Reduction of Heavy Hydrocarbons from Oilfield Produced Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Analysis on Site of Fresh Samples
2.3. Laboratory Analysis of Chemical Composition
2.3.1. Determining Macro- and Meso-components
2.3.2. Determining Specific Parameters
2.4. Oilfield Produced Water Treatment
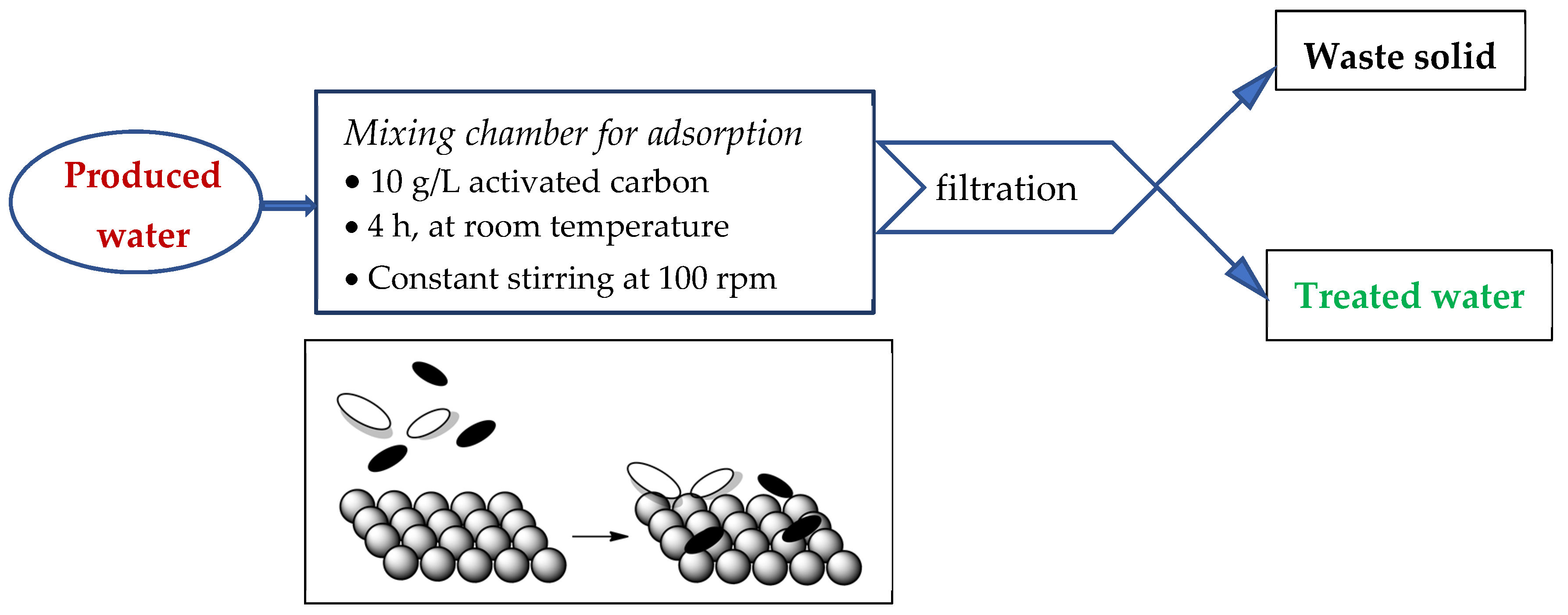
