Simulations of Tesla Valve Micromixer for Water Purification with Fe3O4 Nanoparticles †
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karvelas, E.; Liosis, C.; Karakasidis, T.; Sarris, I. Mixing of Particles in Micromixers under Different Angles and Velocities of the Incoming Water. Proceedings 2018, 2, 577. [Google Scholar] [CrossRef]
- Liosis, C.; Papadopoulou, A.; Karvelas, E.; Karakasidis, T.E.; Sarris, I.E. Heavy Metal Adsorption Using Magnetic Nanoparticles for Water Purification: A Critical Review. Materials 2021, 14, 7500. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Chen, D.-H. Preparation and Adsorption Properties of Monodisperse Chitosan-Bound Fe3O4 Magnetic Nanoparticles for Removal of Cu(II) Ions. J. Colloid Interface Sci. 2005, 283, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Sofos, F.; Karakasidis, T.E.; Spetsiotis, D. Molecular Dynamics Simulations of Ion Separation in Nano-Channel Water Flows Using an Electric Field. Mol. Simul. 2019, 45, 1395–1402. [Google Scholar] [CrossRef]
- Green, J.; Holdø, A.; Khan, A. A Review of Passive and Active Mixing Systems in Microfluidic Devices. Int. J. Multiphys. 2007, 1, 1–32. [Google Scholar] [CrossRef]
- Liosis, C.; Karvelas, E.G.; Karakasidis, T.; Sarris, I.E. Numerical Study of Magnetic Particles Mixing in Waste Water under an External Magnetic Field. J. Water Supply Res. Technol. 2020, 69, 266–275. [Google Scholar] [CrossRef]
