The Abstruse Side of Type I Interferon Immunotherapy for COVID-19 Cases with Comorbidities
Abstract
:1. Introduction
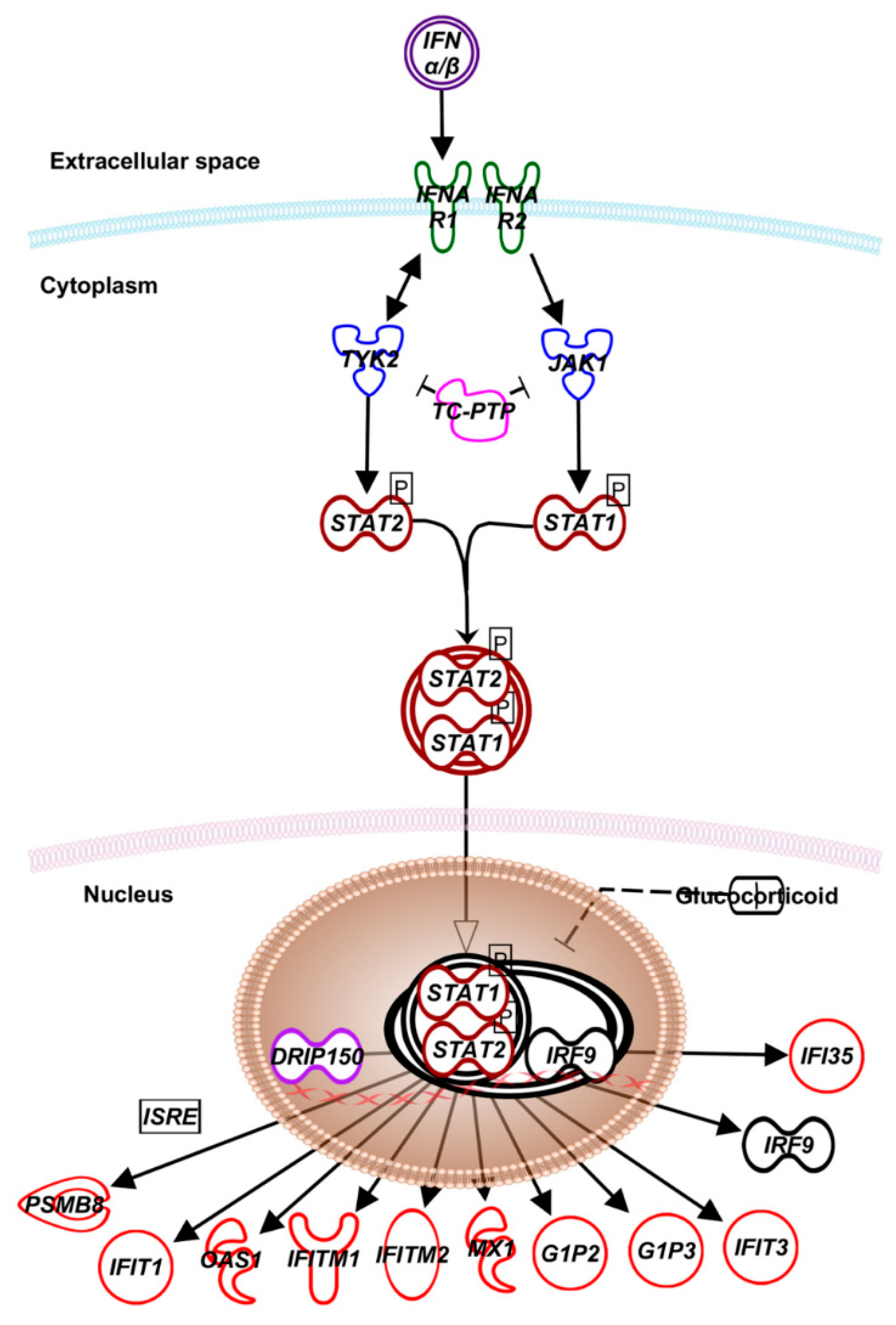
2. Type-I IFN Signaling
3. Type-I IFN Response and IFN-I Immunotherapy for COVID-19
4. Implications of IFN-I Immunotherapy on the Host Response to Other Infectious Diseases
4.1. Viral Diseases
4.2. Nosocomial Infection
4.3. Tuberculosis
5. Implications of IFN-I Immunotherapy on the Host Response to Non-infectious Diseases
5.1. Pulmonary Arterial Hypertension
5.2. Diabetes
5.3. Autoimmune Diseases
6. Summary and Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 8 January 2021).
- Khalaf, K.; Papp, N.; Chou, J.T.; Hana, D.; Mackiewicz, A.; Kaczmarek, M. SARS-CoV-2: Pathogenesis, and Advancements in Diagnostics and Treatment. Front. Immunol. 2020, 11, 570927. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Clinical Trials–COVID19. Available online: https://www.clinicaltrials.gov/ct2/results?cond=COVID-19 (accessed on 7 November 2020).
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Ashkar, A.A. The Dual Nature of Type I and Type II Interferons. Front. Immunol. 2018, 9, 2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teijaro, J.R. Pleiotropic Roles of Type 1 Interferons in Antiviral Immune Responses. Adv. Immunol. 2016, 132, 135–158. [Google Scholar] [PubMed]
- Sa Ribero, M.; Jouvenet, N.; Dreux, M.; Nisole, S. Interplay between SARS-CoV-2 and the type I interferon response. PLoS Pathog. 2020, 16, e1008737. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; To, K.K.; Wong, Y.C.; Liu, L.; Zhou, B.; Li, X.; Huang, H.; Mo, Y.; Luk, T.Y.; Lau, T.T.; et al. Acute SARS-CoV-2 Infection Impairs Dendritic Cell and T Cell Responses. Immunity 2020, 53, 864–877.e5. [Google Scholar] [CrossRef]
- Zhou, Z.; Ren, L.; Zhang, L.; Zhong, J.; Xiao, Y.; Jia, Z.; Guo, L.; Yang, J.; Wang, C.; Jiang, S.; et al. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host Microbe 2020, 27, 883–890.e2. [Google Scholar] [CrossRef]
- Hadjadj, J.; Yatim, N.; Barnabei, L.; Corneau, A.; Boussier, J.; Smith, N.; Pere, H.; Charbit, B.; Bondet, V.; Chenevier-Gobeaux, C.; et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science 2020, 369, 718–724. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e19. [Google Scholar] [CrossRef]
- Park, A.; Iwasaki, A. Type I and Type III Interferons - Induction, Signaling, Evasion, and Application to Combat COVID-19. Cell Host Microbe 2020, 27, 870–878. [Google Scholar] [CrossRef]