2.4.1. Adsorption
2.4.2. Chemical Oxidation
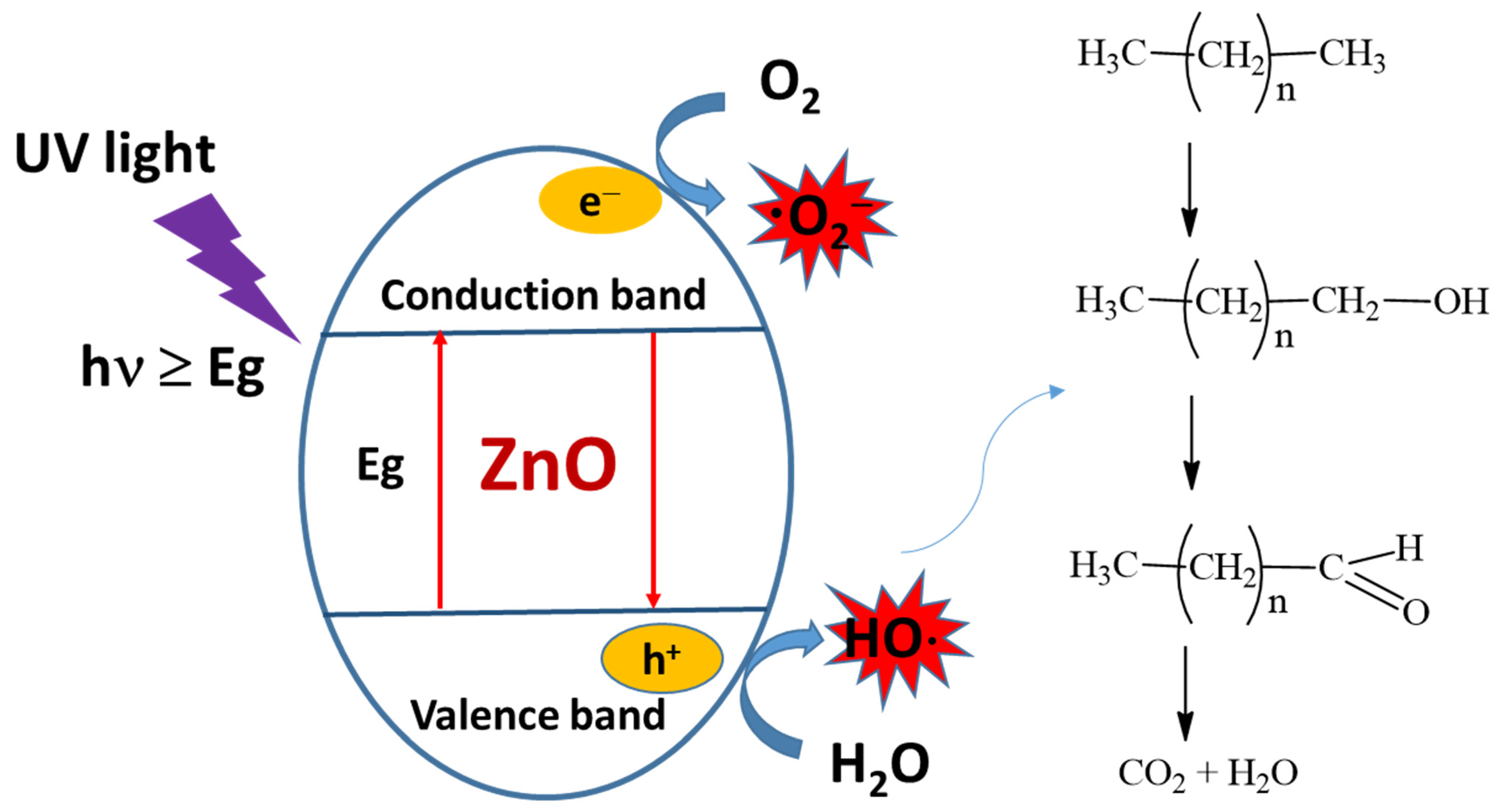
2.4.3. Photocatalysis
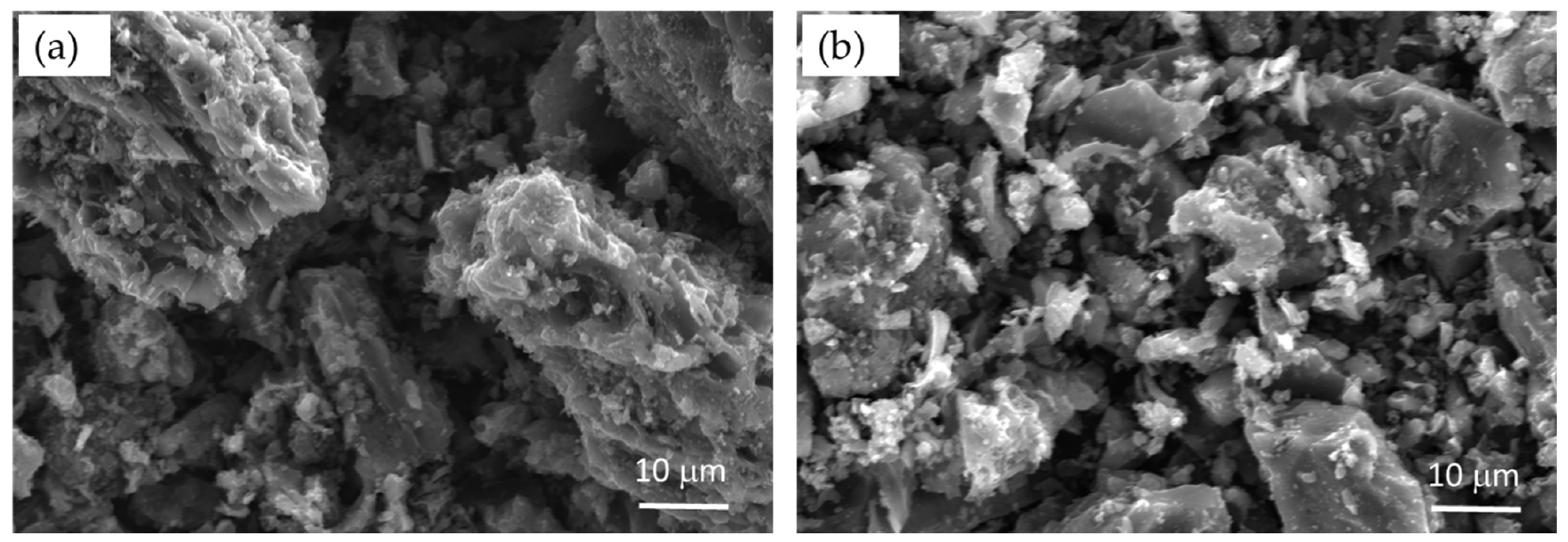
2.5. Materials’ Characterization
3. Results and Discussion
3.1. Produced Water Characterization
3.2. Produced Water Treatment
3.2.1. Adsorption on Activated Charcoal
3.2.2. Chemical Oxidation with Ca(ClO)2
