Photodegradation of Polycyclic Aromatic Hydrocarbons from Coal Tar into Mine Wastewaters and Sewage Wastewater on a Flat-Bed Photoreactor
Abstract
:1. Introduction
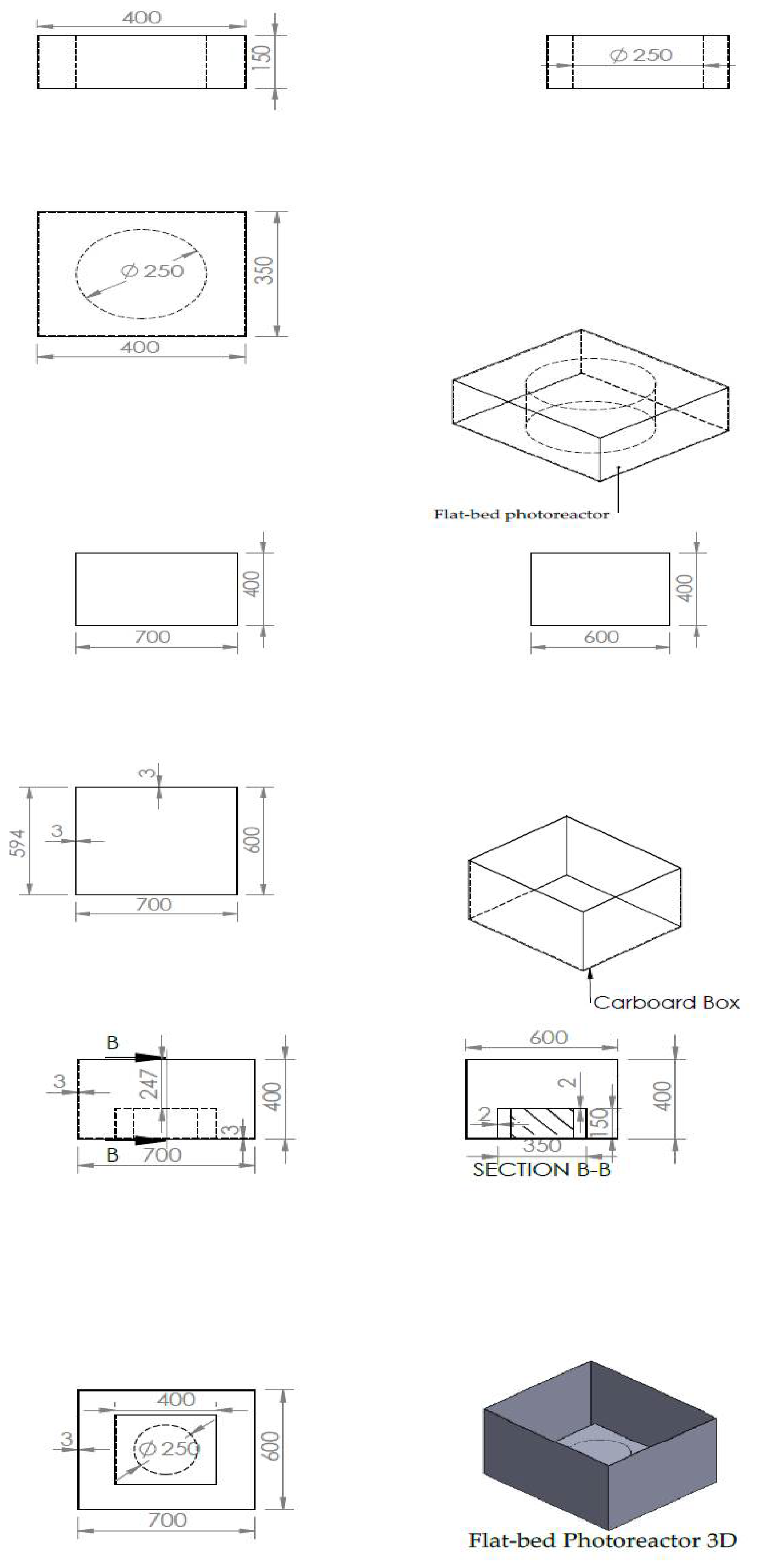
2. Materials and Methods
2.1. Degradation Experiments
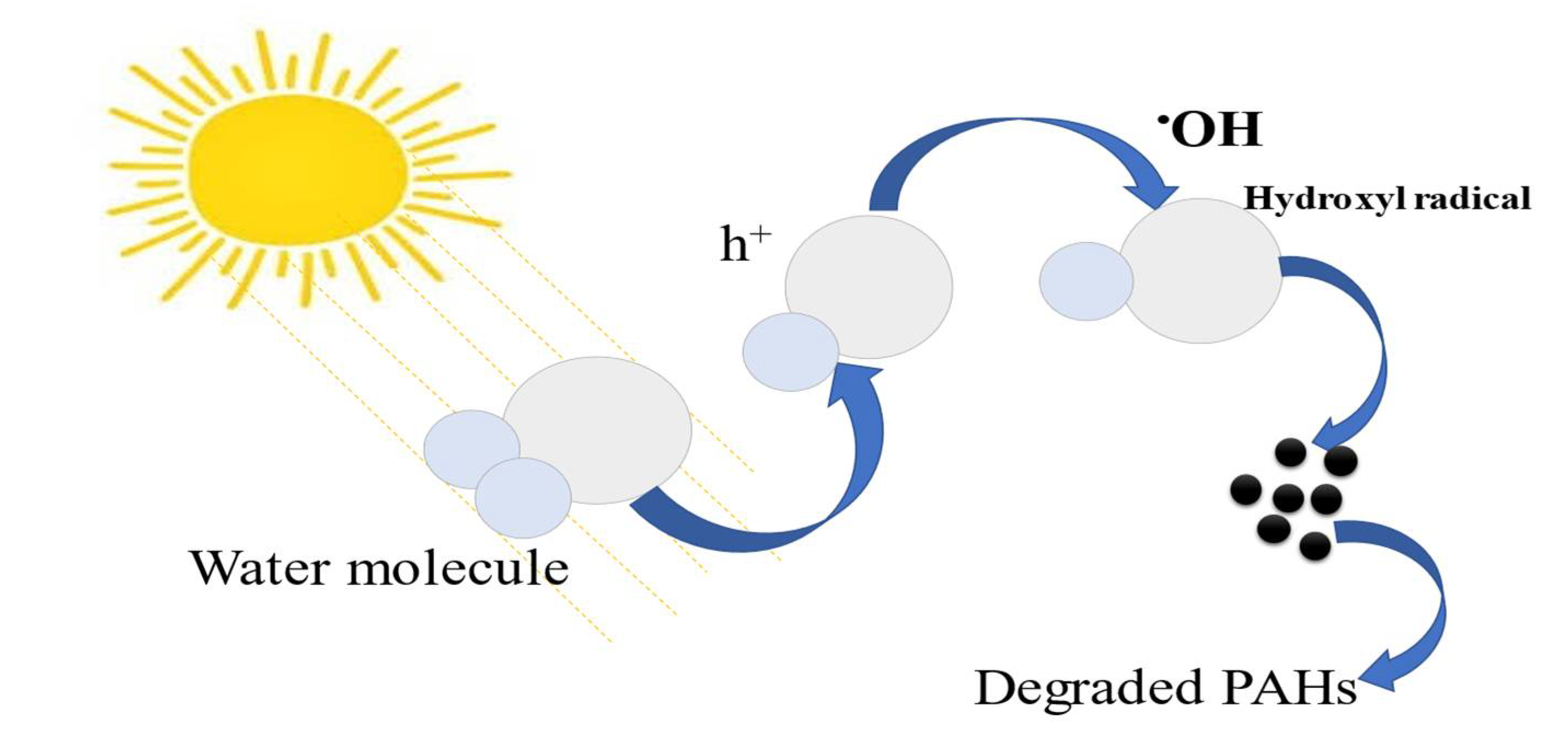
2.2. Photodegradation Mechanism
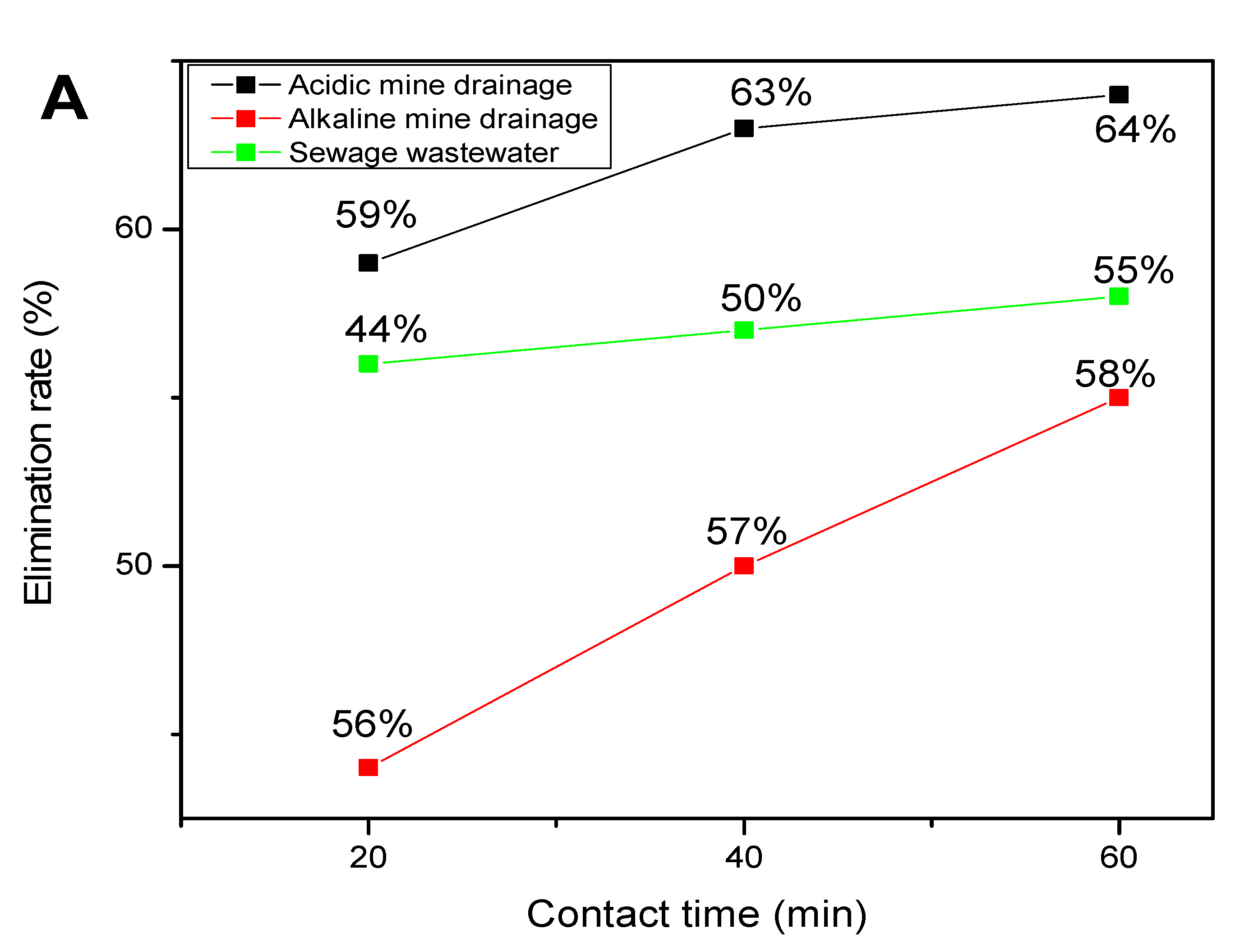
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Note
- Rashed, M.N. Organic Pollutants: Monitoring, Risk and Treatment; BoD–Books on Demand: Norderstedt, Germany, 2013. [Google Scholar]
- Loganath, R.; Mazumder, D. Performance study on organic carbon, total nitrogen, suspended solids removal and biogas production in hybrid UASB reactor treating real slaughterhouse wastewater. J. Environ. Chem. Eng. 2018, 6, 3474–3484. [Google Scholar] [CrossRef]
- Halicki, W.; Halicki, M. From Domestic Sewage to Potable Water Quality: New Approach in Organic Matter Removal Using Natural Treatment Systems for Wastewater. Water 2022, 14, 1909. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Xie, B.; Qin, J.; Sun, H.; Wang, S.; Li, X. Leaching behavior of polycyclic aromatic hydrocarbons (PAHs) from oil-based residues of shale gas drill cuttings. Environ. Pollut. 2021, 288, 117773. [Google Scholar] [CrossRef] [PubMed]
- Mojiri, A.; Zhou, J.L.; Ohashi, A.; Ozaki, N.; Kindaichi, T. Comprehensive review of polycyclic aromatic hydrocarbons in water sources, their effects and treatments. Sci. Total Environ. 2019, 696, 133971. [Google Scholar] [CrossRef]
- Gaurav, G.K.; Mehmood, T.; Kumar, M.; Cheng, L.; Sathishkumar, K.; Kumar, A.; Yadav, D. Review on polycyclic aromatic hydrocarbons (PAHs) migration from wastewater. J. Contam. Hydrol. 2021, 236, 103715. [Google Scholar] [CrossRef]
- Huang, Y.; Li, K.; Liu, H.; Yuan, X.; Li, M.; Xiong, B.; Du, R.; Johnson, D.M.; Xi, Y. Distribution, sources and risk assessment of PAHs in soil from the water level fluctuation zone of Xiangxi Bay, Three Gorges Reservoir. Environ. Geochem. Health 2021, 44, 2615–2628. [Google Scholar] [CrossRef]
- Cai, T.; Ding, Y.; Zhang, Z.; Wang, X.; Wang, T.; Ren, Y.; Dong, Y. Effects of total organic carbon content and leaching water volume on migration behavior of polycyclic aromatic hydrocarbons in soils by column leaching tests. Environ. Pollut. 2019, 254, 112981. [Google Scholar] [CrossRef]
- Batchamen Mougnol, J.B.; Waanders, F.; Fosso-Kankeu, E.; Al Alili, A.R. Leaching of Polycyclic Aromatic Hydrocarbons from the Coal Tar in Sewage Wastewater, Acidic and Alkaline Mine Drainage. Int. J. Environ. Res. Public Health 2022, 19, 4791. [Google Scholar] [CrossRef]
