Removal of Trimethoprim and 5-Fluorouracil by UV/Persulfate and UV/VUV Persulfate Methods †
Abstract
1. Introduction
2. Materials and Methods
2.1. Photochemical Experiments
2.2. Materials and Methods
3. Results and Discussion
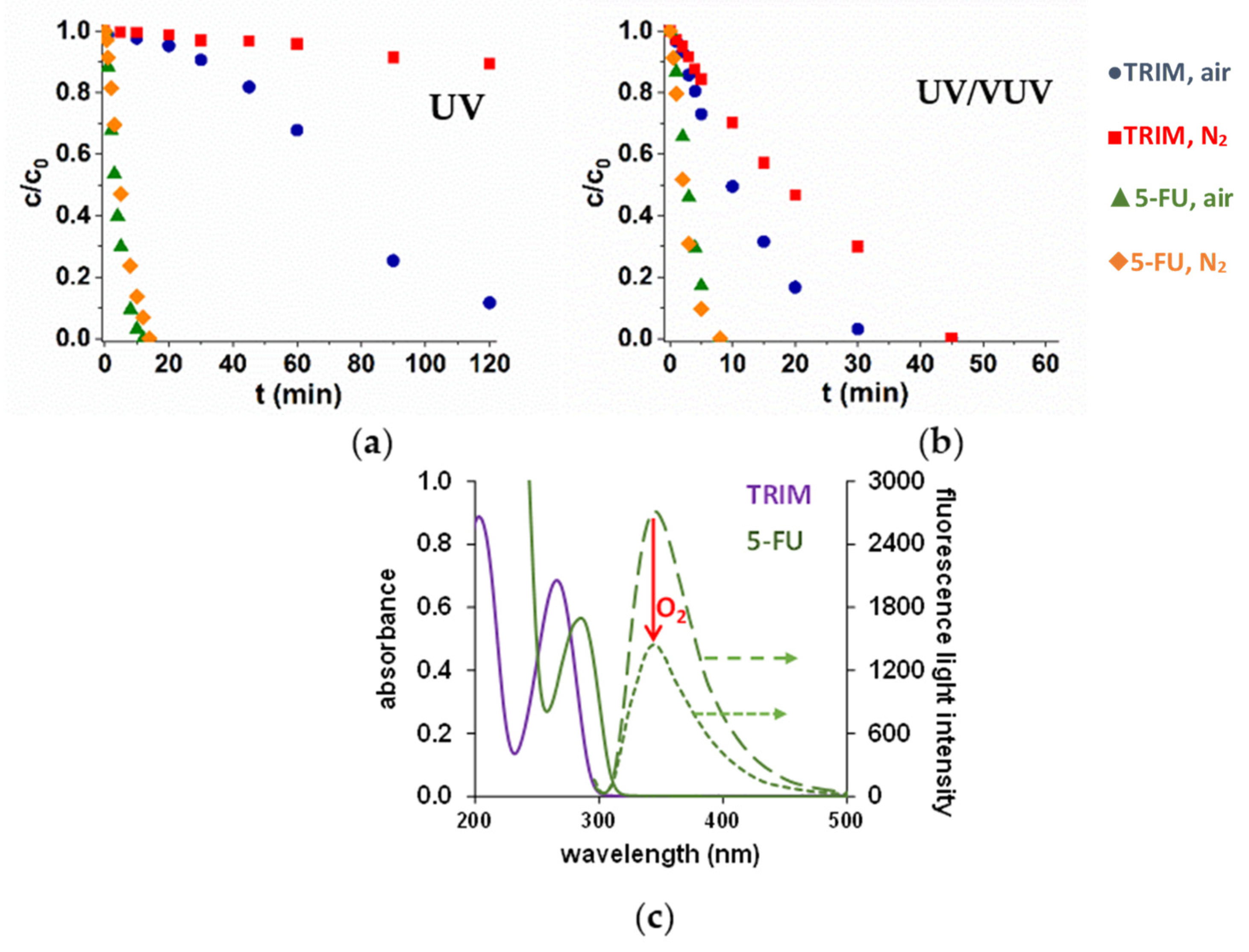
3.1. UV and UV/VUV Photolysis
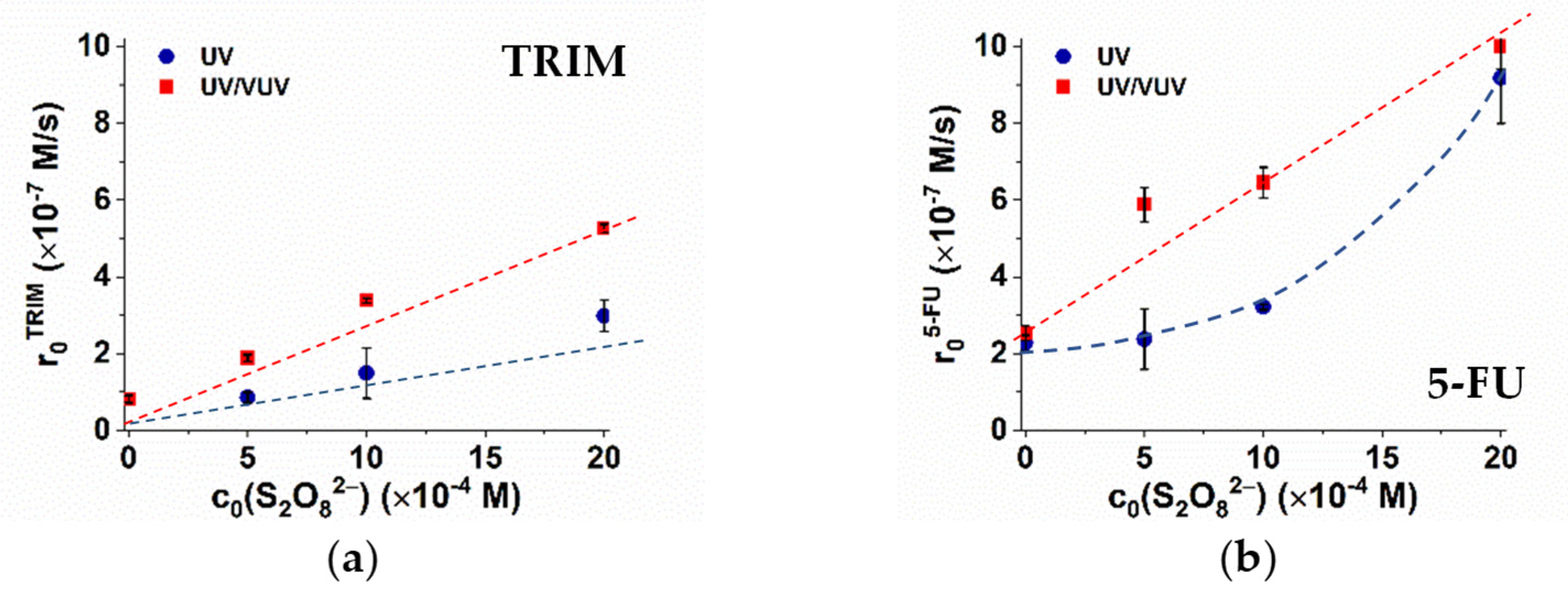
3.2. Effect of S2O82−
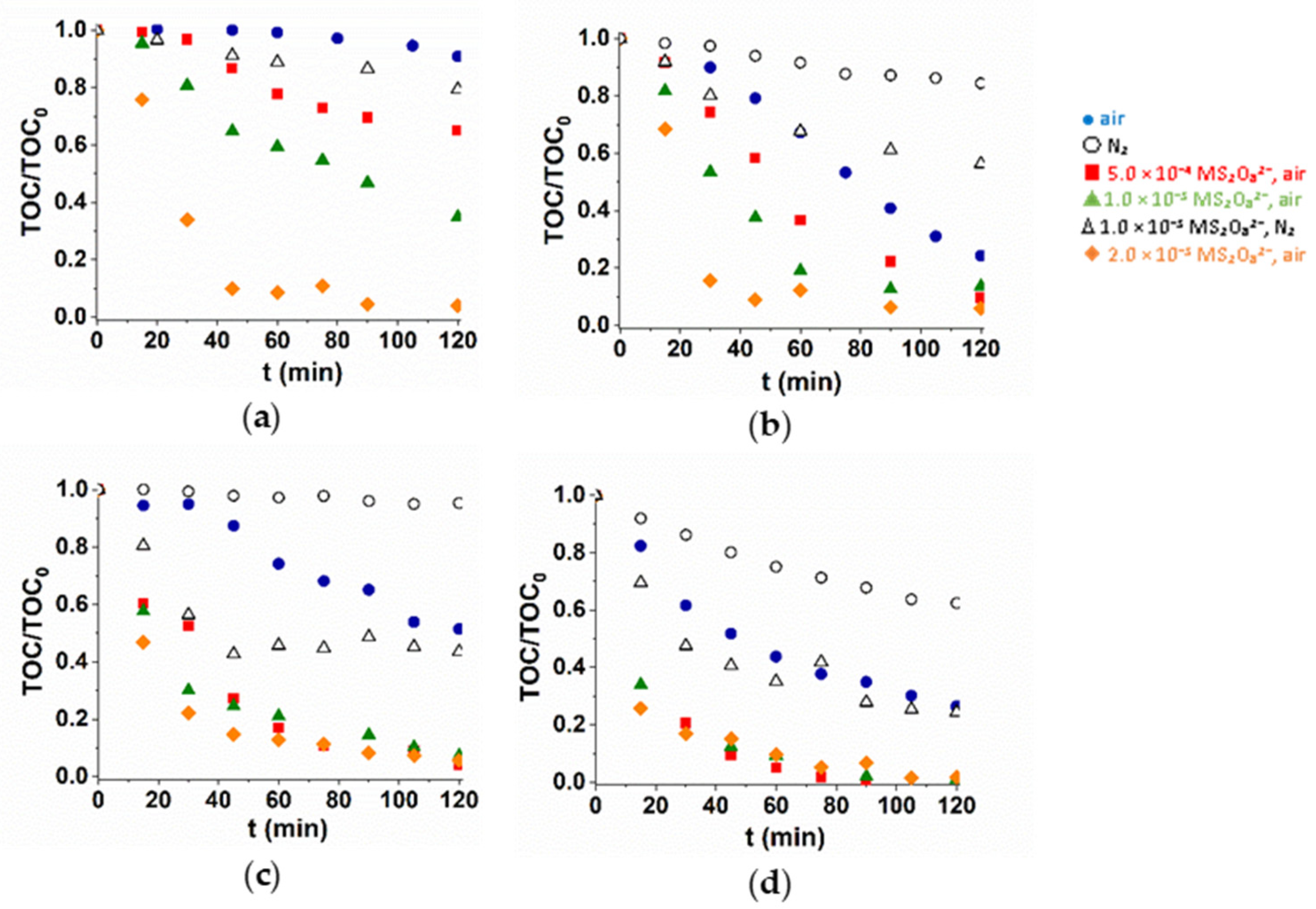
3.3. Radical Scavenger
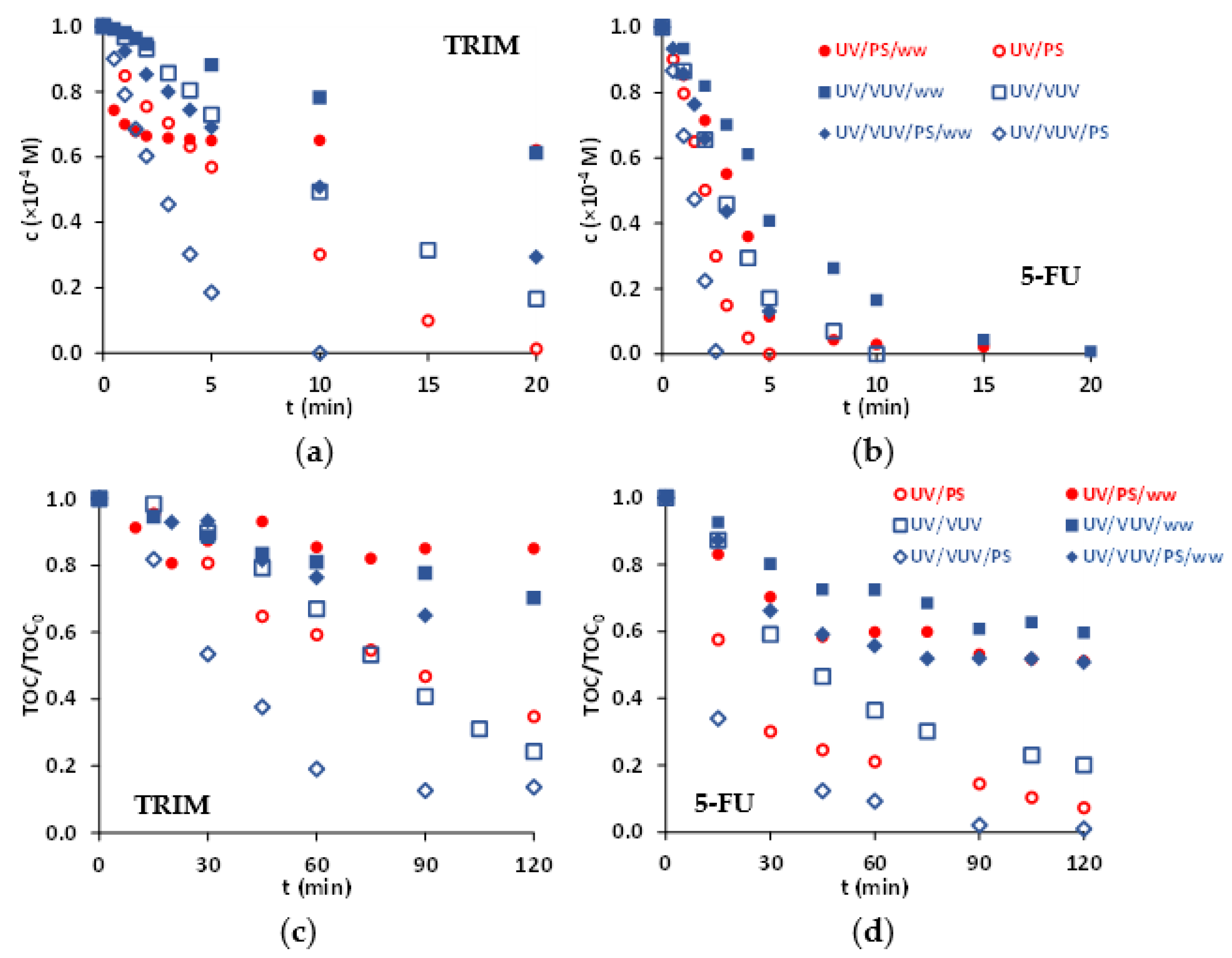
3.4. Matrix Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsieh, S.; Lai, W.W.; Lin, A.Y. Kinetics and mechanism of 4-methylbenzylidene camphor degradation by UV-activated persulfate oxidation. Environ. Sci. Pollut. Res. 2021, 28, 18021–18034. [Google Scholar] [CrossRef] [PubMed]
- Mark, G.; Schuchmann, M.N.; Schuchmann, H.P.; von Sonntag, C. The photolysis of potassium peroxodisulphate in aqueous solution in the presence of tert-butanol: A simple actinometer for 254 nm radiation. J. Photochem. Photobiol. A 1990, 55, 157–168. [Google Scholar] [CrossRef]
- Karpel Vel Leitner, N. Sulfate radical ion—Based AOPs. In Advanced Oxidation Processes for Water Treatment; Stefan, M.I., Ed.; IWA Publishing: London, UK, 2017; pp. 429–460. [Google Scholar]