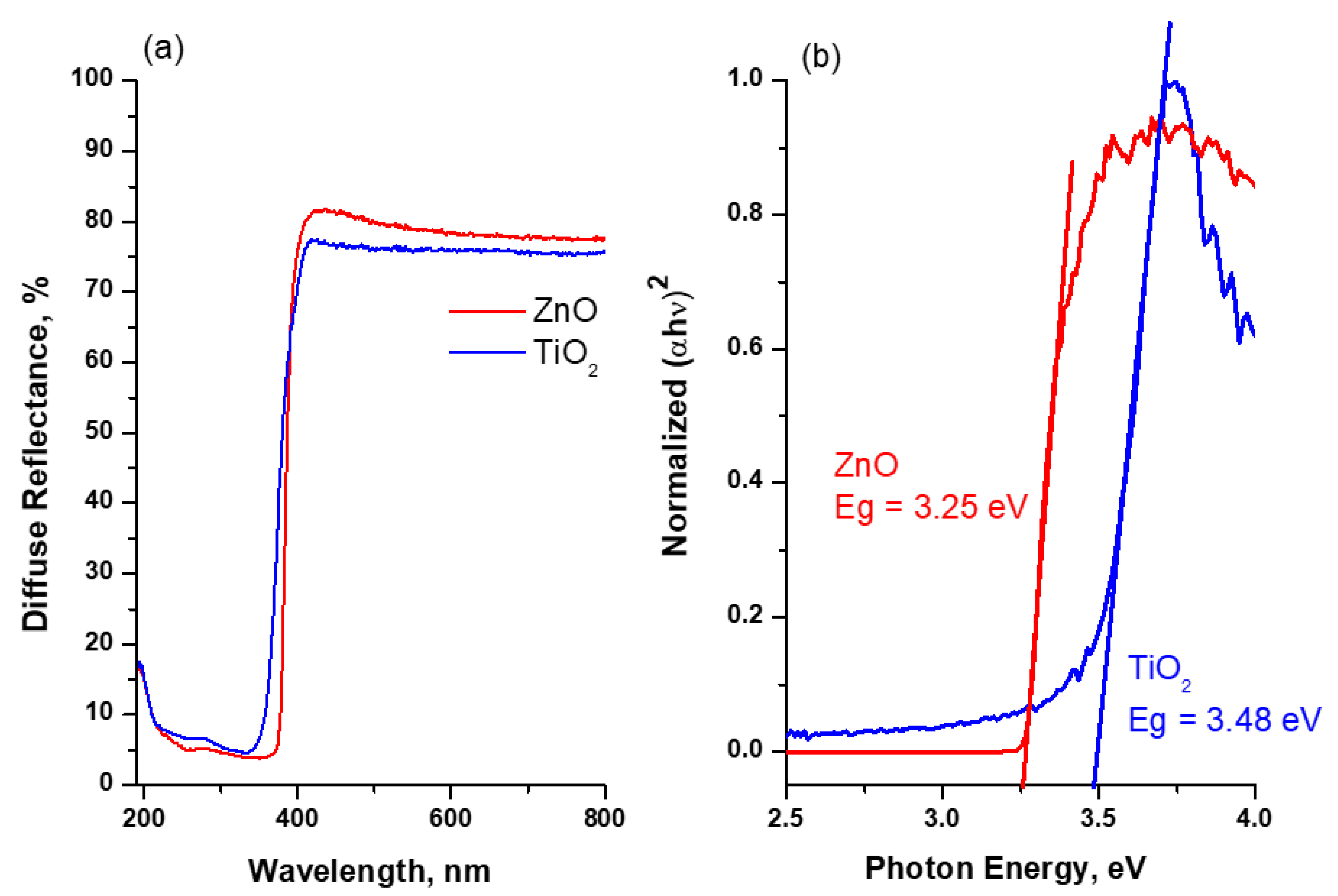
3.2.3. Photocatalysis with TiO2 and ZnO
Characterization of Photocatalysts
Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanchez, B. Fossil Fuels Account for the Largest Share of U.S. Energy Production and Consumption. Available online: https://www.eia.gov/todayinenergy/detail.php?id=45096 (accessed on 10 November 2021).