- Singh, S.; Haritash, A. Polycyclic aromatic hydrocarbons: Soil pollution and remediation. Int. J. Environ. Sci. Technol. 2019, 16, 6489–6512. [Google Scholar]
- Adeniji, A.O.; Okoh, O.O.; Okoh, A.I. Analytical methods for polycyclic aromatic hydrocarbons and their global trend of distribution in water and sediment: A review. Recent Insights Pet. Sci. Eng. 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Smol, M.; Włóka, D.; Włodarczyk-Makuła, M. Influence of integrated membrane treatment on the phytotoxicity of wastewater from the coke industry. Water Air Soil Pollut. 2018, 229, 154. [Google Scholar] [CrossRef] [Green Version]
- Bolden, A.L.; Rochester, J.R.; Schultz, K.; Kwiatkowski, C.F. Polycyclic aromatic hydrocarbons and female reproductive health: A scoping review. Reprod. Toxicol. 2017, 73, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Davie-Martin, C.L.; Stratton, K.G.; Teeguarden, J.G.; Waters, K.M.; Simonich, S.L.M. Implications of bioremediation of polycyclic aromatic hydrocarbon-contaminated soils for human health and cancer risk. Environ. Sci. Technol. 2017, 51, 9458–9468. [Google Scholar] [CrossRef] [PubMed]
- Alghamdi, M.A.; Hassan, S.K.; Al Sharif, M.Y.; Khoder, M.I.; Harrison, R.M. On the nature of polycyclic aromatic hydrocarbons associated with sporting walkways dust: Concentrations, sources and relative health risk. Sci. Total Environ. 2021, 781, 146540. [Google Scholar] [CrossRef] [PubMed]
- Shamsedini, N.; Dehghani, M.; Samaei, M.; Azhdarpoor, A.; Hoseini, M.; Fararouei, M.; Bahrany, S.; Roosta, S. Health risk assessment of polycyclic aromatic hydrocarbons in individuals living near restaurants: A cross-sectional study in Shiraz, Iran. Sci. Rep. 2022, 12, 8254. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Park, R.; Alexandrou, N.; Dryfhout-Clark, H.; Brice, K.; Hung, H. Multi-year analyses reveal different trends, sources, and implications for source-related human health risks of atmospheric polycyclic aromatic hydrocarbons in the Canadian Great Lakes Basin. Environ. Sci. Technol. 2021, 55, 2254–2264. [Google Scholar] [CrossRef]
- Gopinath, A.; Divyapriya, G.; Srivastava, V.; Laiju, A.; Nidheesh, P.; Kumar, M.S. Conversion of sewage sludge into biochar: A potential resource in water and wastewater treatment. Environ. Res. 2021, 194, 110656. [Google Scholar] [CrossRef]
