Effects of Cigarette Butt Leachate on the Growth of White Mustard (Sinapis alba L.) and Soil Properties: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sources of Materials
2.2. Leaching Experiment Procedure
2.3. Test Soil
2.4. Terrestrial Plant Test
- OECD soil alone
- Vertisol soil alone
- OECD soil + SCB1
- OECD soil + SCB2
- OECD soil + SCB3
- OECD soil + USCB1
- OECD soil + USCB2
- OECD soil + USCB3
- Vertisol soil + SCB1
- Vertisol soil + SCB2
- Vertisol soil + SCB3
- Vertisol soil + USCB1
- Vertisol soil + USCB2
- Vertisol soil + USCB3
2.5. Plant Physiological Parameters and Analysis of Heavy Metals in Plant Tissue
2.6. Heavy Metal Indexes
2.7. Soil Analysis
2.8. Statistical Analysis
3. Results
3.1. Physiological Observations
3.2. Elemental Composition of White Mustard Plant
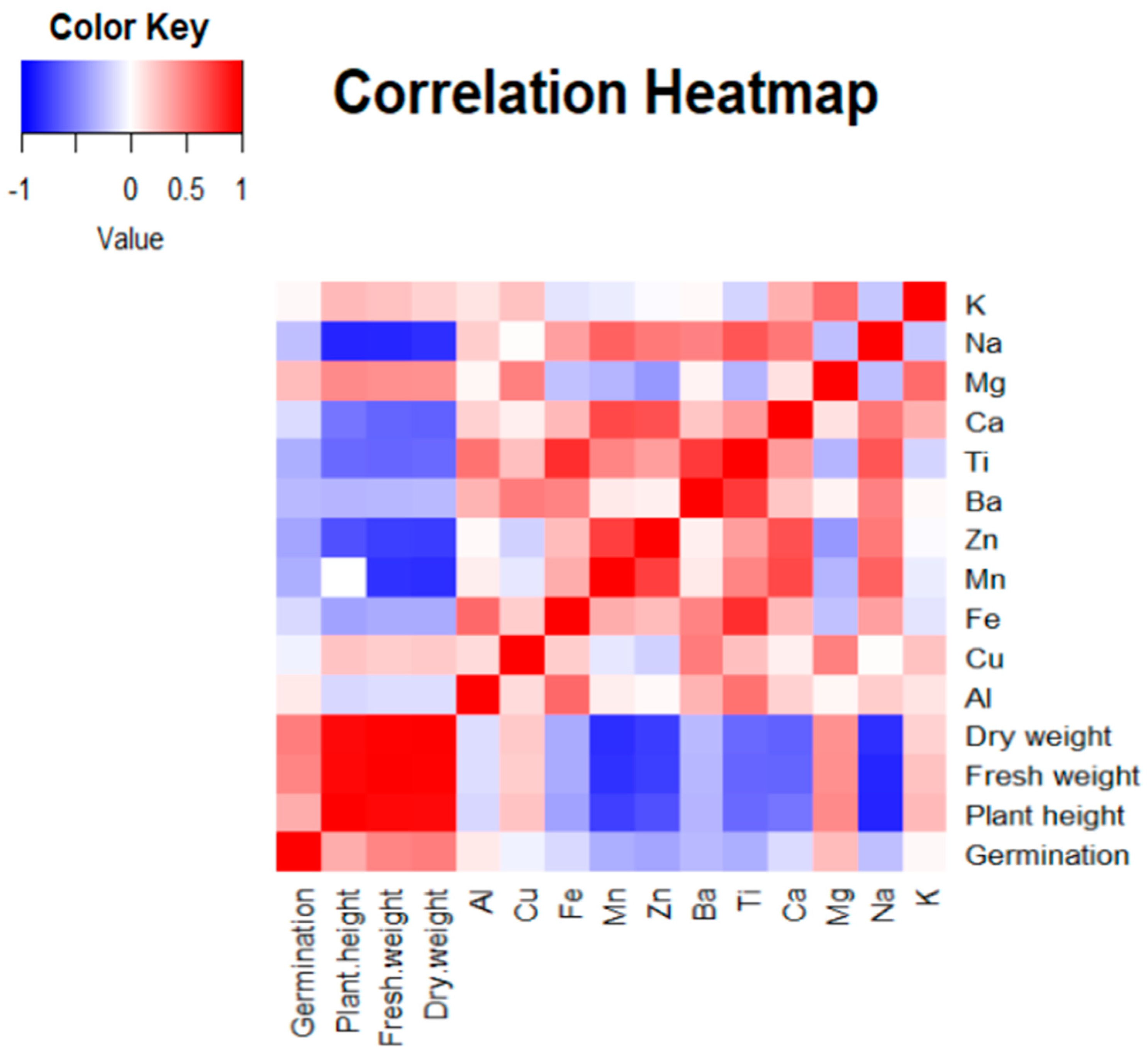
3.3. Correlation Between Plant Biological and Chemical Properties
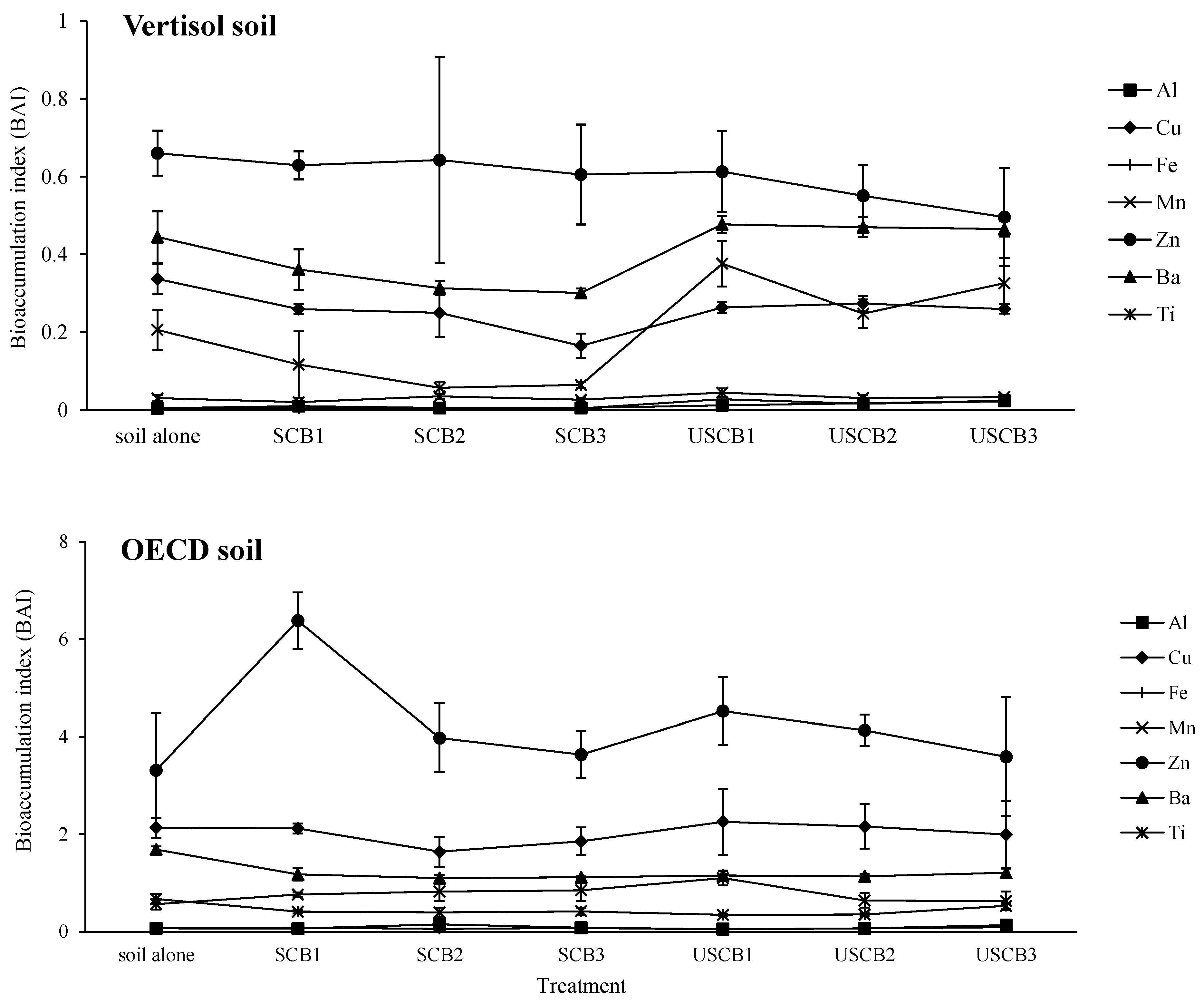
3.3.1. Bioaccumulation Index
3.3.2. Heavy Metal Uptake Index
3.3.3. Tolerance Index
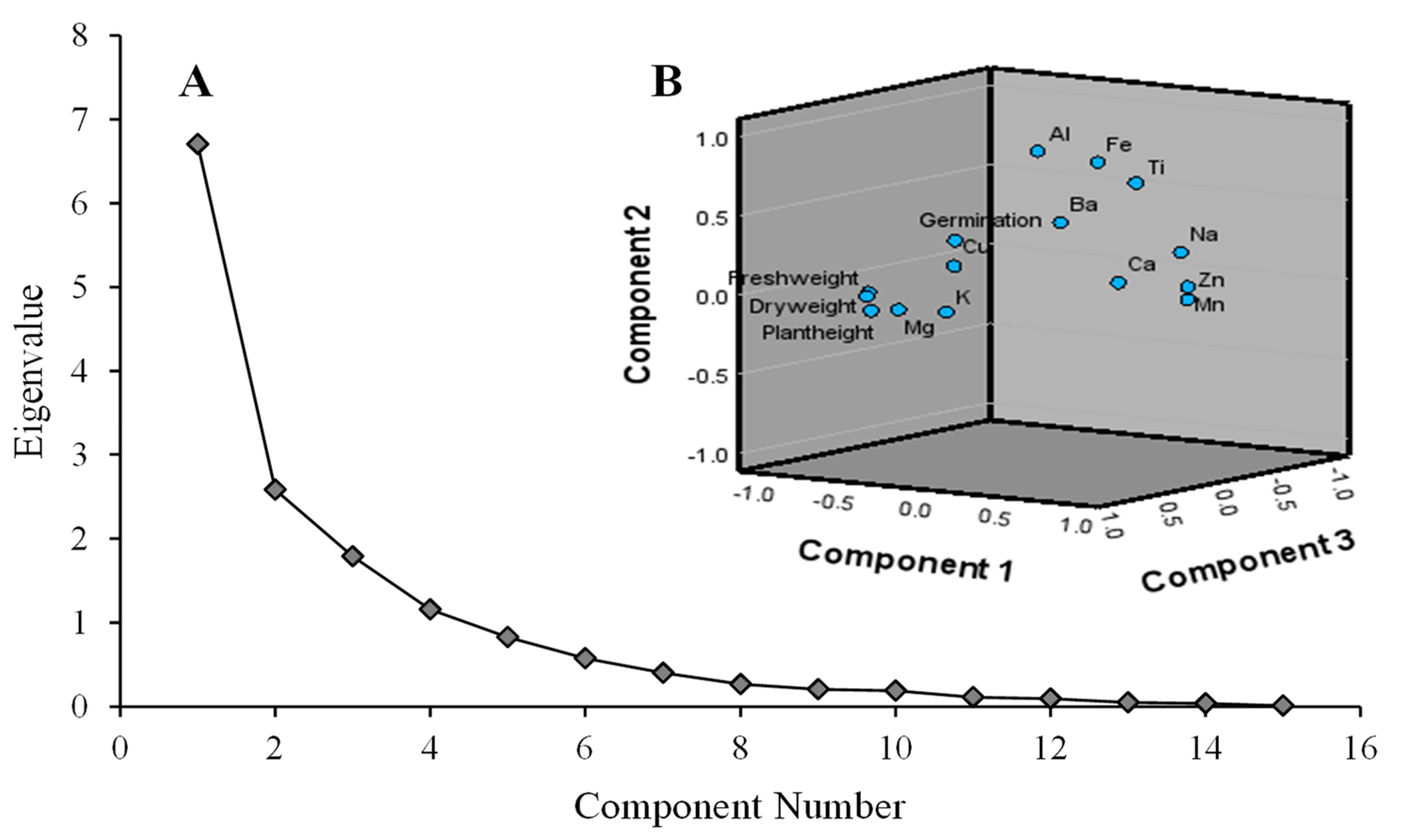
3.4. Factor and Principal Component Analysis
Multivariate Interaction and Main Effects of Indicator Variability
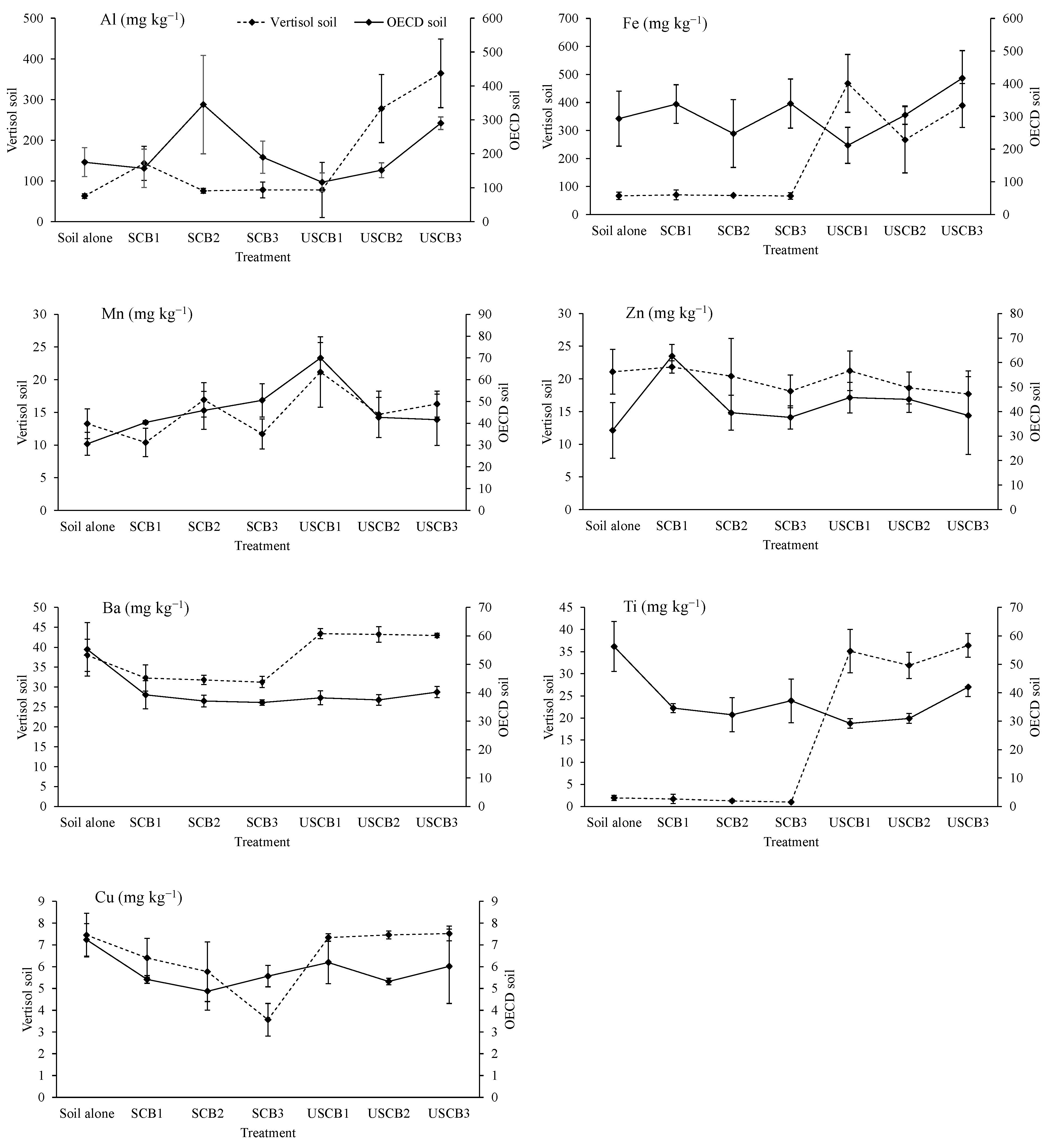
