Comparative Analysis of Photodegradation of Ibuprofen and Clotrimazole Water Pollutant Using UVC Rays in Presence and Absence of ZnO Photocatalyst †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
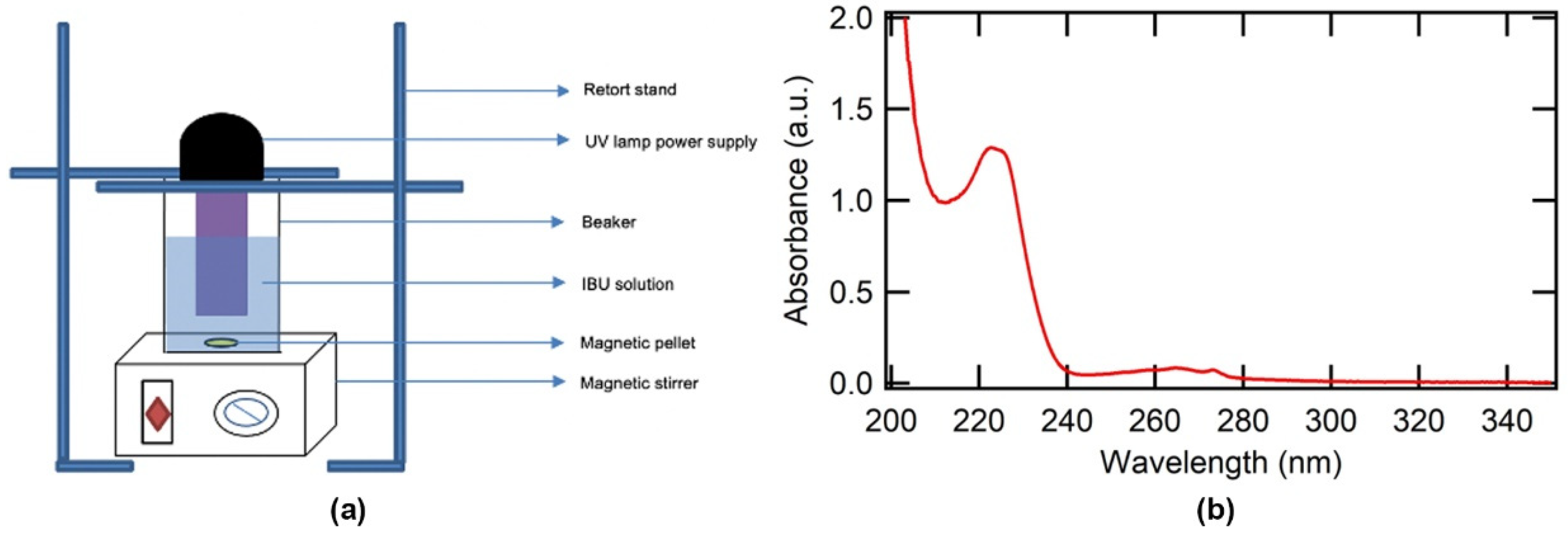
2.2. Experimental Setup
2.3. Measurement and Analysis Using UV–Vis Spectrophotometer
3. Results and Discussion
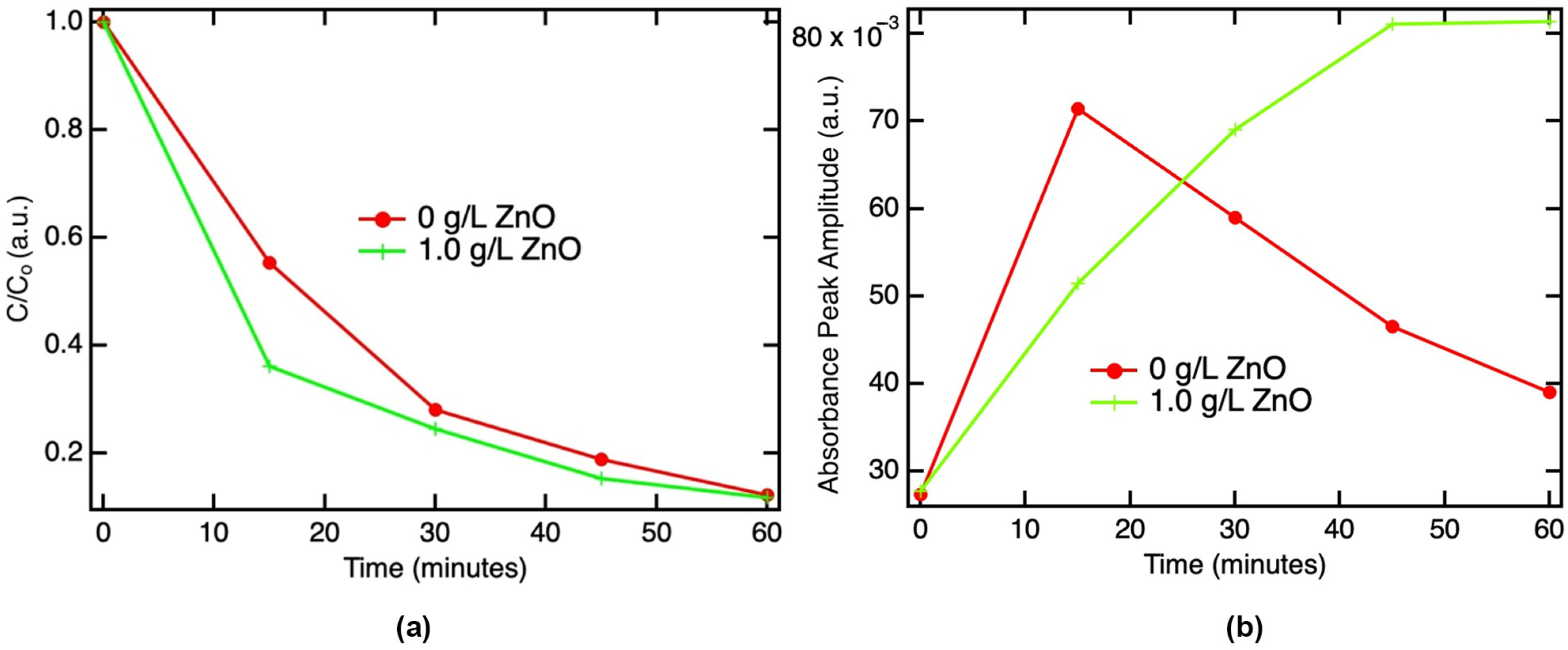
3.1. Time Dependence of Degradation of IBP without and with ZnO Particles
3.2. Extraction of the Rate of IBP Degradation and Maximum IBP Degradation Potential
3.3. Clotrimazole Degradation Rate and Maximum Degradation Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stasinakis, A.; Gatidou, G. Micropollutants and Aquatic Environment. Treatment of Micropollutants Water and Wastewater; IWA Publishing: London, UK, 2016; pp. 1–51. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of Pharmaceuticals and Personal Care Products (PPCPs) from Wastewater: A Review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef] [PubMed]
- Bottoni, P.; Caroli, S.; Caracciolo, A.B. Pharmaceuticals as Priority Water Contaminants. Toxicol. Environ. Chem. 2010, 92, 549–565. [Google Scholar] [CrossRef]
- Thiebault, T.; Boussafir, M.; Le Milbeau, C. Occurrence and Removal Efficiency of Pharmaceuticals in an Urban Wastewater Treatment Plant: Mass Balance, Fate and Consumption Assessment. J. Environ. Chem. Eng. 2017, 5, 2894–2902. [Google Scholar] [CrossRef]
- Żur, J.; Piński, A.; Marchlewicz, A.; Hupert-Kocurek, K.; Wojcieszyńska, D.; Guzik, U. Organic Micropollutants Paracetamol and Ibuprofen-Toxicity, Biodegradation, and Genetic Background of Their Utilization by Bacteria. Environ. Sci. Pollut. Res. Int. 2018, 25, 21498–21524. [Google Scholar] [CrossRef] [PubMed]
- Daughton, C. Pharmaceuticals as Environmental Pollutants: The Ramifications for Human Exposure. In International Encyclopedia of Public Health; Academic Press: Cambridge, MA, USA, 2008; pp. 66–102. [Google Scholar] [CrossRef]