- Bora, A.P.; Gupta, D.P.; Durbha, K.S. Sewage sludge to bio-fuel: A review on the sustainable approach of transforming sewage waste to alternative fuel. Fuel 2020, 259, 116262. [Google Scholar] [CrossRef]
- Mondala, A.H.; Hernandez, R.; French, T.; McFarland, L.; Santo Domingo, J.W.; Meckes, M.; Ryu, H.; Iker, B. Enhanced lipid and biodiesel production from glucose-fed activated sludge: Kinetics and microbial community analysis. AIChE J. 2012, 58, 1279–1290. [Google Scholar] [CrossRef]
- Agoro, M.A.; Adeniji, A.O.; Adefisoye, M.A.; Okoh, O.O. Heavy metals in wastewater and sewage sludge from selected municipal treatment plants in Eastern Cape Province, South Africa. Water 2020, 12, 2746. [Google Scholar] [CrossRef]
- Titilawo, Y.; Adeniji, A.; Adeniyi, M.; Okoh, A. Determination of levels of some metal contaminants in the freshwater environments of Osun State, Southwest Nigeria: A risk assessment approach to predict health threat. Chemosphere 2018, 211, 834–843. [Google Scholar] [CrossRef]
- Nyamukamba, P.; Moloto, M.J.; Tavengwa, N.; Ejidike, I.P. Evaluating physicochemical parameters, heavy metals, and antibiotics in the influents and final effluents of South African wastewater treatment plants. Pol. J. Environ. Stud. 2019, 28, 1305–1312. [Google Scholar] [CrossRef] [Green Version]
- Grobelak, A.; Grosser, A.; Kacprzak, M.; Kamizela, T. Sewage sludge processing and management in small and medium-sized municipal wastewater treatment plant-new technical solution. J. Environ. Manag. 2019, 234, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Antonio, S.; Puente-Luna, I.; Lagüela-López, S.; Veiga-Ríos, M. Techniques to correct and prevent acid mine drainage: A review. Dyna 2014, 81, 73–80. [Google Scholar] [CrossRef]
- Dold, B. Evolution of acid mine drainage formation in sulphidic mine tailings. Minerals 2014, 4, 621–641. [Google Scholar] [CrossRef] [Green Version]
- Simate, G.S.; Ndlovu, S. Acid mine drainage: Challenges and opportunities. J. Environ. Chem. Eng. 2014, 2, 1785–1803. [Google Scholar] [CrossRef]
- Rodríguez-Galán, M.; Baena-Moreno, F.M.; Vázquez, S.; Arroyo-Torralvo, F.; Vilches, L.F.; Zhang, Z. Remediation of acid mine drainage. Environ. Chem. Lett. 2019, 17, 1529–1538. [Google Scholar] [CrossRef]
- Kaur, G.; Couperthwaite, S.J.; Hatton-Jones, B.W.; Millar, G.J. Alternative neutralisation materials for acid mine drainage treatment. J. Water Process Eng. 2018, 22, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Skousen, J.; Zipper, C.E.; Rose, A.; Ziemkiewicz, P.F.; Nairn, R.; McDonald, L.M.; Kleinmann, R.L. Review of passive systems for acid mine drainage treatment. Mine Water Environ. 2017, 36, 133–153. [Google Scholar] [CrossRef] [Green Version]