3.5. Post-Harvest Soil Chemical Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ajibade, S.; Nnadozie, E.C.; Gulyás, M.; Simon, B. Cigarette Butts as Water Emerging Contaminants: Possible Method for Their Remediation. J. Central Eur. Green Innov. 2023, 11, 105–117. [Google Scholar] [CrossRef]
- Kaszubska, G. Rmit.edu.au. Hämtat från. 2020. Available online: https://www.rmit.edu.au/news/media-releases-and-expert-comments/2020/sep/cigarette-butt-bricks (accessed on 31 October 2024).
- Hernandez, C.G. Cigarette Litter Leachates: A Statistical Study of Elements in Freshwater and Saltwater. Bachelor’s Thesis, University of Tennessee at Chattanooga, Chattanooga, TN, USA, 2018. [Google Scholar]
- Green, D.S.; Boots, B.; Da Carvalho, J.D.; Starkey, T. Cigarette butts have adverse effects on initial growth of perennial ryegrass (gramineae: Lolium perenne L.) and white clover (leguminosae: Trifolium repens L.). Ecotoxicol. Environ. Saf. 2019, 182, 109418. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, N.; Etebari, M.; Ebrahimi, A.; Ebrahimpour, K.; Rahimi, B.; Hassanzadeh, A. Genotoxicity and phytotoxicity comparison of cigarette butt with cigarette ash. Environ. Sci. Pollut. Res. 2020, 27, 40383–40391. [Google Scholar] [CrossRef] [PubMed]