- Rehman, M.S.U.; Rashid, N.; Ashfaq, M.; Saif, A.; Ahmad, N.; Han, J.-I. Global Risk of Pharmaceutical Contamination from Highly Populated Developing Countries. Chemosphere 2015, 138, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Rudd, N.D.; Wang, H.; Fuentes-Fernandez, E.M.A.; Teat, S.J.; Chen, F.; Hall, G.; Chabal, Y.J.; Li, J. Highly Efficient Luminescent Metal-Organic Framework for the Simultaneous Detection and Removal of Heavy Metals from Water. ACS Appl. Mater. Interfaces 2016, 8, 30294–30303. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Drewek, A.; Klimaszyk, P. Pharmaceutical Pollution of Aquatic Environment: An Emerging and Enormous Challenge. Limnol. Rev. 2017, 17, 97–107. [Google Scholar] [CrossRef]
- Chopra, S.; Kumar, D. Ibuprofen as an Emerging Organic Contaminant in Environment, Distribution and Remediation. Heliyon 2020, 6, e04087. [Google Scholar] [CrossRef] [PubMed]
- Tanveer, M.; Guyer, G.T.; Abbas, G. Photocatalytic Degradation of Ibuprofen in Water Using TiO2 and ZnO under Artificial UV and Solar Irradiation. Water Environ. Res. 2019, 91, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Sabouni, R.; Gomaa, H. Photocatalytic Degradation of Pharmaceutical Micro-Pollutants Using ZnO. Environ. Sci. Pollut. Res. Int. 2019, 26, 5372–5380. [Google Scholar] [CrossRef] [PubMed]
- Jallouli, N.; Pastrana-Martínez, L.M.; Ribeiro, A.R.; Moreira, N.F.F.; Faria, J.L.; Hentati, O.; Silva, A.M.T.; Ksibi, M. Heterogeneous Photocatalytic Degradation of Ibuprofen in Ultrapure Water, Municipal and Pharmaceutical Industry Wastewaters Using a TiO2/UV-LED System. Chem. Eng. J. 2018, 334, 976–984. [Google Scholar] [CrossRef]
- Prayitno; Kusuma, Z.; Yanuwiadi, B.; Laksmono, R.W.; Kamahara, H.; Daimon, H. Hospital Wastewater Treatment Using Aerated Fixed Film Biofilter—Ozonation (Af2b/O3). Adv. Environ. Biol. 2014, 8, 1251–1260. [Google Scholar]
- Gonçalves, N.P.F.; del Puerto, O.; Medana, C.; Calza, P.; Roslev, P. Degradation of the Antifungal Pharmaceutical Clotrimazole by UVC and Vacuum-UV Irradiation: Kinetics, Transformation Products and Attenuation of Toxicity. J. Environ. Chem. Eng. 2021, 9, 106275. [Google Scholar] [CrossRef]
- The Risks of Environmental Effects of Pharmaceutical and Medicinal Products. Available online: https://www.greenfacts.org/en/pharmaceuticals-environment/index.htm (accessed on 9 May 2022).
- Khalaf, S.; Hasan, J.; Lelario, F.; Scrano, L.; Bufo, S.; Karaman, R. TiO2 and Active Coated Glass Photodegradation of Ibuprofen. Catalysts 2020, 10, 560. [Google Scholar] [CrossRef]