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef] [PubMed]
- Olajire, A.A. Recent advances on the treatment technology of oil and gas produced water for sustainable energy industry-mechanistic aspects and process chemistry perspectives. Chem. Eng. J. Adv. 2020, 4, 100049. [Google Scholar] [CrossRef]
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low-Carbon Technol. 2014, 9, 157–177. [Google Scholar] [CrossRef] [Green Version]
- Nasiri, M.; Jafari, I. Produced water from oil-gas plants: A short review on challenges and opportunities. Period. Polytech. Chem. Eng. 2017, 61, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Wang, X.; Shan, B.; Li, X.; Wang, W. Analysis of chemical compositions contributable to chemical oxygen demand (COD) of oilfield produced water. Chemosphere 2006, 62, 322–331. [Google Scholar] [CrossRef]
- Hu, G.; Li, J.; Zeng, G. Recent development in the treatment of oily sludge from petroleum industry: A review. J. Hazard. Mater. 2013, 261, 470–490. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Lo, W.H.; Chan, G.Y. Physico-chemical treatments for removal of recalcitrant contaminants from landfill leachate. J. Hazard. Mater. 2006, 129, 80–100. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Alhaija, M.A.; Al-Zuhair, S. Evaluation of a three-step process for the treatment of petroleum refinery wastewater. J. Environ. Chem. Eng. 2014, 2, 56–62. [Google Scholar] [CrossRef]
- Golestanbagh, M.; Parvini, M.; Pendashteh, A. Integrated systems for oilfield produced water treatment: The state of the art. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 3404–3411. [Google Scholar] [CrossRef]
- Dai, X.; Fang, J.; Li, L.; Dong, Y.; Zhang, J. Enhancement of COD removal from oilfield produced wastewater by combination of advanced oxidation, adsorption and ultrafiltration. Int. J. Environ. Res. Public Health 2019, 16, 3223. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Guo, S.; Li, F. Treatment of oilfield produced water by anaerobic process coupled with micro-electrolysis. J. Environ. Sci. 2010, 22, 1875–1882. [Google Scholar] [CrossRef]
- Dwyer, B.P.; McDonald, F. Treatment of Oil & Gas Produced Water; Sandia National Laboratories: Livermore, CA, USA, 2016. [Google Scholar]
- Yu, L.; Han, M.; He, F. A review of treating oily wastewater. Arab. J. Chem. 2017, 10, S1913–S1922. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Wei, L.; Guo, X.; Guo, S.; Yan, G. Investigation of heavy oil refinery wastewater treatment by integrated ozone and activated carbon-supported manganese oxides. Fuel Process. Technol. 2014, 124, 165–173. [Google Scholar] [CrossRef]
- Wenhu, Z.; Dejin, W.; Ruoyu, F.; Feng, L. Studies on affecting factors and mechanism of oily wastewater by wet hydrogen peroxide oxidation. Arab. J. Chem. 2017, 10, S2402–S2405. [Google Scholar] [CrossRef] [Green Version]
- Stepnowski, P.; Siedlecka, E.M.; Behrend, P.; Jastorff, B. Enhanced photo-degradation of contaminants in petroleum refinery wastewater. Water Res. 2002, 36, 2167–2172. [Google Scholar] [CrossRef]
- Shokrollahzadeh, S.; Golmohammad, F.; Naseri, N.; Shokouhi, H.; Arman-mehr, M. Chemical oxidation for removal of hydrocarbons from gas–field produced water. Proced. Eng. 2012, 42, 942–947. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.J.; Kim, B.H.; Hong, J.H.; Pyo, H.S.; Park, S.J.; Lee, D.W. A study on the distribution of chlorination by-products (CBPs) in treated water in Korea. Water Res. 2001, 35, 2861–2872. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Grzechulska, J.; Hamerski, M.; Morawski, A.W. Photocatalytic decomposition of oil in water. Water Res. 2000, 34, 1638–1644. [Google Scholar] [CrossRef]
- Saien, J.; Shahrezaei, F. Organic pollutants removal from petroleum refinery wastewater with nanotitania photocatalyst and UV light emission. Int. J. Photoenergy 2012, 2012, 703074. [Google Scholar] [CrossRef]
- Azevedo, E.B.; Tôrres, A.R.; Aquino Neto, F.R.; Dezotti, M. TiO2-photocatalyzed degradation of phenol in saline media in an annular reactor: Hydrodynamics, lumped kinetics, intermediates, and acute toxicity. Braz. J. Chem. Eng. 2009, 26, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Bessa, E.; Sant’Anna, G.L., Jr.; Dezotti, M. Photocatalytic/H2O2 treatment of oil field produced waters. Appl. Catal. B Environ. 2001, 29, 125–134. [Google Scholar] [CrossRef]
- Soltanian, G.R.; Havaee Behbahani, M. The effect of metal oxides on the refinery effluent treatment. Iran. J. Environ. Health Sci. Eng. 2011, 8, 169–174. [Google Scholar]