- Jakimiuk, A.; Bulak, A.; Barroso, P.M.; Podlasek, A.; Vaverková, M.D. Impact of Cigarette Butts on Plant Germination Based on Sinapis alba L. and Hordeum vulgare L. Seeds. J. Ecol. Eng. 2022, 23, 226–237. [Google Scholar] [CrossRef]
- Mohamed, A.R. Impacts of Used Cigarette Butts on Soil Properties and Local Plants Growth. Sirte Univ. Sci. J. 2023, 13, 83–89. [Google Scholar]
- Shah, G.; Bhatt, U.; Singh, H.; Kumar, D.; Sharma, J.; Strasser, R.J.; Soni, V. Ecotoxicological assessment of cigarette butts on morphology and photosynthetic potential of Azolla pinnata. BMC Plant Biol. 2024, 24, 300. [Google Scholar] [CrossRef] [PubMed]
- Koroleva, E.; Mqulwa, A.Z.; Norris-Jones, S.; Reed, S.; Tambe, Z.; Visagie, A.; Jacobs, K. Impact of cigarette butts on bacterial community structure in soil. Environ. Sci. Pollut. Res. 2021, 28, 33030–33040. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, G.; Maisto, G.; De Marco, A.; Cesarano, G.; Zotti, M.; Mazzei, P.; Libralato, G.; Staropoli, A.; Siciliano, A.; De Filippis, F.; et al. The fate of cigarette butts in different environments: Decay rate, chemical changes and ecotoxicity revealed by a 5-years decomposition experiment. Environ. Pollut. 2020, 261, 114108. [Google Scholar] [CrossRef] [PubMed]