- Mahmood, S.; Ahmad, Z.; Aslam, M.; Naeem, F.; Hussain, A.; Kumar, N. Method Development and Validation for the Estimation and Evaluation of Clotrimazole (an-Antifungal Drug) in Tablet Preparation by UV-VIS Spectroscopy. Int. J. Pharm. Sci. Rev. Res. 2015, 32, 55–58. [Google Scholar]
- Wang, Z.; Srivastava, V.; Ambat, I.; Safaei, Z.; Sillanpää, M. Degradation of Ibuprofen by UV-LED/Catalytic Advanced Oxidation Process. J. Water Process Eng. 2019, 31, 100808. [Google Scholar] [CrossRef]
- Ruggeri, G.; Ghigo, G.; Maurino, V.; Minero, C.; Vione, D. Photochemical Transformation of Ibuprofen into Harmful 4-Isobutylacetophenone: Pathways, Kinetics, and Significance for Surface Waters. Water Res. 2013, 47, 6109–6121. [Google Scholar] [CrossRef] [PubMed]
| ZnO Concentration (g/L) | k (min−1) | Maximum Degradation Potential (%) |
|---|---|---|
| 0 | 0.045 | 94.4 |
| 1 | 0.083 | 86.6 |
| ZnO Concentration (g/L) | k (min−1) | Maximum Degradation Potential (%) |
|---|---|---|
| 0 | 0.025 | 92.0 |
| 0.5 | 0.034 | 95.2 |
| 1.0 | 0.051 | 99.9 |
| 1.5 | 0.047 | 95.9 |
| 2.0 | 0.023 | 93.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesik, S.; Jobiliong, E.; Steven, E. Comparative Analysis of Photodegradation of Ibuprofen and Clotrimazole Water Pollutant Using UVC Rays in Presence and Absence of ZnO Photocatalyst. Environ. Sci. Proc. 2023, 25, 49. https://doi.org/10.3390/ECWS-7-14176
Pesik S, Jobiliong E, Steven E. Comparative Analysis of Photodegradation of Ibuprofen and Clotrimazole Water Pollutant Using UVC Rays in Presence and Absence of ZnO Photocatalyst. Environmental Sciences Proceedings. 2023; 25(1):49. https://doi.org/10.3390/ECWS-7-14176
Chicago/Turabian StylePesik, Shania, Eric Jobiliong, and Eden Steven. 2023. "Comparative Analysis of Photodegradation of Ibuprofen and Clotrimazole Water Pollutant Using UVC Rays in Presence and Absence of ZnO Photocatalyst" Environmental Sciences Proceedings 25, no. 1: 49. https://doi.org/10.3390/ECWS-7-14176
APA StylePesik, S., Jobiliong, E., & Steven, E. (2023). Comparative Analysis of Photodegradation of Ibuprofen and Clotrimazole Water Pollutant Using UVC Rays in Presence and Absence of ZnO Photocatalyst. Environmental Sciences Proceedings, 25(1), 49. https://doi.org/10.3390/ECWS-7-14176