- Tetteh, E.K.; Rathilal, S.; Naidoo, D.B. Photocatalytic degradation of oily waste and phenol from a local South Africa oil refinery wastewater using response methodology. Sci. Rep. 2020, 10, 8850. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.Z.; Najeeb, I.; Tuiyebayeva, M.; Makhtayeva, Z. Refinery wastewater degradation with titanium dioxide, zinc oxide, and hydrogen peroxide in a photocatalytic reactor. Process Saf. Environ. Prot. 2015, 94, 479–486. [Google Scholar] [CrossRef]
- Santos, F.V.; Azevedo, E.B.; Sant’Anna, G.L., Jr.; Dezotti, M. Photocatalysis as a tertiary treatment for petroleum refinery wastewaters. Braz. J. Chem. Eng. 2006, 23, 451–460. [Google Scholar] [CrossRef]
- Dionysiou, D.D.; Suidan, M.T.; Baudin, I.; Laîné, J.M. Effect of hydrogen peroxide on the destruction of organic contaminants-synergism and inhibition in a continuous-mode photocatalytic reactor. Appl. Catal. B Environ. 2004, 50, 259–269. [Google Scholar] [CrossRef]
- Hassan, A.A.; Naeema, H.T.; Hadi, R.T. Degradation of oily waste water in aqueous phase using solar (ZnO, TiO2 and Al2O3) catalysts. Pak. J. Biotechnol. 2018, 15, 909–916. [Google Scholar]
- Ali, D.; Hosein, G.M. Photocatalytic degradation of kermanshah refinery wastewater using nano-TiO2 supported on bentonite. World Appl. Sci. J. 2012, 19, 874–879. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Bahrami, M. Investigation of the photocatalytic activity of supported ZnO–TiO2 on clinoptilolite nano-particles towards photodegradation of wastewater-contained phenol. Desalin. Water Treat. 2015, 55, 1096–1104. [Google Scholar] [CrossRef]
- Aljuboury, D.A.; Palaniandy, P.; Abdul Aziz, H.B.; Feroz, S.; Abu Amr, S.S. Evaluating photo-degradation of COD and TOC in petroleum refinery wastewater by using TiO2/ZnO photo-catalyst. Water Sci. Technol. 2016, 74, 1312–1325. [Google Scholar] [CrossRef] [PubMed]
- Alkhazraji, H.A.J.; Alatabe, M.J.A. Oil recovery from oilfield produced water using zinc oxide nano particle as catalyst in batch and continuous system. J. Ecol. Eng. 2021, 22, 278–286. [Google Scholar] [CrossRef]
- Al-Sabahi, J.; Bora, T.; Al-Abri, M.; Dutta, J. Efficient visible light photocatalysis of benzene, toluene, ethylbenzene and xylene (BTEX) in aqueous solutions using supported zinc oxide nanorods. PLoS ONE 2017, 12, e0189276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haiqi, O.A.; Nour, A.H.; Bargaa, R.; Ayodele, B.V. Effect of process parameters on the photocatalytic degradation of phenol in oilfield produced wastewater using ZnO/Fe2O3 nanocomposites. Bull. Chem. React. Eng. Catal. 2020, 15, 128–136. [Google Scholar] [CrossRef] [Green Version]
- Okiel, K.; El-Sayed, M.; El-Kady, M.Y. Treatment of oil–water emulsions by adsorption onto activated carbon, bentonite and deposited carbon. Egypt. J. Pet. 2011, 20, 9–15. [Google Scholar] [CrossRef] [Green Version]
- El-Naas, M.H.; Al-Zuhair, S.; Alhaija, M.A. Reduction of COD in refinery wastewater through adsorption on date-pit activated carbon. J. Hazard. Mater. 2010, 173, 750–757. [Google Scholar] [CrossRef]
- Anisuzzaman, S.M.; Joseph, C.G.; Taufiq-Yap, Y.H.; Krishnaiah, D.; Tay, V.V. Modification of commercial activated carbon for the removal of 2,4-dichlorophenol from simulated wastewater. J. King Saud Univ.-Sci. 2015, 27, 318–330. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Tai, N.-H. Carbon materials as oil sorbents: A review on the synthesis and performance. J. Mater. Chem. A 2016, 4, 1550–1565. [Google Scholar] [CrossRef]
- Sun, H.; Li, A.; Zhu, Z.; Liang, W.; Zhao, X.; La, P.; Deng, W. Superhydrophobic activated carbon-coated sponges for separation and absorption. ChemSusChem 2013, 6, 1057–1062. [Google Scholar] [CrossRef]
- Zhan, B.; Liu, Y.; Zhou, W.-T.; Li, S.-Y.; Chen, Z.-B.; Stegmaier, T.; Aliabadi, M.; Han, Z.W.; Ren, L.Q. Multifunctional 3D GO/g-C3N4/TiO2 foam for oil-water separation and dye adsorption. Appl. Surf. Sci. 2021, 541, 148638. [Google Scholar] [CrossRef]
- Yang, H.; Sun, J.; Zhang, Y.; Xue, Q.; Xia, S. Preparation of hydrophobic carbon aerogel using cellulose extracted from luffa sponge for adsorption of diesel oil. Ceram. Int. 2021, 47, 33827–33834. [Google Scholar] [CrossRef]
- Kusworo, T.D.; Aryanti, N.; Qudratun; Utomo, D.P. Oilfield produced water treatment to clean water using integrated activated carbon-bentonite adsorbent and double stages membrane process. Chem. Eng. J. 2018, 347, 462–471. [Google Scholar] [CrossRef]
- Liu, X.; Ruan, W.; Wang, W.; Zhang, X.; Liu, Y.; Liu, J. The perspective and challenge of nanomaterials in oil and gas wastewater treatment. Molecules 2021, 26, 3945. [Google Scholar] [CrossRef] [PubMed]
- Bulgarian State Standards: Environment Protection, 1989 or Bulgarian State Standards: Environment Protection 1989, v.1; Bulgarian Institute for Standardization: Sofia, Bulgaria, 1989.