- Torkashvand, J.; Godini, K.; Norouzi, S.; Gholami, M.; Yeganeh, M.; Farzadkia, M. Effect of cigarette butt on concentration of heavy metals in landfill leachate: Health and ecological risk assessment. J. Environ. Health Sci. Eng. 2021, 19, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Farzadkia, M.; Nia, M.Y.; Nia, M.Y.; Shacheri, F.; Nourali, Z.; Torkashvand, J. Reduction of the environmental and health consequences of cigarette butt recycling by removal of toxic and carcinogenic compounds from its leachate. Environ. Sci. Pollut. Res. 2024, 31, 23942–23950. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Xu, J.; Huang, C. Substrate utilization pattern, biomass and activity of microbial communities in a sequence of heavy metal-polluted paddy soils. Geoderma 2003, 115, 139–148. [Google Scholar] [CrossRef]
- Rasheed, A.; Khan, A.A.; Nawaz, M.; Mahmood, A.; Arif, U.; Hassan, M.U.; Iqbal, J.; Saleem, M.H.; Ali, B.; Fahad, S. Development of Aluminium (Al)-Tolerant Soybean Using Molecular Tools: Limitations and Future Directions. J. Plant Growth Regul. 2023, 42, 7403–7417. [Google Scholar] [CrossRef]
- Hadia-e-Fatima, A.A. Heavy metal pollution–A mini review. J. Bacteriol. Mycol. Open Access 2018, 6, 179–181. [Google Scholar]
- Statista. Tobacco Products—Hungary. Statista. 2024. Available online: https://fr.statista.com/outlook/cmo/tobacco-products/hungary (accessed on 30 October 2024).
