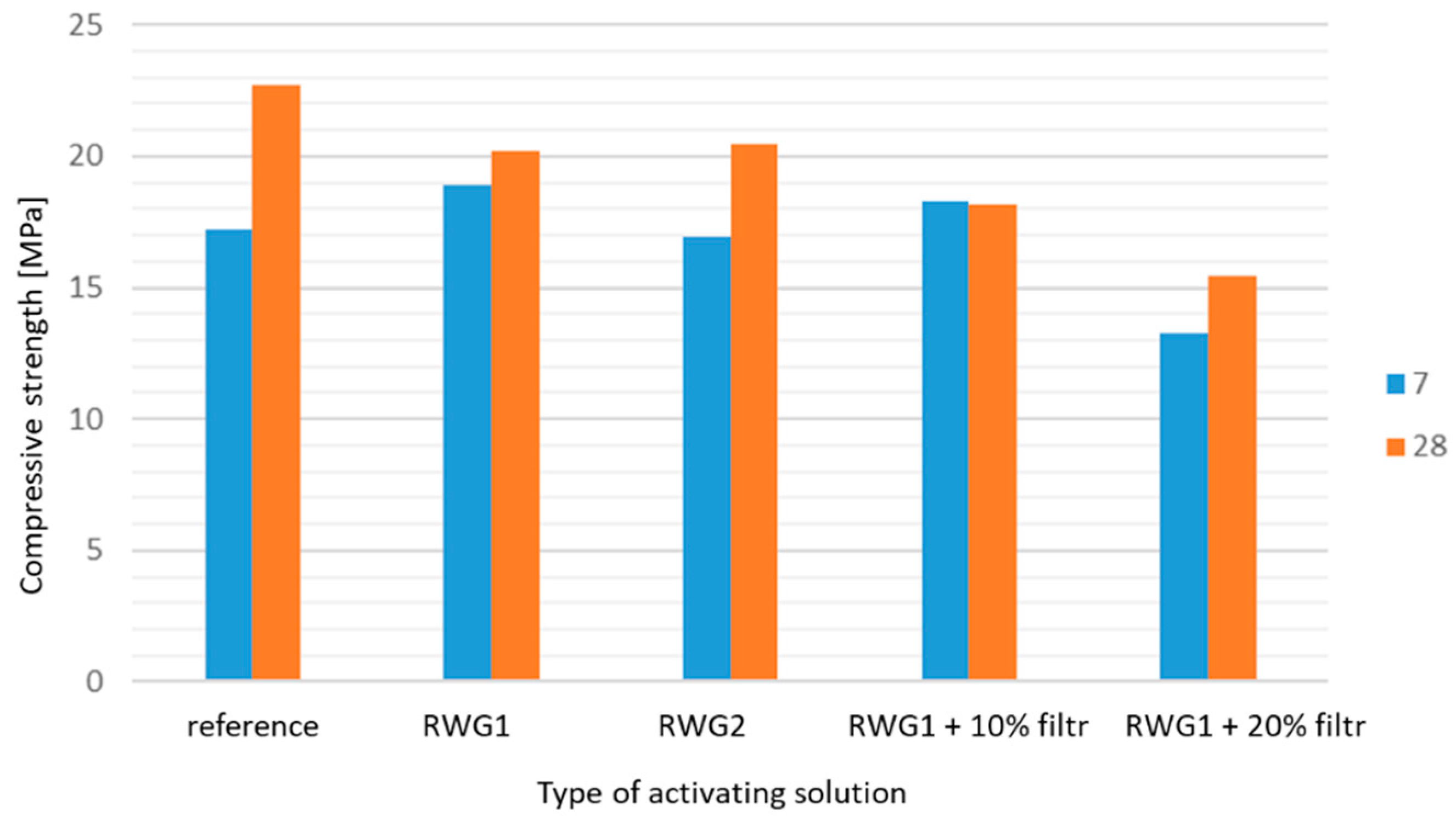
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witherspoon, R.; Wang, H.; Aravinthan, T.; Omar, T. Energy and emissions analysis of fly ash based geopolymers. In Proceedings of the SSEE 2009: Solutions for a Sustainable Planet, Barton, ACT, Australia, 1 January 2009; pp. 311–321. [Google Scholar]
- Peys, A. Inorganic Polymers from CaO-FeO-SiO2 Slags. Ph.D. Thesis, KU Leuven, Leuven, Belgium, 2018; p. 206. [Google Scholar]
- Tchakouté, H.K.; Rüscher, C.H.; Kong, S.; Kamseu, E.; Leonelli, C. Geopolymer binders from metakaolin using sodium waterglass from waste glass and rice husk ash as alternative activators: A comparative study. Constr. Build. Mater. 2016, 114, 276–289. [Google Scholar] [CrossRef]
- Fawer, M.; Concannon, M.; Rieber, W. Life Cycle Inventories for the Production of Sodium Silicates. Int. J. Life Cycle Assess. 1999, 4, 207–2012. [Google Scholar] [CrossRef] [Green Version]
- Peys, A.; Rahier, H.; Pontikes, Y. Potassium-rich biomass ashes as activators in metakaolin-based inorganic polymers. Appl. Clay Sci. 2016, 119, 401–409. [Google Scholar] [CrossRef]
- Puertas, F.; Torres-Carrasco, M. Use of glass waste as an activator in the preparation of alkali-activated slag. Mechanical strength and paste characterisation. Cem. Concr. Res. 2014, 57, 95–104. [Google Scholar] [CrossRef]