- Zipper, C.E.; Skousen, J.G.; Jage, C.R. Passive treatment of acid-mine drainage. 2018. [Google Scholar]
- Vidal, R.; Moraes, J. Removal of organic pollutants from wastewater using chitosan: A literature review. Int. J. Environ. Sci. Technol. 2019, 16, 1741–1754. [Google Scholar] [CrossRef]
- Nisticò, R.; Cesano, F.; Franzoso, F.; Magnacca, G.; Scarano, D.; Funes, I.G.; Carlos, L.; Parolo, M.E. From biowaste to magnet-responsive materials for water remediation from polycyclic aromatic hydrocarbons. Chemosphere 2018, 202, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Rashed, M.N. Adsorption technique for the removal of organic pollutants from water and wastewater. Org. Pollut.Monit. Risk Treat. 2013, 7, 167–194. [Google Scholar]
- Roy, A.; Murthy, H.A.; Ahmed, H.M.; Islam, M.N.; Prasad, R. Phytogenic synthesis of metal/metal oxide nanoparticles for degradation of dyes. J. Renew. Mater. 2022, 10, 1911. [Google Scholar] [CrossRef]
- Varjani, S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef]
- Akinpelu, A.A.; Ali, M.E.; Johan, M.R.; Saidur, R.; Qurban, M.A.; Saleh, T.A. Polycyclic aromatic hydrocarbons extraction and removal from wastewater by carbon nanotubes: A review of the current technologies, challenges and prospects. Process Saf. Environ. Prot. 2019, 122, 68–82. [Google Scholar] [CrossRef]
- Patel, A.B.; Shaikh, S.; Jain, K.R.; Desai, C.; Madamwar, D. Polycyclic aromatic hydrocarbons: Sources, toxicity and remediation approaches. Front. Microbiol. 2020, 11, 2675. [Google Scholar] [CrossRef]
- Taghipour, F. UV-LED Radiation Photoreactor. U.S. Patent 20180201521A1, 5 May 2020. [Google Scholar]
- Sundar, K.P.; Kanmani, S. Progression of Photocatalytic reactors and it’s comparison: A Review. Chem. Eng. Res. Des. 2020, 154, 135–150. [Google Scholar] [CrossRef]
- Purcar, V.; Rădiţoiu, V.; Rădiţoiu, A.; Raduly, F.M.; Manea, R.; Frone, A.; Anastasescu, M.; Ispas, G.C.; Căprărescu, S. Bilayer coatings based on silica materials and iron (III) phthalocyanine–Sensitized TiO2 photocatalyst. Mater. Res. Bull. 2021, 138, 111222. [Google Scholar] [CrossRef]
- Bandala, E.R.; Arancibia-Bulnes, C.A.; Orozco, S.L.; Estrada, C.A. Solar photoreactors comparison based on oxalic acid photocatalytic degradation. Sol. Energy 2004, 77, 503–512. [Google Scholar] [CrossRef]
- Chen, M.; He, Y.; Zhu, J.; Wen, D. Investigating the collector efficiency of silver nanofluids based direct absorption solar collectors. Appl. Energy 2016, 181, 65–74. [Google Scholar] [CrossRef]