- Global Data. Hungary Cigarettes Market by Segments, Production, Distribution, Tax and Pricing, Competitive Landscape and Forecast to 2027. 2023. Available online: https://www.globaldata.com/store/report/hungary-cigarettes-market-analysis/ (accessed on 25 September 2024).
- Kaul, K.E. How to Reduce the Harmful Impact of Cigarettes on Environment. 2022. Available online: https://publikaciotar.uni-bge.hu/id/eprint/1872/1/75_PDFsam_Sus_Conference_k%C3%B6tet_2021.pdf (accessed on 26 September 2024).
- Slaughter, E.; Gersberg, R.M.; Watanabe, K.; Rudolph, J.; Stransky, C.; Novotny, T.E. Toxicity of cigarette butts, and their chemical components, to marine and freshwater fish. Tob. Control 2011, 20 (Suppl. 1), i25–i29. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Rowe, D.; Reid, M.J.; Thomas, K.V.; Galloway, T.S. Bioaccumulation and biological effects of cigarette litter in marine worms. Sci. Rep. 2015, 5, 14119. [Google Scholar] [CrossRef] [PubMed]
- Moerman, J.W.; Potts, G.E. Analysis of metals leached from smoked cigarette litter. Tob. Control 2011, 20 (Suppl. 1), i30–i35. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.; Rogers, K.; Rehman, B.; Moynihan, J.; Bergey, E.A. Cigarette butts may have low toxicity to soil-dwelling invertebrates: Evidence from a land snail. Sci. Total Environ. 2018, 628–629, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Jośko, I.; Oleszczuk, P. Influence of soil type and environmental conditions on ZnO, TiO2 and Ni nanoparticles phytotoxicity. Chemosphere 2013, 92, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Pontoni, L.; Van Hullebusch, E.D.; Fabbricino, M.; Esposito, G.; Pirozzi, F. Assessment of trace heavy metals dynamics during the interaction of aqueous solutions with the artificial OECD soil: Evaluation of the effect of soil organic matter content and colloidal mobilization. Chemosphere 2016, 163, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Qiao, Y.; Sun, Z.; Sun, X.; Li, Y. Molecular toxicity of earthworms induced by cadmium contaminated soil and biomarkers screening. J. Environ. Sci. 2012, 24, 1504–1510. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development. OECD Guideline for the Testing of Chemicals; Organisation for Economic Co-operation and Development: Paris, France, 2016. [Google Scholar]
- OECD. Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test (OECD Test No. 208). 2014. Available online: https://www.oecd-ilibrary.org/environment/test-no-208-terrestrial-plant-test-seedling-emergence-and-seedling-growth-test_9789264070066-en (accessed on 29 April 2024).
- MSZ-08-0205; A talaj fizikai és vízgazdálkodási tulajdonságainak vizsgálata. MSZH–Nyomda: Budapest, Hungary, 1978.
- Blaylock, M.J.; Huang, J.W. Phytoextraction of metals. In Raskin I Phytoremediation of Toxic Metals: Using Plants to Clean the Environment; Ensley, B.D., Ed.; J. Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- MSZ 21470-50; Environmental Testing of Soils. Determination of Total and Soluble Toxic Element, Heavy Metal and Chromium (VI) Content. Hungarian Standards Board: Budapest, Hungary, 2006. (In Hungarian)
- Różyło, K.; Świeca, M.; Gawlik-Dziki, U.; Kwiecińska-Poppe, E.; Andruszczak, S.; Kraska, P. Phytochemical prop-erties and heavy metal accumulation in wheat grain after three years’ fertilization with biogas digestate and mineral waste. Agric. Food Sci. 2017, 26, 148–159. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Reeves, R.D.; Hajar, A.S.M. Heavy metal accumulation and tolerance in British populations of the metallophyte Thlaspi caerulescens J. & C. Presl (Brassicaceae). New Phytol. 1994, 127, 61–68. [Google Scholar] [PubMed]
- MSZ-08-0206-2; A talaj egyes kémiai tulajdonságainak vizsgálata. Laboratóriumi vizsgálatok: Budapest, Hungary, 1978.