- Petrova, Y.; Aleksiev, A.; Momchilova, S.; Zdravkova, Z. Laboratory Practicals in Analysis of Toxic Compounds; Sofia University press: Sofia, Bulgaria, 2004. [Google Scholar]
- Panayotova, M.; Gicheva, G.; Dzherahov, L.; Mintcheva, N. Liquid phase oxidation as a possibility for the removal of oil compounds from produced water. J. Min. Geol. Sci. Part II Min. Technol. Miner. Process. 2017, 60, 110–115. [Google Scholar]
- Bulgarian Council of Ministers. Decree 6/9.11.2000 on Emission Standards for Permissible Content of Harmful and Dangerous Substances in Wastewater Discharged into Water Bodies, Bulgarian State Gazette No. 97/28.11.2000 and No. 24/23.03.2004; National Assembly of the Republic of Bulgaria: Sofia, Bulgaria, 2000. [Google Scholar]
- Tellez, G.T.; Nirmalakhandan, N.; Gardea-Torresdey, J.L. Performance evaluation of an activated sludge system for removing petroleum hydrocarbons from oilfield produced water. Adv. Environ. Res. 2002, 6, 455–470. [Google Scholar] [CrossRef]
- Tully, E.J. A study of calcium hypochlorite as a disinfectant of water. Am. J. Public Health 1914, 4, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Fukuzaki, S. Mechanisms of actions of sodium hypochlorite in cleaning and disinfection processes. Biocontrol Sci. 2006, 11, 147–157. [Google Scholar] [CrossRef]
- Khandaker, N.R.; Afreen, I.; Diba, D.S.; Huq, F.B.; Akter, T. Treatment of textile wastewater using calcium hypochlorite oxidation followed by waste iron rust aided rapid filtration for color and COD removal for application in resources challenged Bangladesh. Groundw. Sustain. Dev. 2020, 10, 100342. [Google Scholar] [CrossRef]
- Mintcheva, N.; Gicheva, G.; Panayotova, M.; Kulinich, S.A. Room-temperature synthesis of ZnS nanoparticles using zinc xanthates as molecular precursors. Materials 2020, 13, 171. [Google Scholar] [CrossRef] [Green Version]
- Kubelka, P.; Munk, F. Ein Beitrag Zur Optik der Farbanstriche. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Sáenz-Trevizo, A.; Amézaga-Madrid, P.; Pizá-Ruiz, P.; Antúnez-Flores, W.; Miki-Yoshida, M. Optical band gap estimation of ZnO nanorods. Mater. Res. 2016, 19, 33–38. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; de Coss, R.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Berberidou, C.; Galeckas, A.; Bazioti, C.; Sagstuen, E.; Norby, T.; Poulios, I.; Chatzitakis, A. Visible light driven photocatalytic decolorization and disinfection of water employing reduced TiO2 nanopowders. Catalysts 2021, 11, 228. [Google Scholar] [CrossRef]