- Johnston, S.L. IFN Therapy in Airway Disease: Is Prophylaxis a New Approach in Exacerbation Prevention? Am. J. Respir. Crit. Care Med. 2020, 201, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Scagnolari, C.; Antonelli, G. Type I interferon and HIV: Subtle balance between antiviral activity, immunopathogenesis and the microbiome. Cytokine Growth Factor Rev. 2018, 40, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, S.; Thomas, D.L.; Balagopal, A. HIV-1 Infection and Type 1 Interferon: Navigating Through Uncertain Waters. AIDS Res. Hum. Retroviruses 2019, 35, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Parker, D. Impact of Type I and III Interferons on Respiratory Superinfections Due to Multidrug-Resistant Pathogens. J. Infect. Dis. 2017, 215, S58–S63. [Google Scholar] [CrossRef] [Green Version]
- Moreira-Teixeira, L.; Mayer-Barber, K.; Sher, A.; O’Garra, A. Type I interferons in tuberculosis: Foe and occasionally friend. J. Exp. Med. 2018, 215, 1273–1285. [Google Scholar] [CrossRef] [Green Version]
- Rivas-Santiago, C.E.; Guerrero, G.G. IFN-alpha Boosting of Mycobacterium bovis Bacillus Calmette Guerin-Vaccine Pro-moted Th1 Type Cellular Response and Protection against M. tuberculosis Infection. Biomed. Res. Int. 2017, 2017, 8796760. [Google Scholar] [CrossRef]
- Savale, L.; Chaumais, M.C.; O’Connell, C.; Humbert, M.; Sitbon, O. Interferon-induced pulmonary hypertension: An update. Curr. Opin. Pulm. Med. 2016, 22, 415–420. [Google Scholar] [CrossRef]
- Nakamura, K.; Kawasaki, E.; Imagawa, A.; Awata, T.; Ikegami, H.; Uchigata, Y.; Kobayashi, T.; Shimada, A.; Nakanishi, K.; Makino, H.; et al. Type 1 diabetes and interferon therapy: A nationwide survey in Japan. Diabetes Care 2011, 34, 2084–2089. [Google Scholar] [CrossRef] [Green Version]
- Newby, B.N.; Mathews, C.E. Type I Interferon Is a Catastrophic Feature of the Diabetic Islet Microenvironment. Front. Endocrinol. (Lausanne) 2017, 8, 232. [Google Scholar] [CrossRef] [Green Version]
- Schulthess, F.T.; Paroni, F.; Sauter, N.S.; Shu, L.; Ribaux, P.; Haataja, L.; Strieter, R.M.; Oberholzer, J.; King, C.C.; Maedler, K. CXCL10 impairs beta cell function and viability in diabetes through TLR4 signaling. Cell Metab. 2009, 9, 125–139. [Google Scholar] [CrossRef] [Green Version]
- Psarras, A.; Emery, P.; Vital, E.M. Type I interferon-mediated autoimmune diseases: Pathogenesis, diagnosis and targeted therapy. Rheumatology (Oxford) 2017, 56, 1662–1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Tatouli, I.P.; Rosen, L.B.; Hasni, S.; Alevizos, I.; Manna, Z.G.; Rivera, J.; Jiang, C.; Siegel, R.M.; Holland, S.M.; et al. Distinct Functions of Autoantibodies Against Interferon in Systemic Lupus Erythematosus: A Comprehensive Analysis of Anticytokine Autoantibodies in Common Rheumatic Diseases. Arthritis Rheumatol. 