- Walker-Black, I.A. An examination of the Degtajareff method for soil organic matter determination and a proposed modification of the chronic acid titration. Soil Sci. 1934, 37, 29–38. [Google Scholar]
- Kaiser, H.F. The application of electronic computers to factor analysis. Educ. Psychol. Meas. 1960, 20, 141–151. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- Jalali, M.; Hemati, N. Chemical fractionation of seven heavy metals (Cd, Cu, Fe, Mn, Ni, Pb, and Zn) in selected paddy soils of Iran. Paddy Water Environ. 2013, 11, 299–309. [Google Scholar] [CrossRef]
- Gao, L.; Wang, Z.; Shan, J.; Chen, J.; Tang, C.; Yi, M.; Zhao, X. Distribution characteristics and sources of trace metals in sediment cores from a trans-boundary watercourse: An example from the Shima River, Pearl River Delta. Ecotoxicol. Environ. Saf. 2016, 134, 186–195. [Google Scholar] [CrossRef]
- Kumari, A.; Parida, A.K. Metabolomics and network analysis reveal the potential metabolites and biological pathways involved in salinity tolerance of the halophyte Salvadora persica. Environ. Exp. Bot. 2018, 148, 85–99. [Google Scholar] [CrossRef]
- Islam, M.; Jahan, K.; Sen, A.; Urmi, T.A.; Haque, M.M.; Ali, H.M.; Siddiqui, M.H.; Murata, Y. Exogenous Application of Calcium Ameliorates Salinity Stress Tolerance of Tomato (Solanum lycopersicum L.) and Enhances Fruit Quality. Antioxidants 2023, 12, 558. [Google Scholar] [CrossRef]
- Selmar, D.; Radwan, A.; Abdalla, N.; Taha, H.; Wittke, C.; El-Henawy, A.; Alshaal, T.; Amer, M.; Kleinwächter, M.; Nowak, M.; et al. Uptake of nicotine from discarded cigarette butts–A so far unconsidered path of contamination of plant–derived commodities. Environ. Pollut. 2018, 238, 972–976. [Google Scholar] [CrossRef] [PubMed]
- Ishfaq, M.; Wang, Y.; Yan, M.; Wang, Z.; Wu, L.; Li, C.; Li, X. Physiological essence of magnesium in plants and its widespread deficiency in the farming system of China. Front. Plant Sci. 2022, 13, 802274. [Google Scholar] [CrossRef] [PubMed]
- Holubík, O.; Vaněk, A.; Mihaljevič, M.; Vejvodová, K. Higher Tl bioaccessibility in white mustard (hyper-accumulator) grown under the soil than hydroponic conditions: A key factor for the phytoextraction use. J. Environ. Manag. 2020, 255, 109880. [Google Scholar] [CrossRef] [PubMed]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Wilhelm, K.P.; Zhai, H.; Maibach, H.I. OECD guidelines for testing of chemicals. In Dermatotoxicology; CRC press: Boca Raton, FL, USA, 2012; pp. 509–511. [Google Scholar]
- Vasile, G.-G.; Tenea, A.-G.; Dinu, C.; Iordache, A.M.M.; Gheorghe, S.; Mureseanu, M.; Pascu, L.F. Bioavailability, Accumulation and Distribution of Toxic Metals (As, Cd, Ni and Pb) and Their Impact on Sinapis alba Plant Nutrient Metabolism. Int. J. Environ. Res. Public Health 2021, 18, 12947. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, Q.; Diao, C. Effects of soil amended with sewage sludge on growth, physiological response, and stress resistance of Zea mays. J. Hazard. Mater. 2009, 172, 1032–1038. [Google Scholar]



| Elements | Vertisol Soil |
|---|---|
| pH (H2O) | 6.88 |
| pH (KCl) | 6.30 |
| EC (µS·cm−1) | 95.63 |
| N (%) | 0.18 |
| C (%) | 1.83 |
| C: N | 10.34 |
| OM (%) | 4.90 |
| CEC (cmol+·kg−1) | 40.1 |
| Sand (%) | 3.00 |