- Criquet, J.; Leitner, N.K.V. Degradation of acetic acid with sulfate radical generated by persulfate ion photolysis. Chemosphere 2009, 77, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, G.; Zoh, K. Benzophenone-3 degradation via UV/H2O2 and UV/persulfate reactions, Benzophenone-3 degradation via UV/H2O2 and UV/persulfate reaction. J. Hazard. Mater. 2021, 403, 123591. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Lu, S.; Wang, Z.; Wang, Y.; Zhang, G.; Guo, X.; Guo, W.; Zhang, T.; Xi, B. Degradation difference of ofloxacin and levofloxacin by UV/H2O2 and UV/PS (persulfate): Efficiency, factors and mechanism. Chem. Eng. J. 2020, 385, 123987. [Google Scholar] [CrossRef]
- Karthikeyan, K.G.; Meyer, M.T. Occurrence of antibiotics in wastewater treatment facilities in Wisconsin, USA. Sci. Total Environ. 2006, 361, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Wojnárovits, L.; Tóth, T.; Takács, E. Critical evaluation of rate coefficients for hydroxyl radical reactions with antibiotics: A review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 575–613. [Google Scholar] [CrossRef]
- Ganzenko, O.; Oturan, N.; Sirés, I.; Huguenot, D.; van Hullebusch, E.D.; Esposito, G.; Oturan, M.A. Fast and complete removal of the 5-fluorouracil drug from water by electro-Fenton oxidation. Environ. Chem. Lett. 2018, 16, 281–286. [Google Scholar] [CrossRef]