- Cai, G.; Xue, L.; Zhang, H.; Lin, J. A Review on Micromixers. Micromachines 2017, 8, 274. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, J.S.; Mathioulakis, D.S. Numerical Simulation and Hydrodynamic Design Optimization of a Tesla-Type Valve for Micropumps. In Proceedings of the 3rd IASME/WSEAS International Conference Fluid Dynamic Aerodynamic, Corfu, Greece, 20–22 August 2005; pp. 195–201. [Google Scholar]
- Fairley, J.D.; Thompson, S.M.; Anderson, D. Time-Frequency Analysis of Flat-Plate Oscillating Heat Pipes. Int. J. Therm. Sci. 2015, 91, 113–124. [Google Scholar] [CrossRef]
- Wang, C.T.; Chen, Y.M.; Hong, P.A.; Wang, Y.T. Tesla Valves in Micromixers. Int. J. Chem. React. Eng. 2014, 12, 397–403. [Google Scholar] [CrossRef]
- Hossain, S.; Ansari, M.A.; Husain, A.; Kim, K.Y. Analysis and Optimization of a Micromixer with a Modified Tesla Structure. Chem. Eng. J. 2010, 158, 305–314. [Google Scholar] [CrossRef]
- Weng, X.; Yan, S.; Zhang, Y.; Liu, J.; Shen, J. Design, Simulation and Experimental Study of a Micromixer Based on Tesla Valve Structure. Huagong Jinzhan/Chem. Ind. Eng. Prog. 2021, 40, 4173–4178. [Google Scholar] [CrossRef]
- Teja, A.S.; Koh, P.-Y. Synthesis, Properties, and Applications of Magnetic Iron Oxide Nanoparticles. Prog. Cryst. Growth Charact. Mater. 2009, 55, 22–45. [Google Scholar] [CrossRef]
- Chicot, D.; Mendoza, J.; Zaoui, A.; Louis, G.; Lepingle, V.; Roudet, F.; Lesage, J. Mechanical Properties of Magnetite (Fe3O4), Hematite (α-Fe2O3) and Goethite (α-FeO·OH) by Instrumented Indentation and Molecular Dynamics Analysis. Mater. Chem. Phys. 2011, 129, 862–870. [Google Scholar] [CrossRef]