| Silt (%) | 45.05 |
| Clay (%) | 51.95 |
| Textural class | Silty clay |
| Plasticity index (ml) | 43.4 |
| P2O5 | 23.75 |
| Ca | 4509.08 |
| Mg | 2218.24 |
| K | 1747.49 |
| Na | 84.54 |
| Al | 9492 |
| Co | 7.83 |
| Cr | 11.22 |
| Cu | 23.17 |
| Fe | 15,342 |
| Mn | 570.07 |
| Ni | 18.87 |
| Pb | 10.71 |
| Zn | 32.04 |
| Ba | 77.44 |
| Ti | 134.66 |
| V | 17.73 |
| OECD SOIL | ||||||||
|---|---|---|---|---|---|---|---|---|
| Germination (%) | Plant Height (cm) | Fresh Weight (g) | Dry Weight (g) | |||||
| CB use | Used | Unused | Used | Unused | Used | Unused | Used | Unused |
| Soil alone | 55.00 ± 17.53 | 5.69 ± 1.37 | 1.01 ± 0.37 | 0.27 ± 0.09 | ||||
| CB brand | ||||||||
| Pall Mall (1) | 60.00 ± 13.33 | 70.00 ± 15.87 | 9.88 ± 2.32 | 9.78 ± 1.87 | 2.13 ± 0.23 | 1.92 ± 0.18 | 0.40 ± 0.06 | 0.41 ± 0.07 |
| Philip Morris (2) | 78.33 ± 18.36 | 73.33 ± 14.40 | 9.48 ± 3.11 | 7.56 ± 0.83 | 1.92 ± 0.71 | 1.63 ± 0.58 | 0.38 ± 0.14 | 0.37 ± 0.09 |
| Marlboro (3) | 65.00 ± 11.39 | 65.00 ± 17.53 | 8.40 ± 1.24 | 9.32 ± 4.62 | 1.63 ± 0.49 | 1.65 ± 0.45 | 0.38 ± 0.10 | 0.36 ± 0.06 |
| VERTISOL SOIL | ||||||||
| Soil alone | 68.33 ± 17.53 | 23.43 ± 3.97 | 7.13 ± 1.23 | 1.50 ± 0.37 | ||||
| CB brand | ||||||||
| Pall Mall (1) | 90.00 ± 3.85 | 76.67 ± 8.61 | 22.90 ± 4.11 | 23.45 ± 5.24 | 8.04 ± 1.33 | 8.09 ± 1.15 | 1.68 ± 0.18 | 1.65 ± 0.20 |
| Philip Morris (2) | 81.67 ± 16.67 | 81.67 ± 6.38 | 21.63 ± 1.67 | 23.31 ± 3.08 | 7.96 ± 0.90 | 7.94 ± 0.41 | 1.58 ± 0.29 | 1.58 ± 0.22 |
| Marlboro (3) | 81.67 ± 14.78 | 85.00 ± 11.39 | 21.80 ± 3.11 | 24.01 ± 4.88 | 8.01 ± 0.92 | 8.50 ± 1.17 | 1.57 ± 0.17 | 1.78 ± 0.20 |
| Factors | Germination | Plant Height | Fresh Weight | Dry Weight |
|---|---|---|---|---|
| Soil type | 11.047 a | 41.269 a | 41.269 a | 41.310 a |
| CB use | 4.637 | 0.386 | 1.854 | 1.103 |
| CB brand | 5.482 | 0.652 | 2.003 | 1.310 |
| Factors | Al | Cu | Fe | Mn | Zn | Ba | Ti | Ca | Mg | K | Na |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil type | 3.613 | 4.331 c | 6.452 c | 41.053 a | 37.762 a | 1.170 | 13.594 a | 20.455 a | 16.118 a | 39.185 a | 3.997 c |
| CB use | 10.372 b | 20.805 a | 15.143 a | 3.132 | 0.496 | 24.134 a | 9.496b | 0.858 | 1.803 | 1.260 | 0.522 |
| CB brand | 6.736 | 10.056c | 5.317 | 2.106 | 4.671 | 5.776 | 3.299 | 1.987 | 1.120 | 1.232 | 0.582 |
| BAI | |||||||
|---|---|---|---|---|---|---|---|
| Factors | Al | Cu | Fe | Mn | Zn | Ba | Ti |
| Soil type | 0.155 | 0.845 | 0.069 | 3.625 | 1.724 | 9.931 b | 8.728 b |
| CB use | 2.049 | 3.336 | 2.078 | 0.856 | 0.478 | 5.597 | 5.160 |
| CB brand | 1.856 | 2.740 | 1.248 | 0.787 | 2.543 | 2.317 | 4.023 |
| UI | |||||||
| Soil type | 4.142 c | 0.276 | 5.280 c | 9.121 b | 6.897 b | 0.155 | 0.069 |
| CB use | 7.213 c | 1.915 | 16.505 a | 10.518 b | 1.289 | 2.654 | 22.614 a |
| CB brand | 6.701 | 0.630 | 10.321 c | 6.984 | 5.277 | 0.285 | 2.146 |
| Rotated Component Loadings | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Communality Estimates | |
| Dry weight | −0.931 | 0.949 | |||
| Fresh weight | −0.919 | 0.948 | |||
| Mn | 0.888 | 0.796 | |||
| Zn | 0.865 | 0.753 | |||
| Plant height | −0.862 | 0.878 | |||
| Ca | 0.793 | 0.472 | 0.883 | ||
| Na | 0.737 | 0.304 | 0.735 | ||
| Al | 0.878 | 0.785 | |||
| Fe | 0.793 | 0.769 | |||
