1. Introduction
Tungsten (W) is a rare metal with a wide range of applications such as rocket engines, aircrafts, magnetic trains, cutting tools, and pacemakers. There are two economically relevant tungsten mineral ores: wolframite, (Fe,Mn)WO4, and scheelite (CaWO4). Compared to other metals, the global reserves of tungsten are very low (7 million t), and mostly located in China, which accounts for 80% of the 81,100 t global production in 2018. In the same year, EU production was 3200 t (4% of global production), while its consumption was estimated at about 10,000 t per year. Due to the high demand and scarcity of this metal in the EU, tungsten has been included among the Critical Raw Materials (CRM) since 2011.
A strong research effort is being dedicated to reduce the dependence on external countries. In practice, tungsten grade is expressed as %WO3. Naturally occurring scheelite and wolframite grades are very low (0.5% WO3) and they have to be upgraded for further W extraction. Scheelite ores are upgraded by gravity/flotation techniques, and wolframite ores by gravity/magnetic separation, due to their paramagnetic properties. By these technologies, the tungsten grade is increased from 0.5% (as WO3) to 65–70% (as WO3). Tungsten is then produced from concentrates in five steps: (i) Decomposition of tungstates by acid leaching (to H2WO4), caustic leaching, or alkali roasting (to Na2WO4), (ii) Digestion of H2WO4 in aqueous ammonia or Na2WO4 in water, (iii) Precipitation of tungsten as ammonium paratungstate, (iv) Calcination to WO3, and (v) WO3 reduction to tungsten in hydrogen. The first step requires toxic acids (HCl/H2SO4) or high temperatures (Na2CO3 roasting at 800–900 °C).
In this study, Deep Eutectic Solvents (DES) were proposed as an environmentally friendly leaching alternative for the extraction of the tungsten from the concentrate material. Tungsten can be later recovered from the DES leachate by a liquid-liquid extraction process. This work is focused in the DES leaching step.
2. Materials and Methods
2.1. Materials
High-grade scheelite concentrate (57% W) from the Barruecopardo mine (Spain), supplied by the mining company Saloro, was used for the experimental tests. The material is composed mainly of calcium tungstate (scheelite) in 99%, with some impurities.
For digestion and analysis of solid samples, 65% ExpertQ® HNO3, and 37% ExpertQ® HCl supplied by Scharlab (Spain), and 48% ExpertQ® HF were used.
For preparing the DES, choline chloride ≥98% was supplied by Merck, and oxalic acid dihydrate 99% for analysis ExpertQ® was supplied by Scharlab. The rest of the reactives were ethylene glycol ReagentPlus ≥99%, lactic acid 85% FCC, citric acid ACS reagent ≥99.5, malic acid ≥95%, malonic acid ReagentPlus 99%, formic acid reagent grade ≥95%, and levulinic acid 98%, supplied by Merck.
2.2. Methods
2.2.1. DES Preparation
The DES were prepared by making a homogenous mixture of their components in a flask. The mixture was then heated up to a temperature above the melting point of the DES (used preferably 80 °C) under mild stirring (200 rpm). Then, the DES was cooled to ambient temperature.
2.2.2. Leaching Reaction
First, high-grade scheelite concentrate (57% W) was weighed in a flask (0.25–0.5 g). DES was added to the solid in the liquid state (5 g). The flask was closed and kept under stirring (300 rpm) at the required leaching temperature and time. At the end of the reaction, a leachate sample was taken for analysis.
To perform kinetic studies, 100 g of DES was heated to the leaching temperature under stirring (700 rpm). High-grade scheelite concentrate (57% W) was added to DES at once. Leachate samples were taken from leaching media at different times.
Leachate samples were centrifuged at 15,000× g rpm for 1 min to remove any trace of solid. The liquid leachate was weighed (approx. 0.5 g) in a 25 mL volumetric flask and diluted with HNO3 1% w/v.
2.2.3. Characterization
All leachates were characterized by Inductively Coupled Plasma Mass Spectrometry (ICP-MS) using a spectrophotometer mod. 7900 of Agilent. W was measured in the mass 182W, using 205Tl as internal standard. A calibration line was previously built from known standards made from a certified standard of CPAchem (W 1000 mg L−1), in a concentration range (5 µg L−1 to 10 mg L−1) where a linear correlation exists between the signal intensity and the concentration.
For solid materials, microwave-assisted acid digestion was carried out to dissolve. A representative sample of approximately 0.1 g was digested in 4 mL of concentrated nitric acid, 4 mL hydrochloric acid, and 2 mL hydrofluoric acid using microwave heating. Both sample and acid mixture were placed in inert PTFE-sealed microwave vessels. The temperature profile was set to reach 220 ± 10 °C in 20 min and to remain at 220 ± 10 °C for 15 min. After cooling, the vessel content was diluted to 50 mL, and analyzed by ICP-MS.