- Srikant, V.; Clarke, D.R. On the optical band gap of zinc oxide. J. Appl. Phys. 1998, 83, 5447–5451. [Google Scholar] [CrossRef]
- Pawar, V.; Jha, P.K.; Panda, S.K.; Jha, P.A.; Singh, P. Band-gap engineering in ZnO thin films: A combined experimental and theoretical study. Phys. Rev. Appl. 2018, 9, 054001. [Google Scholar] [CrossRef]
- Sakthivel, S.; Neppolian, B.; Shankar, M.V.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Aggelopoulos, C.A.; Dimitropoulos, M.; Govatsi, A.; Sygellou, L.; Tsakiroglou, C.D.; Yannopoulos, S.N. Influence of the surface-to-bulk defects ratio of ZnO and TiO2 on their UV-mediated photocatalytic activity. Appl. Catal. B 2017, 205, 292–301. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, C.; Zhan, Y.; Lin, X.; Zheng, Q.; Wei, K.; Zhu, J.; Zhu, Y. Luminescence and photocatalytic activity of ZnO nanocrystals: Correlation between structure and property. Inorg. Chem. 2007, 46, 6675–6682. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]



| Sample № | T °C | pH | Eh mV | χ mS/cm | ΔpH | Dissolved O2 mg/L |
|---|---|---|---|---|---|---|
| I | 20.2 | 7.40 | −12 | 5.65 | +0.10 | <0.08 |
| II | 19.8 | 7.38 | −15 | 3.55 | −0.01 | <0.08 |
| III | 22.2 | 7.57 | −81 | 4.41 | −0.01 | <0.08 |
| IV | 25.3 | 7.18 | −139 | 5.70 | −0.08 | <0.08 |
| Sample № | I | II | III | IV |
|---|---|---|---|---|
| Ca2+ | 110 | 77 | 88 | 103 |
| Mg2+ | 57 | 43 | 50 | 60 |
| Na+ | 1260 | 805 | 1020 | 1201 |
| K+ | 18 | 16 | 17 | 19 |
| Cl− | 1777 | 975 | 1310 | 1761 |
| HCO3− | 440 | 510 | 577 | 585 |
| SO42− | 448 | 445 | 460 | 470 |
| S2− | 2.40 | 3.42 | 1.96 | 9.76 |
| Hardness * | 5.09 | 3.70 | 4.26 | 5.05 |
| Sample № | COD, mg/L | TOH, mg/L | Phenols, mg/L |
|---|---|---|---|
| I | 49 | 363 | 0.11 |
| II | 39 | 317 | 0.18 |
| III | 39 | 336 | 0.07 |
| IV | 58 | 152 | 0.21 |
| Sample № | TOH Initial | Adsorption | Photocatalysis | Chemical Oxidation | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Charcoal 1 | Charcoal 2 | TiO2 | ZnO | Ca(ClO)2 | |||||||
| TOH | Removal | TOH | Removal | TOH | Removal | TOH | Removal | TOH | Removal | ||
| I | 363 | 91 | 75 | 138 | 62 | 271 | 25 | 65 | 82 | 27 | 93 |
| II | 317 | 203 | 36 | 262 | 17 | 120 | 62 | 47 | 85 | 19 | 94 |
| III | 336 | 52 | 85 | 56 | 83 | 96 | 71 | 28 | 92 | 66 | 80 |
| IV | 152 | 58 | 62 | 109 | 28 | 33 | 78 | 24 | 84 | 27 | 82 |
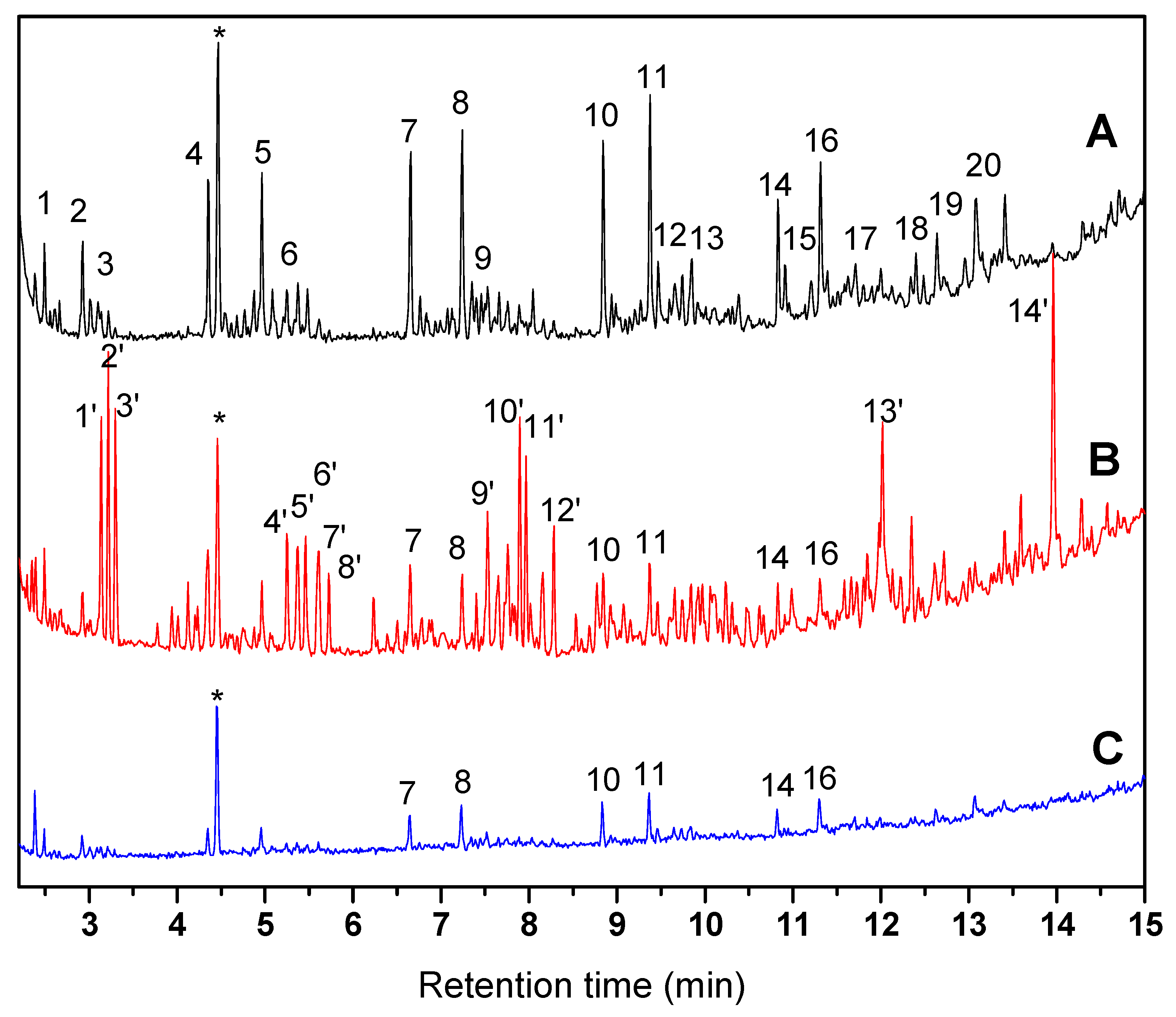
| Peak № | Name and Formula (Chromatogram A) | Peak № | Name and Formula (Chromatogram B) |
|---|---|---|---|