2016, 68, 1677–1687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moll, H.P.; Freudenthaler, H.; Zommer, A.; Buchberger, E.; Brostjan, C. Neutralizing type I IFN antibodies trigger an IFN-like response in endothelial cells. J. Immunol. 2008, 180, 5250–5256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Beziat, V.; et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Pioggia, G.; Negrini, S. Vitamin D and Covid-19: An update on evidence and potential therapeutic implications. Clin. Mol. Allergy 2020, 18, 23. [Google Scholar] [CrossRef]
- Munoz-Fontela, C.; Dowling, W.E.; Funnell, S.G.P.; Gsell, P.S.; Riveros-Balta, A.X.; Albrecht, R.A.; Andersen, H.; Baric, R.S.; Carroll, M.W.; Cavaleri, M.; et al. Animal models for COVID-19. Nature 2020, 586, 509–515. [Google Scholar] [CrossRef]
| Trial ID | IFN-I Molecule | Adjunctive Drug # | Route of IFN-I Administration | Study Sponsor | Reference/Status * |
|---|---|---|---|---|---|
| ChiCTR2000029387 | IFNα1β | Lopinavir/Ritonavir or Ribavirin | Subcutaneous | Chongqing Public Health Medical Center, China | Recruiting |
| ChiCTR2000029638 | IFNα (“super-compound”) | Umifenovir or Lopinavir and Ritonavir | Nebulization | West China Hospital, China | Recruiting |
| ChiCTR2000030262 | IFNκ | Trefoil Factor Family 2 | Aerosol inhalation | Shanghai Medical College, China | Recruiting |
| ChiCTR2000030013 | IFNα1β | Standard care | Nasal spray | Chinese PLA General Hospital, China | Not recruiting |
| ChiCTR2000030166 | IFNα2β | Lopinavir/Ritonavir or Qing-Wen Bai-Du-Yin granules | Injection (route not disclosed) | The 5th Medical Center Chinese PLA General Hospital, China | Not recruiting |
| ChiCTR2000030922 | IFNα2α | Ribavirin | Route not disclosed | Foshan First People’s Hospital, China | Recruiting |
| ChiCTR2000029989 | IFNα1β | Standard care | Eye drops | Chinese PLA General Hospital, China | Not recruiting |
| ChiCTR2000030082 | IFNα | Dihydroartemisinin/Piperaquine or Ardibdol | Route not disclosed | The First Affiliated Hospital of Nanchang University, China | Suspended |
| ChiCTR2000029600 | IFNα | Lopinavir/Ritonavir or Favipiravir | Atomization (nasal spray) | The Third People’s Hospital of Shenzhen, China | Recruiting |
| ChiCTR2000030535 | IFNα | Ebastine and Lopinavir | Aerosol inhalation | Mianyang Central Hospital, China | Recruiting |
| IRCT20100228003449N27 | IFNβ1α | Hydroxychloroquine, Lopinavir/Ritonavir | Subcutaneous | Tehran University of Medical Sciences, Iran | Recruiting |