- Luo, Y.; Su, R.; Yao, H.Y.; Zhang, A.; Xiang, S.; Huang, L. Degradation of trimethoprim by sulfate radical-based advanced oxidation processes: Kinetics, mechanisms, and effects of natural water matrices. Environ. Sci. Pollut. Res. 2021, 28, 62572–62582. [Google Scholar] [CrossRef] [PubMed]
- Mahdi-Ahmed, M.; Barbati, S.; Doumenq, P.; Chiron, S. Sulfate radical anion oxidation of diclofenac and sulfamethoxazole for water decontamination. Chem. Eng. J. 2012, 197, 440–447. [Google Scholar] [CrossRef]
- Mahnik, S.N.; Lenz, K.; Weissenbacher, N.; Mader, R.M.; Fuerhacker, M. Fate of 5-fluorouracil, doxorubicin, epirubicin, and daunorubicin in hospital wastewater and their elimination by activated sludge and treatment in a membrane-bio-reactor system. Chemosphere 2007, 66, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Al-Gharabli, S.; Engeßer, P.; Gera, D.; Klein, S.; Oppenländer, T. Engineering of a highly efficient Xe2*-excilamp (xenon excimer lamp, λmax = 172 nm, η = 40%) and qualitative comparison to a low-pressure mercury lamp (LP-Hg, λ=185/254 nm) for water purification. Chemosphere 2016, 144, 811–815. [Google Scholar] [CrossRef]