- Bouchikhi, A.; Mamindy-Pajany, Y.; Maherzi, W.; Albert-Mercier, C.; El-Moueden, H.; Benzerzour, M.; Peys, A.; Abriak, N.-E. Use of residual waste glass in an alkali-activated binder—Structural characterization, environmental leaching behavior and comparison of reactivity. J. Build. Eng. 2021, 34, 101903. [Google Scholar] [CrossRef]
- van de Sande, J.; Kriskova, L.; Pontikes, Y.; Rahier, H. Fire-resistant inorganic polymer foams made from Fe-rich slags. In Proceedings of the 7th International Slag Valorisation Symposium, Virtual, 27–29 April 2021; pp. 250–255. [Google Scholar]
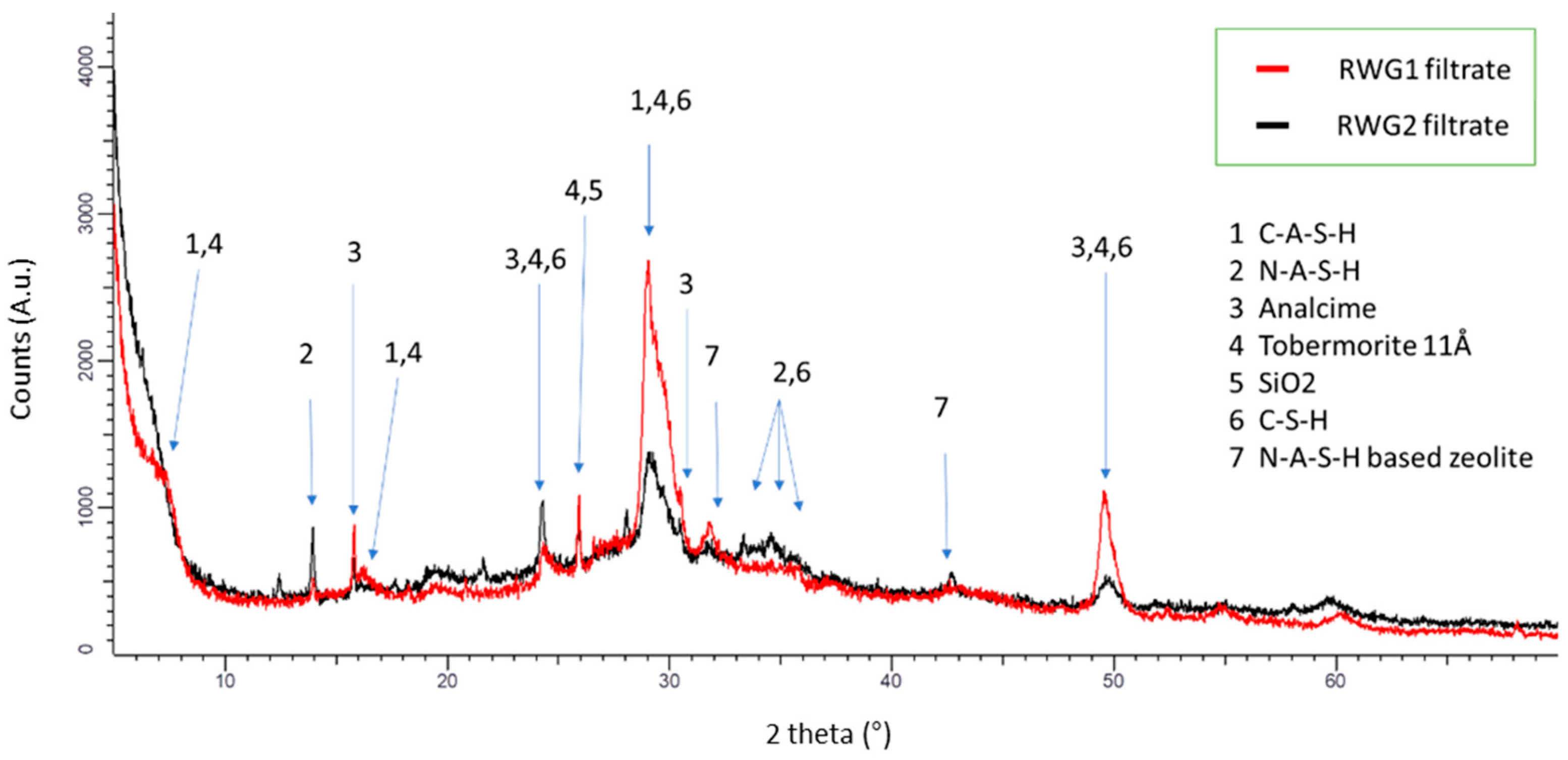
| RWG1 | RWG1 Filtrate | RWG2 | RWG2 Filtrate | |
|---|---|---|---|---|
| SiO2 | 62 | 51 | 58 | 46 |
| CaO | 15 | 27 | 8 | 8 |
| Na2O | 12 | 5 | 13 | 23 |
| Al2O3 | 3 | 7 | 6 | 9 |
| MgO | 2 | 3 | 2 | 2 |
| SO3 | 1 | 1 | <1 | <1 |
| Fe2O3 | 1 | 1 | 7 | 6 |
| K2O | 1 | <1 | 2 | 2 |
| MnO | - | - | 3 | 3 |
| others | 3 | 4 | 1 | 1 |
| RWG1 | RWG2 | |||||
|---|---|---|---|---|---|---|
| Achieved Molarity | Solid Content wt% | Dissolved RWG wt% | Achieved Molarity | Solid Content wt% | Dissolved RWG wt% | |
| Time | 16.5% NaOH (aq), 160 °C | |||||
| 2 h | 1.16 | 23 | 45 | |||
| 3 h | 1.25 | 29 | 50 | |||
| 5 h | 1.25 | 26 | 51 | |||
| NaOH (aq) | 205 °C, 3.5 h | 140 °C, 3 h | ||||
| 10% | 1.48 | 18 | 1.47 | 18 | ||