- Sofos, F.; Liakopoulos, A.; Karakasidis, T.E. Particle-Based Modeling and Meshless Simulation of Flows with Smoothed Particle Hydrodynamics. Glob. Nest J. 2019, 21, 513–518. [Google Scholar] [CrossRef]
- Endaylalu, S.A.; Tien, W.-H. A Numerical Investigation of the Mixing Performance in a Y-Junction Microchannel Induced by Acoustic Streaming. Micromachines 2022, 13, 338. [Google Scholar] [CrossRef] [PubMed]
- Weller, H.G.; Tabor, G.; Jasak, H.; Fureby, C. A Tensorial Approach to Computational Continuum Mechanics Using Object-Oriented Techniques. Comput. Phys. 1998, 12, 620. [Google Scholar] [CrossRef]
- Ramesha, D.K.; Anvekar, A.; Raj, A.; Vighnesh, J.; Tripathi, S. A DSMC Analysis of Gas Flow in Micro Channels Using OpenFOAM. In Proceedings of the International Conference on Advances in Mechanical Engineering Sciences (ICAMES-17), PES College of Engineering, Mandya, India, 21–22 April 2017. [Google Scholar]
- Karvelas, E.; Liosis, C.; Karakasidis, T.; Sarris, I. Micromixing Nanoparticles and Contaminated Water Under Different Velocities for Optimum Heavy Metal Ions Adsorption. Environ. Sci. Proc. 2020, 2, 65. [Google Scholar] [CrossRef]
- Karvelas, E.; Liosis, C.; Benos, L.; Karakasidis, T.; Sarris, I. Micromixing Efficiency of Particles in Heavy Metal Removal Processes under Various Inlet Conditions. Water 2019, 11, 1135. [Google Scholar] [CrossRef]
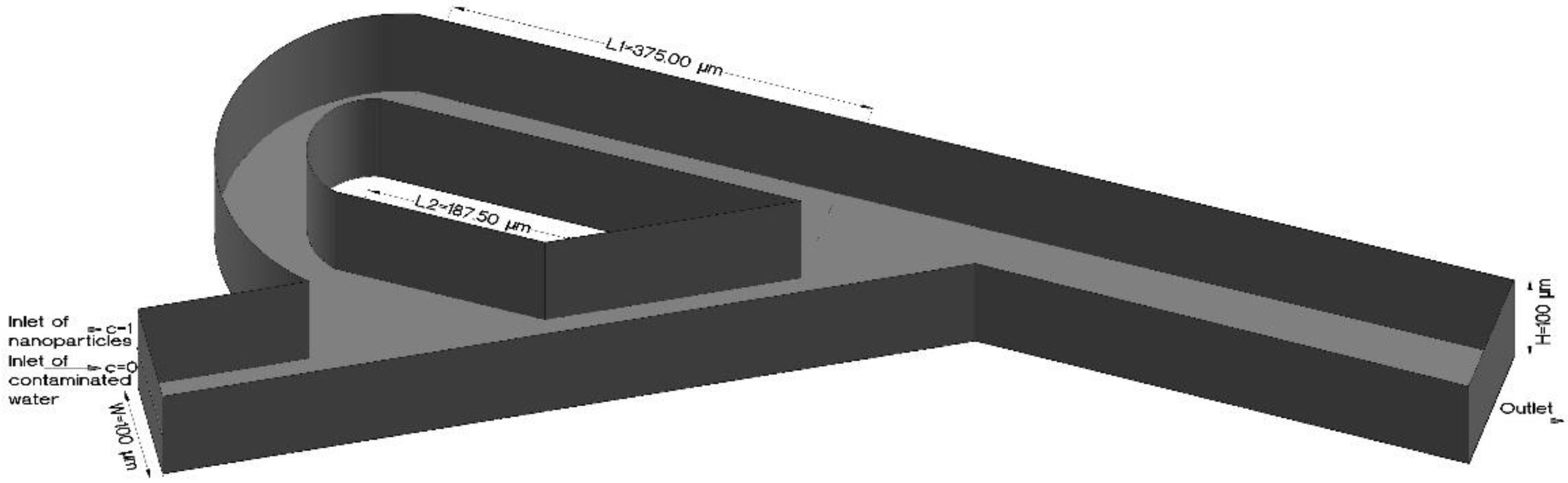
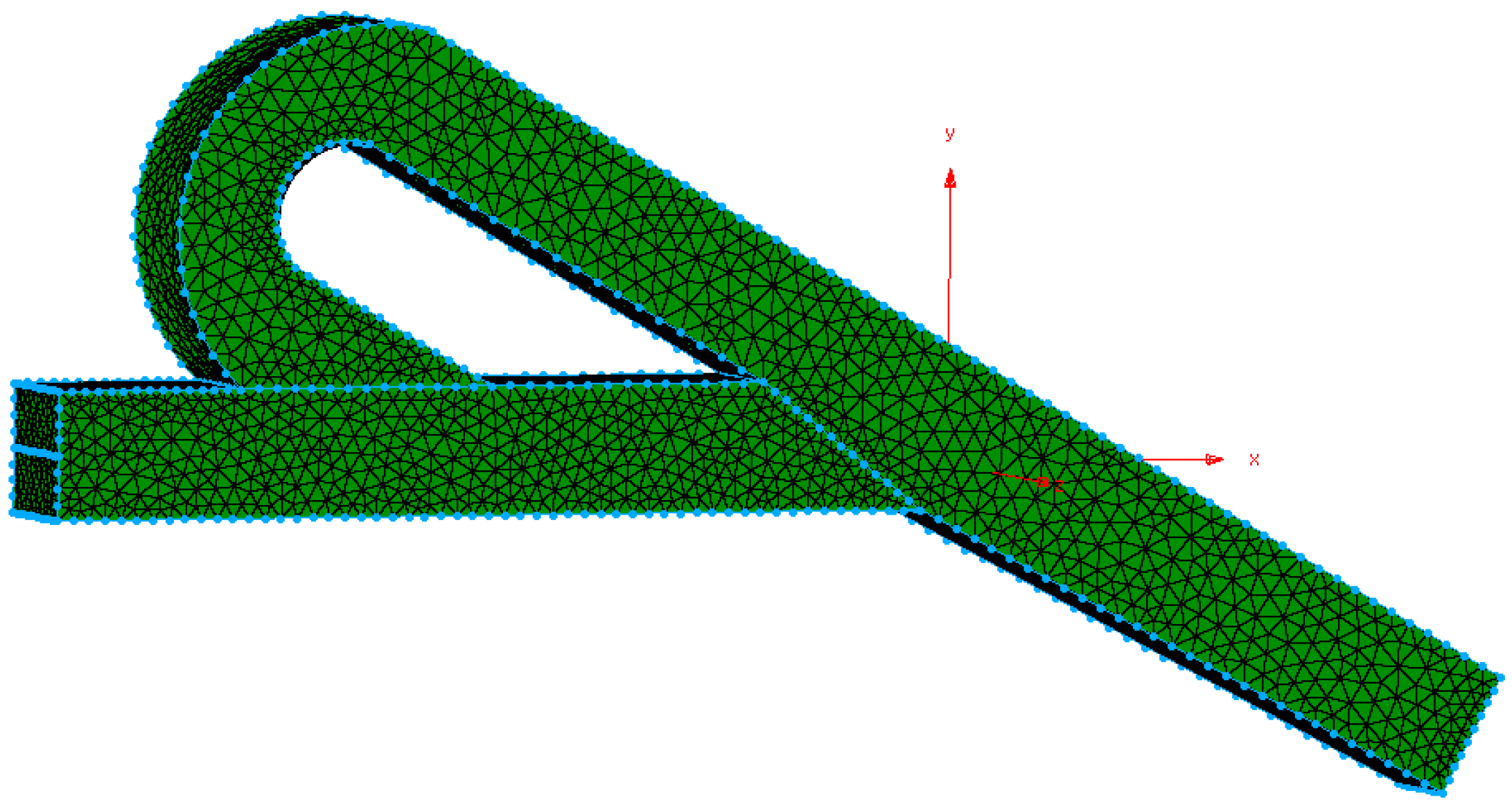


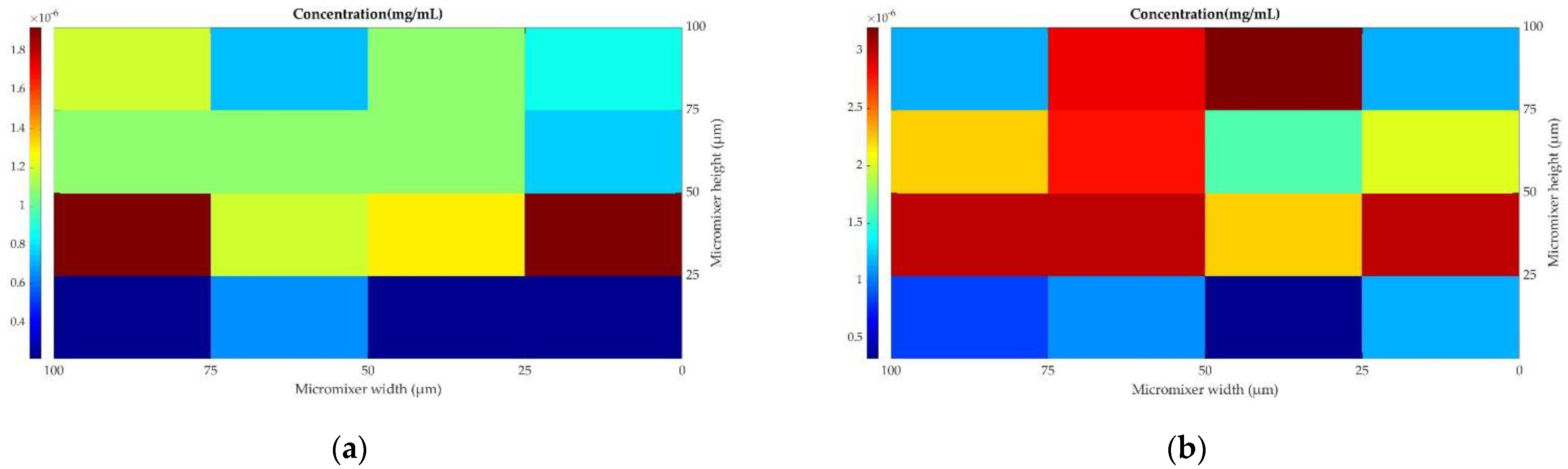