- Tzivanidis, C.; Bellos, E.; Korres, D.; Antonopoulos, K.; Mitsopoulos, G. Thermal and optical efficiency investigation of a parabolic trough collector. Case Stud. Therm. Eng. 2015, 6, 226–237. [Google Scholar] [CrossRef] [Green Version]
- Matamoros, V.; Gutiérrez, R.; Ferrer, I.; García, J.; Bayona, J.M. Capability of microalgae-based wastewater treatment systems to remove emerging organic contaminants: A pilot-scale study. J. Hazard. Mater. 2015, 288, 34–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.; Dong, D.; Kong, L.; Zheng, Y.; Li, X. Photocatalytic degradation of phenanthrene on soil surfaces in the presence of nanometer anatase TiO2 under UV-light. J. Environ. Sci. 2012, 24, 2122–2126. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts. Catalysts 2019, 9, 430. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Kim, S.; Jin, J.; Do, H.C.; Park, J.H. Efficient photodegradation of volatile organic compounds by iron-based metal-organic frameworks with high adsorption capacity. Appl. Catal. B Environ. 2020, 263, 118284. [Google Scholar] [CrossRef]
- Sudhaik, A.; Raizada, P.; Shandilya, P.; Jeong, D.-Y.; Lim, J.-H.; Singh, P. Review on fabrication of graphitic carbon nitride based efficient nanocomposites for photodegradation of aqueous phase organic pollutants. J. Ind. Eng. Chem. 2018, 67, 28–51. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Tan, H.; Zhang, A.; Xie, Y.; Wu, B.; Xu, H. A novel microbe consortium, nano-visible light photocatalyst and microcapsule system to degrade PAHs. Chem. Eng. J. 2019, 359, 1065–1074. [Google Scholar] [CrossRef]
- Eker, G.; Hatipoglu, M. Effect of UV wavelength, temperature and photocatalyst on the removal of PAHs from industrial soil with photodegradation applications. Environ. Technol. 2018, 40, 3793–3803. [Google Scholar] [CrossRef]
- Sliem, M.A.; Salim, A.Y.; Mohamed, G.G. Photocatalytic degradation of anthracene in aqueous dispersion of metal oxides nanoparticles: Effect of different parameters. J. Photochem. Photobiol. A Chem. 2019, 371, 327–335. [Google Scholar] [CrossRef]
- Rani, C.N.; Karthikeyan, S. Synergic effects on degradation of a mixture of polycyclic aromatic hydrocarbons in a UV slurry photocatalytic membrane reactor and its cost estimation. Chem. Eng. Processing-Process Intensif. 2021, 159, 108179. [Google Scholar] [CrossRef]
- Qiao, Y. Preparation, Characterization, and Evaluation of Photocatalytic Properties of a Novel NaNbO3/Bi2WO6 Heterostructure Photocatalyst for Water Treatment. Ph.D. Thesis, University of Ottawa, Ottawa, ON, Canada, 2018. [Google Scholar]