| 1 | 2-methylundecane (C12H26) | 1’ | 2-isopropyl-5-methylheptan-1-ol (C11H24O)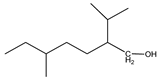 |
| 2 | 2,6-dimethyldecane (C12H26)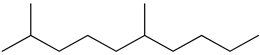 | 2’ | 2-methyldecan-1-ol (C11H24O)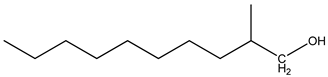 |
| 3 | n-Tridecane (C13H28) | 3’ | 2-methylundecan-1-ol (C12H26O)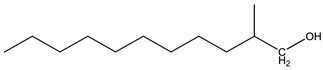 |
| 4 | 2-methyltetradecane (C15H32)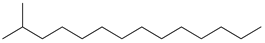 | 4’ | 2-hexyloctan-1-ol (C14H30O)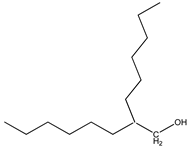 |
| 5 | 2,6,11-trimethyldodecane (C15H32)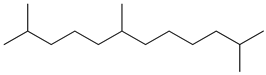 | 5’ | 2-ethyldodecan-1-ol (C14H30O) |
| 6 | n-Pentadecane (C15H32) | 6’ | 3,7,11-trimethyldodecan-1-ol (C15H32O)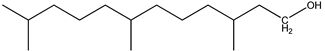 |
| 7 | 2-methyloctadecane (C19H40)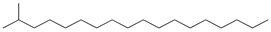 | 7’ | Pentadecene (C15H30) |
| 8 | 2,6,10,14-tetramethylhexadecane (C20H42)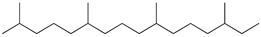 | 8’ | Hexadecene (C16H32) |
| 9 | n-Heptadecane (C17H36) | 9’ | 2-hexyldecan-1-ol (C16H34O)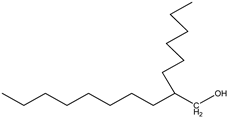 |
| 10 | 2-methylnonadecane (C20H42) | 10’ | hexadecan-1-ol (C16H34O) |
| 11 | 2,6,11,15-tetramethylheptadecane (C21H44)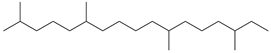 | 11’ | heptadecan-1-ol (C17H36O) |
| 12 | n-Nonadecane (C19H40) | 12’ | 6,10,14-trimethylpentadecan-2-ol (C18H38O) |
| 13 | 2,6,10,15-tetramethylheptadecane (C21H44)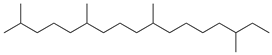 | 13’ | Hexadecanal (C16H32O)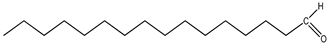 |
| 14 | 2-methyleicosane (C21H44)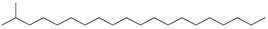 | 14’ | Heptadecanal (C17H34O) |
| 15 | n-Eicosane (C20H42) | ||
| 16 | 8-hexylpentadecane (C21H44)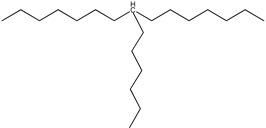 | ||
| 17 | n-Heneicosane (C21H44) | ||
| 18 | 2-methylheneicosane (C22H46) | ||
| 19 | n-Docosane (C22H46) | ||
| 20 | 8-heptylpentadecane (C22H46)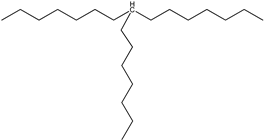 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mintcheva, N.; Gicheva, G.; Panayotova, M. Reduction of Heavy Hydrocarbons from Oilfield Produced Water. Pollutants 2022, 2, 234-251. https://doi.org/10.3390/pollutants2020016
Mintcheva N, Gicheva G, Panayotova M. Reduction of Heavy Hydrocarbons from Oilfield Produced Water. Pollutants. 2022; 2(2):234-251. https://doi.org/10.3390/pollutants2020016
Chicago/Turabian StyleMintcheva, Neli, Gospodinka Gicheva, and Marinela Panayotova. 2022. "Reduction of Heavy Hydrocarbons from Oilfield Produced Water" Pollutants 2, no. 2: 234-251. https://doi.org/10.3390/pollutants2020016