| Inlet, outlet dimensions of geometry | Height (H) = Width (W) = 1 × 10−4 m | |
| Diameter of nanoparticles | 13.5 nm | |
| Nanoparticles per second | 500 and 1000 | |
| Boundary conditions | Velocity (U) (m/s) | Pressure (p) (pa) |
| Contaminated water–heavy metals (Vc) | 0.0005, 0.00005 | zero gradient |
| Nanoparticles (Vp) | 0.0005 | zero gradient |
| Outlet | zero gradient | 0 |
| Walls | 0 | zero gradient |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liosis, C.; Sofiadis, G.; Karvelas, E.; Karakasidis, T.; Sarris, I. Simulations of Tesla Valve Micromixer for Water Purification with Fe3O4 Nanoparticles. Environ. Sci. Proc. 2022, 21, 82. https://doi.org/10.3390/environsciproc2022021082
Liosis C, Sofiadis G, Karvelas E, Karakasidis T, Sarris I. Simulations of Tesla Valve Micromixer for Water Purification with Fe3O4 Nanoparticles. Environmental Sciences Proceedings. 2022; 21(1):82. https://doi.org/10.3390/environsciproc2022021082
Chicago/Turabian StyleLiosis, Christos, George Sofiadis, Evangelos Karvelas, Theodoros Karakasidis, and Ioannis Sarris. 2022. "Simulations of Tesla Valve Micromixer for Water Purification with Fe3O4 Nanoparticles" Environmental Sciences Proceedings 21, no. 1: 82. https://doi.org/10.3390/environsciproc2022021082
APA StyleLiosis, C., Sofiadis, G., Karvelas, E., Karakasidis, T., & Sarris, I. (2022). Simulations of Tesla Valve Micromixer for Water Purification with Fe3O4 Nanoparticles. Environmental Sciences Proceedings, 21(1), 82. https://doi.org/10.3390/environsciproc2022021082