| ~15% | 1.21 | 24 | 1.21 | 26 | ||
| ~20% | 0.93 | 28 | 1.02 | 27 | ||
| Temperature | 16.5% NaOH (aq), 3.5 h | 16.5% NaOH (aq), 3 h | ||||
| 140 °C | 1.18 | 25 | 47 | 1.21 | 25.8 | 43 |
| 160 °C | 1.27 | 26 | 51 | 1.20 | 24.7 | - |
| 180 °C | 1.23 | 26 | 51 | - | - | - |
| 205 °C | 1.19 | 22.5 | 45 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kriskova, L.; Tatsis, E.; Afsar, M.; Delaere, D.; Pontikes, Y. Towards Sustainable Inorganic Polymers: Production and Use of Alternative Activator. Mater. Proc. 2021, 5, 127. https://doi.org/10.3390/materproc2021005127
Kriskova L, Tatsis E, Afsar M, Delaere D, Pontikes Y. Towards Sustainable Inorganic Polymers: Production and Use of Alternative Activator. Materials Proceedings. 2021; 5(1):127. https://doi.org/10.3390/materproc2021005127
Chicago/Turabian StyleKriskova, Lubica, Efthymios Tatsis, Muhammad Afsar, David Delaere, and Yiannis Pontikes. 2021. "Towards Sustainable Inorganic Polymers: Production and Use of Alternative Activator" Materials Proceedings 5, no. 1: 127. https://doi.org/10.3390/materproc2021005127
APA StyleKriskova, L., Tatsis, E., Afsar, M., Delaere, D., & Pontikes, Y. (2021). Towards Sustainable Inorganic Polymers: Production and Use of Alternative Activator. Materials Proceedings, 5(1), 127. https://doi.org/10.3390/materproc2021005127