- Nagaraju, P.; Puttaiah, S.H.; Wantala, K.; Shahmoradi, B. Preparation of modified ZnO nanoparticles for photocatalytic degradation of chlorobenzene. Appl. Water Sci. 2020, 10, 137. [Google Scholar] [CrossRef]
- Gupta, A.; Pandey, O. Visible irradiation induced photodegradation by NbC/C nanocomposite derived from smoked cigarette litter (filters). Sol. Energy 2018, 163, 167–176. [Google Scholar] [CrossRef]


| PAHs | Chemical Formula | Molecular Weight (g/mol) | Rings Number | Melting Point (°C) | Boiling Point (°C) | Structures |
|---|---|---|---|---|---|---|
| Naphthalene (NAP) | C10H8 | 128 | 2 | 80.2 | 218 | 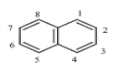 |
| Acenaphthylene (ACY) | C12H8 | 152 | 3 | 92.5 | 280 | 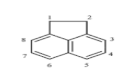 |
| Acenaphthene (ACE) | C12H10 | 152 | 3 | 93.4 | 279 | 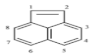 |
| Fluorene (FL) | C13H10 | 166 | 3 | 115 | 295 |  |
| Phenanthrene (PHE) | C14H10 | 178 | 3 | 99.2 | 340 | 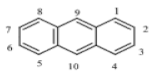 |
| Anthracene (ANT) | C14H10 | 178 | 4 | 215 | 340 | 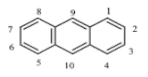 |
| Fluoranthene (FLU) | C16H10 | 202 | 4 | 108 | 384 | 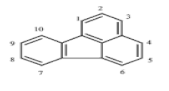 |
| Pyrene (PYR) | C16H10 | 202 | 4 | 151 | 404 |  |
| Benzo[a]anthracene (BaA) | C18H12 | 228 | 4 | 167 | 435 | 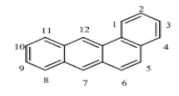 |
| Chrysene (CHY) | C18H12 | 228 | 4 | 258 | 448 |  |
| Benzo[b]fluoranthene (BbF) | C20H12 | 252 | 5 | 168 | 481 | 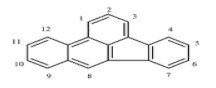 |
| Benzo[k]fluoranthene (Blkf) | C20H12 | 252 | 5 | 217 | 480 | 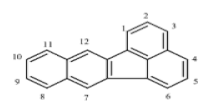 |
| Benzo[a]pyrene (BaP) | C20H12 | 252 | 5 | 177 | 495 | 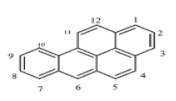 |
| Dibenzo[a,h]anthracene (DahA) | C22H14 | 278 | 5 | 270 | 524 | 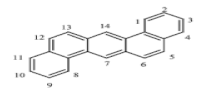 |
| Indenol [1,2,3-cd] pyrene (IP) | C22H12 | 276 | 6 | 164 | 536 | 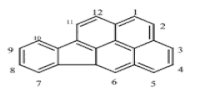 |
| Benzo[g,h,i]perylene (Bghip) | C22H12 | 276 | 6 | 278 | 550 | 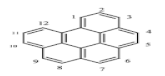 |
| PAHs (Pollutants) | Concentration (mg/L) |
|---|---|
| NAP | 788 |
| ACY | 356 |
| ACE | 18 |
| FL | 327 |
| PHE | 632 |
| ANT | 245 |
| FLU | 395 |
| PYR | 266 |
| BaA | 91 |
| CHY | 126 |
| BbF | 75 |
| Blkf | 71 |
| BaP | 92 |
| DahA | 56 |
| IP | 8 |
| Bghip | 21 |
| PAHs | Initial Concentration |  | 20 min | 40 min | 60 min | 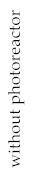 | 20 min | 40 min | 60 min |