| IRCT20100228003449N28 | IFNβ1α | Hydroxychloroquine, lopinavir/ritonavir | Subcutaneous | Tehran University of Medical Sciences, Iran | Recruiting |
| 2020-001023-14 | IFNβ1α | Standard care | Aerosol inhalation | Synairgen Ltd., United Kingdom | Recruiting |
| 2020-001113-21 (RECOVERY Trial) | IFNβ1α | Standard care | Aerosol inhalation | University of Oxford, United Kingdom | Recruiting |
| 2020-000936-23 (DisCoVeRy Trial) | IFNβ1α | Standard care | Subcutaneous | INSERM, France | Recruiting |
| NCT04315948 (DisCoVeRy Trial) | IFNβ1α | Lopinavir/Ritonavir or Standard care | Subcutaneous | Institut National de la Sante Et de la Recherche Medicale, France | Recruiting |
| NCT04276688 | IFNβ1β | Lopinavir/Ritonavir, Ribavirin | Subcutaneous | The University of Hong Kong, Hong Kong. | Recruitment completed |
| NCT04254874 | IFNα2β | Abidol hydrochloride | Atomization (nasal spray) | Tongji Hospital, China | Recruiting |
| NCT04293887 | IFNα1β | Lopinavir/Ritonavir, Remdesivir | Nebulization inhalation | Tongji Hospital, China | Not recruiting |
| NCT04552379 | IFNβ1α | Standard care | Subcutaneous | Pontificia Universidad Catolica de Chile | Recruiting |
| NCT04273763 | IFNα2β | Bromhexine Hydrochloride, Abidol hydrochloride and Standard care | Atomization (nasal spray) | WanBangDe Pharmaceuticals, China | Not recruiting |
| NCT04291729 | IFNα2 | Danoprevir/Ritonavir | Spray inhalation | The Ninth Hospital of Nanchang, China | Completed |
| NCT04251871 | IFNα | Lopinavir/Ritonavir or Traditional Chinese Medicines | Aerosol inhalation | Beijing 302 Hospital, China | Recruiting |
| NCT04275388 | IFNα | Lopinavir/Ritonavir and Abidor Hydrochloride | Nebulization inhalation | Jiangxi Qingfeng Pharmaceutical Co. Ltd. | Not recruiting |
| NCT04469491 | IFNβ1β | Standard care | Nebulization inhalation | Centre Hospitalier Universitaire, Amiens | Suspended |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subbian, S. The Abstruse Side of Type I Interferon Immunotherapy for COVID-19 Cases with Comorbidities. J. Respir. 2021, 1, 49-59. https://doi.org/10.3390/jor1010005
Subbian S. The Abstruse Side of Type I Interferon Immunotherapy for COVID-19 Cases with Comorbidities. Journal of Respiration. 2021; 1(1):49-59. https://doi.org/10.3390/jor1010005
Chicago/Turabian StyleSubbian, Selvakumar. 2021. "The Abstruse Side of Type I Interferon Immunotherapy for COVID-19 Cases with Comorbidities" Journal of Respiration 1, no. 1: 49-59. https://doi.org/10.3390/jor1010005
APA StyleSubbian, S. (2021). The Abstruse Side of Type I Interferon Immunotherapy for COVID-19 Cases with Comorbidities. Journal of Respiration, 1(1), 49-59. https://doi.org/10.3390/jor1010005