3. Results and Discussion
3.1. DES Screening
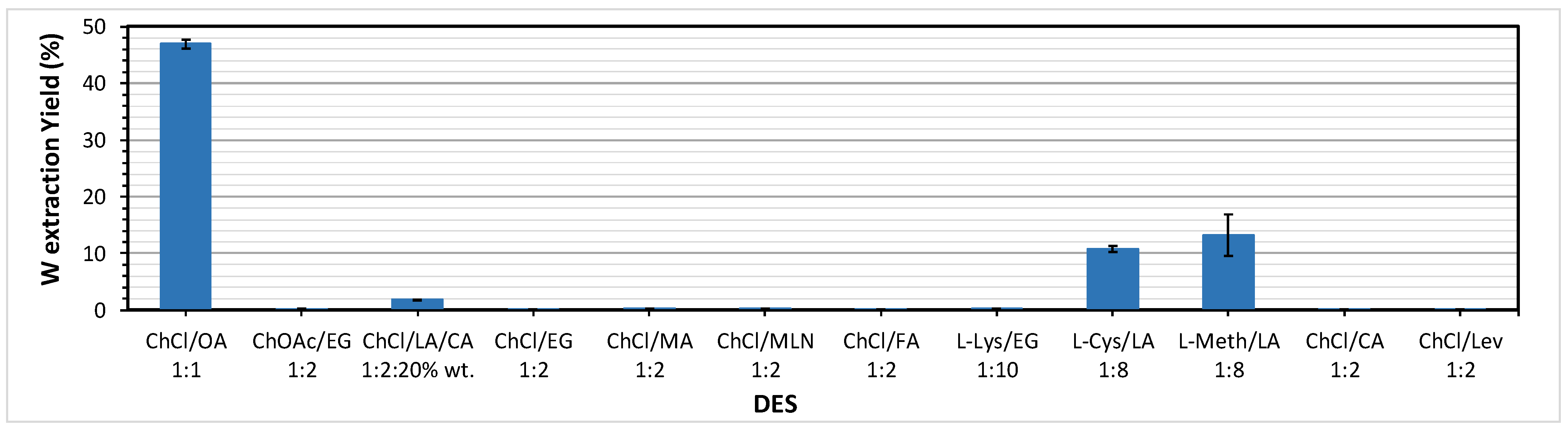
In order to select the best DES formulation for W leaching from scheelite concentrate, a first screening was performed to evaluate the W leaching performance of different DES.
Table 1 shows a relation of the DES tested.
As seen in
Figure 1, a DES based on choline chloride and oxalic acid in an equimolar ratio showed the best W leaching results (46.9% W ext. yield). The rest of DES showed very low or no capacity to leach W. The maximum yield (46.9%) is still too low to justify a process based on DES leaching technology. The next experiments were focused on the optimization of the leaching variables for ChCl/OA-based DES.
3.2. Optimization of Variables
ChCl/OA ratio, L/S ratio, temperature, and time were optimized in order to achieve higher W extraction yields.
3.2.1. ChCl/OA Ratio
In the same conditions as in the screening tests (T 80 °C, L/S 10, leaching time 24 h), the ChCl/OA ratio was studied. The results are shown in
Figure 2. The W extraction yields increased significantly (to 70%) for ChCl/OA 1:2, when the oxalic acid concentration was higher in the DES.
3.2.2. Temperature
Leaching experiments at two temperatures (60 °C and 80 °C) were performed for the three DES studied in
Section 3.2.1. Results are shown in
Figure 3.
An increase in W extraction yield (%) was expected with temperature. However, this was only observed for the DES with lower OA concentration (ChCl/OA 2:1). For this DES, the W extraction yield was very low (13%) at 60 °C due to its low OA concentration. An increase in temperature from 60 to 80 °C led to a higher W extraction yield (45%).
However, this enhancement does not occur for higher OA contents (ChCl/OA 1:1 and 1:2). This behavior can be explained by the fact that W leaching is governed by the equilibrium reaction (1):
For DES ChCl/OA 1:1 and 1:2, equilibrium was reached after 24 h. The equilibrium is described by Equation (1), where the precipitation of calcium oxalate favors CaWO4 decomposition to form calcium oxalate and tungstic acid. As the calcium oxalate precipitation is promoted at lower temperatures, the equilibrium is shifted to the right and higher W extraction yields in the equilibrium may be obtained at lower temperatures.
3.2.3. L/S Ratio
Leaching experiments at different L/S Ratio were performed for two DES, ChCl/OA 2:1 and 1:2. The rest of variables were kept constant (T 60 °C, time 24 h). With only for DES ChCl/OA 1:2, the W extraction yield at L/S 20 was increased to 95% at 24 h (
Figure 4).
Surprisingly, W extraction yield did not increase with L/S for DES ChCl/OA 2:1. The hypothesis is that, at this high ChCl/OA ratio, an excess of choline chloride molecules surrounds oxalic acid, interacting through H-bonds, and makes them less available to react with calcium tungstate. Both effects together, lower presence of calcium tungstate to be leached (due to lower L/S) and lower availability of oxalic acid for leaching, would be responsible of the lower W extraction yield observed for this DES.
3.2.4. Time
The effect of time was studied for both L/S ratios (10 and 20) until 24 h (
Figure 5). The objective was to achieve high W extraction yields (70% or higher) at the lowest time possible. The rate of reaction is higher for the higher L/S ratio as expected. It is possible to achieve 70% W extraction yield after 10 h of leaching at L/S 20.
4. Conclusions
DES based on choline chloride and oxalic acid are a good alternative to the use of strong acid and alkalis and high temperatures in current W leaching processes from concentrates. Both choline chloride (chicken food) and oxalic acid are environmentally friendly products.
The results of this paper show that very high W extraction yields (95%) are obtained under certain experimental conditions (ChCl/OA ratio 1:2, T 60 °C, L/S 20 for 24 h). Furthermore, quite good results (70% yield) are achieved using more moderate values for some of the operating conditions (ChCl/OA ratio 1:2, T 60 °C and L/S 10 for 24 h or L/S 20 for 10 h). For further scale-up activities, a techno-economic feasibility analysis is required to select the most adequate leaching conditions that allow the best performance of the process considering both W extraction yields, and consequently production and the cost associated.
It is also worth mentioning that, according to the experience gained, it is foreseen that the leaching times required to obtain high extraction yields will be significatively reduced for lower-grade concentrates, working in the same conditions for the rest of the parameters. Thus, tungsten recovery from lowest-grade fractions (tailings, mine wastes WO3 < 1%), which are currently stockpiled in mines as residues, could also be a source for tungsten using this DES-based promising technology.