| Ti | 0.447 | 0.678 | 0.507 | 0.947 | |
| K | 0.856 | 0.735 | |||
| Mg | −0.317 | 0.834 | 0.809 | ||
| Ba | 0.428 | 0.801 | 0.844 | ||
| Cu | 0.470 | 0.682 | 0.769 | ||
| Germination | −0.414 | −0.581 | 0.626 | ||
| Eigenvalues | 6.701 | 2.581 | 1.787 | 1.159 | |
| % of Variance | 44.675 | 17.203 | 11.914 | 7.725 | |
| Cumulative % | 44.675 | 61.878 | 73.792 | 81.516 | |
| Effect | Multivariate Test | Value | F | Hypothesis df | Error df | Sig. |
|---|---|---|---|---|---|---|
| CB use | Pillai’s trace | 0.844 | 52.618 | 4 | 39 | <0.001 |
| Wilks’ lambda | 0.156 | 52.618 | 4 | 39 | <0.001 | |
| Hotelling’s trace | 5.397 | 52.618 | 4 | 39 | <0.001 | |
| Roy’s largest root Pillai’s trace | 5.397 | 52.618 | 4 | 39 | <0.001 | |
| CB brand | 0.409 | 2.574 | 8 | 80 | 0.015 | |
| Wilks’ lambda | 0.628 | 2.551 | 8 | 78 | 0.016 | |
| Hotelling’s trace | 0.532 | 2.527 | 8 | 76 | 0.017 | |
| Roy’s largest root | 0.370 | 3.702 | 8 | 40 | 0.012 | |
| Soil type | Pillai’s trace | 0.979 | 445.951 | 4 | 39 | <0.001 |
| Wilks’ lambda | 0.021 | 445.951 | 4 | 39 | <0.001 | |
| Hotelling’s trace | 45.739 | 445.951 | 4 | 39 | <0.001 | |
| Roy’s largest root | 45.739 | 445.951 | 4 | 39 | <0.001 | |
| CB use × CB brand | Pillai’s trace | 0.512 | 3.444 | 8 | 80 | 0.002 |
| Wilks’ lambda | 0.496 | 4.099 | 8 | 78 | <0.001 | |
| Hotelling’s trace | 1.002 | 4.757 | 8 | 76 | <0.001 | |
| Roy’s largest root | 0.985 | 9.852 | 4 | 40 | <0.001 | |
| CB use × Soil type | Pillai’s trace | 0.805 | 40.233 | 4 | 39 | <0.001 |
| Wilks’ lambda | 0.195 | 40.233 | 4 | 39 | <0.001 | |
| Hotelling’s trace | 4.126 | 40.233 | 4 | 39 | <0.001 | |
| Roy’s largest root | 4.126 | 40.233 | 4 | 39 | <0.001 | |
| CB brand × Soil type | Pillai’s trace | 0.586 | 4.146 | 8 | 80 | <0.001 |
| Wilks’ lambda | 0.487 | 4.222 | 8 | 78 | <0.001 | |
| Hotelling’s trace | 0.903 | 4.290 | 8 | 76 | <0.001 | |
| Roy’s largest root | 0.683 | 6.834 | 4 | 40 | <0.001 | |
| CB use × CB brand × Soil type | Pillai’s trace | 0.254 | 1.455 | 8 | 80 | 0.187 |
| Wilks’ lambda | 0.751 | 1.500 | 8 | 78 | 0.171 | |
| Hotelling’s trace | 0.324 | 1.541 | 8 | 76 | 0.157 | |
| Roy’s largest root | 0.301 | 3.013 | 4 | 40 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajibade, S.; Simon, B.; Takács, A.; Gulyás, M. Effects of Cigarette Butt Leachate on the Growth of White Mustard (Sinapis alba L.) and Soil Properties: A Preliminary Study. Pollutants 2024, 4, 515-536. https://doi.org/10.3390/pollutants4040035
Ajibade S, Simon B, Takács A, Gulyás M. Effects of Cigarette Butt Leachate on the Growth of White Mustard (Sinapis alba L.) and Soil Properties: A Preliminary Study. Pollutants. 2024; 4(4):515-536. https://doi.org/10.3390/pollutants4040035
Chicago/Turabian StyleAjibade, Sinazo, Barbara Simon, Anita Takács, and Miklós Gulyás. 2024. "Effects of Cigarette Butt Leachate on the Growth of White Mustard (Sinapis alba L.) and Soil Properties: A Preliminary Study" Pollutants 4, no. 4: 515-536. https://doi.org/10.3390/pollutants4040035
APA StyleAjibade, S., Simon, B., Takács, A., & Gulyás, M. (2024). Effects of Cigarette Butt Leachate on the Growth of White Mustard (Sinapis alba L.) and Soil Properties: A Preliminary Study. Pollutants, 4(4), 515-536. https://doi.org/10.3390/pollutants4040035