- Han, M.; Mohseni, M. Influence of sulfate and the interactions of major organic and inorganic solutes on the formation of nitrite during VUV photolysis of nitrate-rich water. J. Environ. Chem. Eng. 2021, 9, 105756. [Google Scholar] [CrossRef]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1247. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Jayson, G.G.; Parsons, B.J.; Swallow, A.J. Some simple, highly reactive, inorganic chlorine derivatives in aqueous solution. Their formation using pulses of radiation and their role in the mechanism of the Fricke dosimeter. J. Chem. Soc. Faraday Trans. 1 1973, 69, 1597–1607. [Google Scholar] [CrossRef]
- Wols, B.A.; Harmsen, D.J.H.; Beerendonk, E.F.; Hofman-Caris, C.H.M. Predicting pharmaceutical degradation by UV (LP)/H2O2 processes: A kinetic model. Chem. Eng. J. 2014, 255, 334–343. [Google Scholar] [CrossRef]

| Process | r0 (×10−7 M s−1) | |
|---|---|---|
| TRIM | 5-FU | |
| UV/air | – | 2.28 |
| UV/N2 | – | 1.54 |
| UV/air/S2O82− | 1.49 | 3.22 |
| UV/N2/S2O82− | 1.39 | 4.23 |
| UV/VUV/air | 0.82 | 2.53 |
| UV/VUV/N2 | 0.50 | 3.92 |
| UV/VUV/air/S2O82− | 3.38 | 6.45 |
| UV/VUV/N2/S2O82− | 3.28 | 7.12 |
| r0 (×10−7 M/s) | ||
|---|---|---|
| TRIM | 5-FU | |