| NAP | 0.0031 | 0.0030 | 0.0017 | 0.0025 | 0.0019 | 0.0016 | 0.0029 | ||
| ACY | 0.1302 | 0.1092 | 0.1267 | 0.1031 | 0.1271 | 0.1098 | 0.1277 | ||
| ACE | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| FL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| PHE | 0.0181 | 0.0083 | 0.0091 | 0.0133 | 0.008 | 0.0143 | 0.0176 | ||
| ANT | 18.9847 | 7.7006 | 6.7824 | 6.7014 | 10.7141 | 9.5481 | 8.7359 | ||
| FLU | 0.0020 | 0.0017 | 0.0013 | 0.0013 | 0.0018 | 0.0013 | 0.0016 | ||
| PYR | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| BaA | 0.1114 | 0.1049 | 0.1062 | 0.0517 | 0.1109 | 0.1089 | 0.1093 | ||
| CHY | 0.0012 | 0 | 0 | 0.0007 | 0.0010 | 0.0003 | 0.0009 | ||
| BbF | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Blkf | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| BaP | 0.0046 | 0.0042 | 0.0036 | 0.0032 | 0.0042 | 0.0037 | 0.0040 | ||
| DahA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| IP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Bghip | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| PAHs photodegradation | 19.2553 | 7.932 | 7.031 | 6.889 | 10.969 | 9.788 | 8.999 |
| PAHs | Initial Concentration |  | 20 min | 40 min | 60 min | 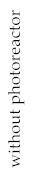 | 20 min | 40 min | 60 min |
| NAP | 0.7615 | 0.5943 | 0.5280 | 0.4212 | 0.6802 | 0.6212 | 0.5212 | ||
| ACY | 0.2596 | 0.0419 | 0.0363 | 0.0139 | 0.2281 | 0.1081 | 0.1281 | ||
| ACE | 0.0020 | 0.0012 | 0.0001 | 0.0001 | 0 | 0 | 0 | ||
| FL | 0.0030 | 0.0013 | 0.0012 | 0.0014 | 0.0027 | 0.0019 | 0.0021 | ||
| PHE | 0.0447 | 0.0381 | 0.0216 | 0.0218 | 0.0418 | 0.0359 | 0.0237 | ||
| ANT | 0.5388 | 0.2329 | 0.2194 | 0.2584 | 0.5071 | 0.4112 | 0.3972 | ||
| FLU | 0.0039 | 0.0027 | 0.0019 | 0.0012 | 0.0037 | 0.0028 | 0.0031 | ||
| PYR | 0.0019 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| BaA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| CHY | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| BbF | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Blkf | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| BaP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| DahA | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| IP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Bghip | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| PAHs photodegradation | 1.6154 | 0.9122 | 0.8085 | 0.7180 | 1.4636 | 1.1811 | 1.0754 |
| PAHs | Initial Concentration |  | 20 min | 40 min | 60 min | 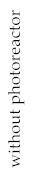 | 20 min | 40 min | 60 min |