| UV | – | 2.28 |
| UV/S2O82− | 1.49 | 3.22 |
| UV/S2O82−/tBuOH | 1.59 | 5.40 |
| UV/VUV | 0.82 | 2.53 |
| UV/VUV/tBuOH | 0.21 | 2.18 |
| UV/VUV/S2O82− | 3.38 | 6.45 |
| UV/VUV/S2O82−/tBuOH | 2.88 | 5.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farkas, L.; Szirmai, A.; Covic, A.; Alapi, T. Removal of Trimethoprim and 5-Fluorouracil by UV/Persulfate and UV/VUV Persulfate Methods. Environ. Sci. Proc. 2022, 21, 52. https://doi.org/10.3390/environsciproc2022021052
Farkas L, Szirmai A, Covic A, Alapi T. Removal of Trimethoprim and 5-Fluorouracil by UV/Persulfate and UV/VUV Persulfate Methods. Environmental Sciences Proceedings. 2022; 21(1):52. https://doi.org/10.3390/environsciproc2022021052
Chicago/Turabian StyleFarkas, Luca, Adrienn Szirmai, Anett Covic, and Tünde Alapi. 2022. "Removal of Trimethoprim and 5-Fluorouracil by UV/Persulfate and UV/VUV Persulfate Methods" Environmental Sciences Proceedings 21, no. 1: 52. https://doi.org/10.3390/environsciproc2022021052
APA StyleFarkas, L., Szirmai, A., Covic, A., & Alapi, T. (2022). Removal of Trimethoprim and 5-Fluorouracil by UV/Persulfate and UV/VUV Persulfate Methods. Environmental Sciences Proceedings, 21(1), 52. https://doi.org/10.3390/environsciproc2022021052