| NAP | 0.0069 | 0.0053 | 0.0045 | 0.0047 | 0.0067 | 0.0062 | 0.0053 | ||
| ACY | 0.0489 | 0.0359 | 0.0310 | 0.0224 | 0.0379 | 0.0358 | 0.0302 | ||
| ACE | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| FL | 0.009 | 0.0067 | 0.0058 | 0.0015 | 0.0011 | 0.0010 | 0.0065 | ||
| PHE | 0.0961 | 0.0177 | 0.0153 | 0.0121 | 0.0821 | 0.0773 | 0.0758 | ||
| ANT | 0.1758 | 0.1502 | 0.0957 | 0.1019 | 0.1508 | 0.1395 | 0.1059 | ||
| FLU | 0.1706 | 0.0197 | 0.0049 | 0.0347 | 0.1607 | 0.1370 | 0.1134 | ||
| PYR | 0.1389 | 0.0149 | 0.0123 | 0.0333 | 0.1297 | 0.1033 | 0.0927 | ||
| BaA | 0.1888 | 0.0158 | 0.0937 | 0.1171 | 0.1251 | 0.1222 | 0.1193 | ||
| CHY | 0.1170 | 0.1002 | 0.0713 | 0.0943 | 0.1078 | 0.0931 | 0.1091 | ||
| BbF | 0.2216 | 0.1506 | 0.1474 | 0.1004 | 0.2004 | 0.1017 | 0.1099 | ||
| Blkf | 0.1563 | 0.0753 | 0.1132 | 0.1007 | 0.1390 | 0.1031 | 0.1013 | ||
| BaP | 0.2185 | 0.1073 | 0.1175 | 0.0372 | 0.2012 | 0.1109 | 0.1197 | ||
| DahA | 0.1278 | 0.0137 | 0.0109 | 0.0273 | 0.1224 | 0.1006 | 0.0710 | ||
| IP | 0.0345 | 0.0063 | 0.0045 | 0.0065 | 0.0217 | 0.0194 | 0.0205 | ||
| Bghip | 0.1018 | 0.0676 | 0.0507 | 0.0451 | 0.0978 | 0.0501 | 0.0612 | ||
| PAHs photodegradation | 1.8125 | 0.7872 | 0.7787 | 0.7572 | 1.5844 | 1.2002 | 1.1113 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batchamen Mougnol, J.B.; Waanders, F.; Fosso-Kankeu, E.; Al Alili, A.R. Photodegradation of Polycyclic Aromatic Hydrocarbons from Coal Tar into Mine Wastewaters and Sewage Wastewater on a Flat-Bed Photoreactor. Pollutants 2022, 2, 333-346. https://doi.org/10.3390/pollutants2030023
Batchamen Mougnol JB, Waanders F, Fosso-Kankeu E, Al Alili AR. Photodegradation of Polycyclic Aromatic Hydrocarbons from Coal Tar into Mine Wastewaters and Sewage Wastewater on a Flat-Bed Photoreactor. Pollutants. 2022; 2(3):333-346. https://doi.org/10.3390/pollutants2030023
Chicago/Turabian StyleBatchamen Mougnol, Jean Bedel, Frans Waanders, Elvis Fosso-Kankeu, and Ali R. Al Alili. 2022. "Photodegradation of Polycyclic Aromatic Hydrocarbons from Coal Tar into Mine Wastewaters and Sewage Wastewater on a Flat-Bed Photoreactor" Pollutants 2, no. 3: 333-346. https://doi.org/10.3390/pollutants2030023
APA StyleBatchamen Mougnol, J. B., Waanders, F., Fosso-Kankeu, E., & Al Alili, A. R. (2022). Photodegradation of Polycyclic Aromatic Hydrocarbons from Coal Tar into Mine Wastewaters and Sewage Wastewater on a Flat-Bed Photoreactor. Pollutants, 2(3), 333-346. https://doi.org/10.3390/pollutants2030023






